Abstract
p-Coumaric acid (pCA), a naturally occurring bioactive, has been chemically incorporated into a poly(anhydride-ester) backbone through solution polymerization following a Knoevenagel synthetic approach to overcome drug delivery issues associated with its short half-life in vivo. Nuclear magnetic resonance and Fourier transform infrared spectroscopies indicated that pCA was successfully incorporated without noticeable alterations in structural integrity. The weight-average molecular weight and thermal properties were determined for the polymer, which exhibited a molecular weight of over 26,000 Da and a glass transition temperature of 57 °C. In addition, in vitro pCA release via hydrolytic anhydride and ester bond cleavage demonstrated pCA release over 30 days and maintained its antioxidant activity, demonstrating its potential as a controlled release system.
Keywords: biodegradable, polymer, poly(anhydride-ester), coumaric acid, drug delivery systems
Introduction
p-Coumaric acid (pCA) is a naturally occurring hydroxycinnamic acid derivative found in grains, teas, and fruits that exhibits many beneficial properties.[1] It is well-known that pCA displays antioxidant, anti-thrombotic, and antimicrobial properties indicating the potential for a variety of applications.[2] Notably, pCA has demonstrated both anti-inflammatory and antioxidant activities in the gastrointestinal system.[3] Research has demonstrated that pCA can reduce oxidative DNA damage and cyclooxygenase-2 (COX 2) overexpression in colonic mucosa of rats and thus has potential for use in treatment of colitis and other related inflammatory conditions.[3a] However, pCA use is currently limited due to its short half-life in vivo, necessitating frequent dosing to maintain therapeutically relevant concentrations.[4]
To control pCA release, researchers have incorporated the bioactive into various matrices. For example, pCA has been physically incorporated into a zinc basic salt hybrid material to achieve pCA release via diffusion, but this system exhibited a burst release and resulted in low pCA concentrations.[5] Additionally, pCA homopolymers[6] and copolymers[7] have been prepared, but either their delivery wasn't studied or their properties were not ideal (i.e., required enzymatic degradation and exhibited low Tg values, limiting in vivo use as the deformation would alter the drug release profile). Therefore, a polymer with a Tg value greater than physiological temperature that releases pCA in a controlled manner over time is desired.
One such method to improve bioactive delivery is through incorporation into a biodegradable polyanhydride backbone, as polyanhydrides undergo surface erosion,[8] unlike the bulk erosion exhibited by polyesters,[9] thus enabling controlled pCA release. This methodology has been demonstrated for ferulic acid, another hydroxycinnamic acid utilizing a Knoevenagel condensation reaction. The resulting poly(anhydride-ester) was shown to protect ferulic acid from premature degradation and release ferulic acid over a period > 1 month. Furthermore, the ferulic acid released from the polymer was shown to exhibit antioxidant and antimicrobial activity equivalent to free ferulic acid.[10]
In this work, a biodegradable poly(anhydride-ester) containing pCA in the backbone was synthesized via solution polymerization method and characterized using proton and carbon nuclear magnetic resonance (1H and 13C NMR) and Fourier transform infrared (FTIR) spectroscopies. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were used to determine thermal properties. Gel permeation chromatography (GPC) and mass spectrometry (MS) were used to determine weight-averaged molecular weight (Mw) of polymer and molecular weight of all polymer precursors, respectively. In addition, in vitro release studies were performed to study the release of pCA via the hydrolytic degradation of the polymer.
Experimental Section
Materials
Silica gel was purchased from VWR (Radnor, PA). 1 N hydrochloric acid (HCl), concentrated HCl, poly(vinylidine fluoride) and poly(tetrafluoroethylene) syringe filters, and Wheaton glass scintillation vials were purchased from Fisher Scientific (Fair Lawn, NJ). All other reagents and solvents were purchased from Aldrich (Milwaukee, WI) and used as received.
Characterization Methods
Chemical composition of polymer and intermediates were synthesized and characterized. 1H and 13C NMR spectra were recorded on a Varian 400 or 500 MHz spectrometer using deuterated chloroform (CDCl3) with TMS as internal reference or deuterated dimethyl sulfoxide (DMSO-d6) as solvent and internal reference. FTIR spectra were obtained using a Thermo Nicolet/Avatar 360 spectrometer of samples ground and pressed with KBr into a disc. Each spectrum was an average of 32 scans. A Finnigan LCQ-DUO equipped with Xcalibur software and an adjustable API-ESI Ion Source was used for mass spectrometry. Samples dissolved in methanol (10 μg/mL) were injected with a glass syringe. Pressure during experiments was 0.8 × 10-5 and 150 °C API temperatures.
A TA Instrument Q200 was used to determine melting (Tm) and glass transition (Tg) temperatures. Measurements on samples (4-6 mg) heated under nitrogen atmosphere from –10 °C to 200 °C at a heating rate of 10 °C/min and cooled to –10 °C at a rate of 10 °C/min with a two-cycle minimum were performed. TA Instruments Universal Analysis 2000 software, version 4.5A was used to analyze the data. A Perkin-Elmer Pyris 1 system with TAC 7/DX instrument controller and Perkin-Elmer Pyris software for data collection was utilized for determining decomposition temperatures (Td) by heating samples (5-10 mg) under nitrogen atmosphere from 25 °C to 400 °C at a heating rate of 10 °C/min. Decomposition temperatures were measured at the onset of thermal decomposition.
Polymer weight-averaged molecular weight and polydispersity were determined using a Perkin-Elmer liquid chromatography system consisting of a Series 200 refractive index detector, a Series 200 LC pump, and an ISS 200 autosampler. Automation of the samples and processing of the data was performed using a Dell OptiPlex GX110 computer running Perkin-Elmer TurboChrom 4 software with a Perkin-Elmer Nelson 900 Series Interface and 600 Series Link. Polymer samples were prepared by dissolving in dichloromethane (DCM, 10 mg/mL) and filtering through 0.45 μm poly(tetrafluoroethylene) syringe filters. Samples were resolved on a Jordi divinylbenzene mixed-bed GPC column (7.8 × 300 mm, Alltech Associates, Deerfield, IL) at 25 °C, with DCM as the mobile phase at a flow rate of 1.0 mL/min. Molecular weights were calibrated relative to broad polystyrene standards (Polymer Source Inc., Dorval, Canada).
Polymer Precursor Synthesis (1-3)
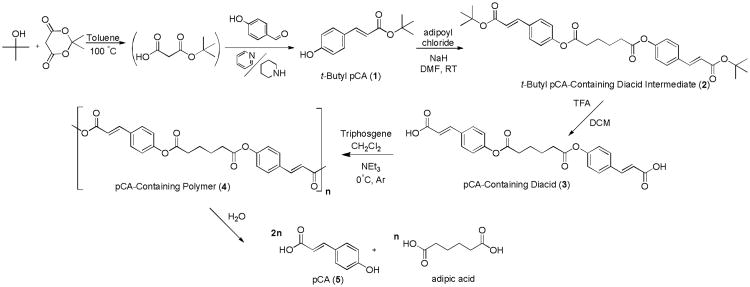
Polymer precursors were synthesized using previously described techniques (Scheme 1);[10] however, a different aldehyde, 4-hydroxybenzaldehyde, was used to prepare the pCA version. In brief, Meldrum's acid was dissolved in toluene, tertiary butanol added, and the reaction heated to 100 °C. The reaction was stopped after complete Meldrum's acid consumption. After cooling to room temperature, aldehyde was added followed by pyridine and piperidine, and then heated to 75 °C for 24 hours. The reaction mixture was dried in vacuo and residue washed and purified to yield 1. Product 1 was dissolved in anhydrous dimethylformamide (DMF) to which sodium hydride (NaH) was then added followed by drop-wise addition of adipoyl chloride and stirred until complete t-butyl pCA consumption. The reaction was washed and then dried in vacuo to yield 2. Compound 2 was dissolved in anhydrous DCM to which trifluoroacetic acid (TFA) was added. After completion, solvent was removed in vacuo and the residue was triturated with DI water, isolated, and dried in vacuo.
Scheme 1.
Synthesis of pCA-containing poly(anhydride-esters) (4) and pCA-containing polymer precursors including diacid (3).
t-Butyl pCA (1)
Yield: 86 % (white crystals). 1H-NMR (400 MHz, CDCl3): δ 7.54 (d, 1H, J=16.0 Hz, R-CH=CH-R), 7.37 (d, 2H, Ar-H), 6.61 (s, 1H, OH), 6.87 (d, 2H, Ar-H), 6.21 (d, 1H, J=16.0 Hz, R-CH=CH-R), 1.55 (s, 9H, 3CH3). 13C-NMR (CDCl3): δ 167.8, 158.3, 144.1, 130.1, 127.3, 117.4, 116.1, 81.1, 28.5. IR (KBr): 3 270 (s, OH, Ph), 1 674 (vs, C=O, ester), 1 630 and 1600 cm-1 (vs, C=C). Tm: 98-101 °C.
t-Butyl pCA-containing Diacid Intermediate (2)
Yield: 70 % (white powder). 1H-NMR (400 MHz, CDCl3): δ 7.58 (d, 2H, J=16.0 Hz, R-CH=CH-R), 7.51 (d, 4H, Ar-H), 7.11 (d, 4H, Ar-H), 6.32 (d, 2H, J=16.0 Hz, R-CH=CH-R), 2.66 (m, 4H, CH2), 1.89 (m, 4H, CH2), 1.53 (s, 18H, 3CH3). 13C-NMR (CDCl3): δ 171.6, 166.4, 152.0, 142.6, 132.7, 129.3, 122.2, 120.6, 80.8, 34.2, 28.4, 24.4. IR (KBr): 1 750 (vs, C=O, ester), 1 700 (vs, C=O, ester), 1 640 and 1 600 cm-1 (vs, C=C). Tm: 150-153 °C.
pCA-containing Diacid (3)
Yield: 99 % (white powder). 1H-NMR (500 MHz, DMSO-d6): δ 12.49 (s, 2H, COOH), 7.82 (d, 4H, Ar-H), 7.67 (d, 2H, J=16.0 Hz, R-CH=CH-R), 7.25 (d, 4H, Ar-H), 6.59 (d, 2H, J=16.0 Hz, R-CH=CH-R), 2.74 (m, 4H, CH2), 1.82 (m, 4H, CH2). 13C-NMR (DMSO-d6): δ 172.2, 168.2, 152.5, 143.6, 132.6, 130.1, 123.0, 120.0, 33.8, 24.3. IR (KBr):2 553 (s, OH, COOH), 1 759 (vs, C=O, ester), 1 677 (vs, C=O, COOH), 1 624 and 1 600 cm-1 (vs, C=C). Tm: 273-274 °C. ESI-MS: m/z 437-.
pCA-containing Poly(anhydride-ester) (4) Synthesis
The polymer (4) was prepared using previously described methods[10] (Scheme 1). Diacid 3 was dissolved in anhydrous DCM under argon and triethylamine (NEt3) added and cooled to 0 °C. Triphosgene dissolved in anhydrous DCM was added drop-wise and reaction progressed for 6 h. The reaction mixture was precipitated over chilled diethyl ether and polymer isolated via vacuum filtration. The residue was dissolved in anhydrous DCM, washed with acidic water, dried over MgSO4, concentrated, and precipitated with an excess of chilled diethyl ether. Polymer was isolated via vacuum filtration and dried in vacuo.
pCA-containing Polymer (4)
Yield: 73 % (white solid). 1H-NMR (500 MHz, DMSO-d6): δ 7.92 (6H, J=16 Hz, R-CH=CH-R, Ar-H), 7.21 (4H, Ar-H), 6.81 (2H, J=16 Hz, R-CH=CH-R), 2.65 (4H, CH2), 1.71 (4H, CH2).13C-NMR (DMSO-d6): δ 172.1, 163.4, 153.4, 148.3, 132.0, 131.1, 123.2, 117.6, 33.8, 24.3. IR (KBr, cm-1): 1 800-1 720 (vs, C=O, anhydride and ester region), 1 630 and 1 600 cm-1 (vs, C=C). Mw = 26,700 Da, PDI = 1.9. Tg = 57 °C. Td = 302 °C.
In VitropCA Release from Polymer
Polymer degradation was monitored via appearance of pCA in phosphate buffered saline (PBS) by high-performance liquid chromatography (HPLC). Polymer discs (n = 3) were prepared by pressing ground polymer (45 ± 5 mg) into 8 mm diameter × 1 mm thick discs in an IR pellet die (International Crystal Laboratories, Garfield, NJ) with a bench-top hydraulic press (Carver model M, Wabash, IN). Pressure of 10,000 psi was applied for 5 min at room temperature. All pH measurements were performed using an Accumet® AR15 pH meter (Fisher Scientific, Fair Lawn, NJ).
Polymer (5) discs were placed in 20 mL Wheaton glass scintillation vials with 10 mL of PBS and incubated at 37 °C with agitation at 60 rpm using a controlled environment incubator-shaker (New Brunswick Scientific Co., Edison, NJ). The degradation media was collected every 2 days until day 12 and then collected every 4 days until day 30. The buffer solution was replaced by fresh solution (10 mL) and the spent media was analyzed over 30 days by HPLC. An XTerra® RP18 5 μm 4.6 × 150 mm column (Waters, Milford, MA) on a Waters 2695 Separations Module equipped with a Waters 2487 Dual λ Absorbance Detector was used to analyze and quantify pCA release via HPLC. The mobile phase was comprised of 0.05 M KH2PO4 with 1 % formic acid in DI water at pH 2.5 (70 %) and acetonitrile (30 %) run at 1 mL/min flow rate at room temperature. Samples were filtered using 0.22 μm poly(vinylidine fluoride) syringe filters and subsequently injected (20 μL) using an autosampler. Absorbance was monitored at λ = 305 nm. At this wavelength, pCA absorbs and minimal to no absorption by the diacid is observed. Amounts were calculated from a calibration curve of known pCA standard solutions.
Antioxidant Assay
To assess bioactivity, an antioxidant assay assessing radical reduction was performed by first preparing a solution of 2,2-diphenyl-1-picryl hydrazyl (DPPH) in methanol (0.024 mg/mL). Polymer sample (0.1 mL) was added to 3.9 mL DPPH solution and incubated at room temperature for one hour. As control, free pCA solution was prepared at specific concentrations corresponding to HPLC data gathered on day-10 and prepared identical to the degradation media samples. After one hour incubation time, the solutions were analyzed using a UV/vis spectrophotometer (λ = 517 nm). DPPH radical reduction (%) was calculated by: [(Abst0 – Abst)/Abst0] × 100, where Abst0 is the initial absorbance and Abst is the absorbance after 1 h. Absorbance values after adding PBS (0.1 mL) to the DPPH solution (3.9 mL) was used as Abst0. All radical quenching assays were performed in triplicate and student's t-tests were used to determine significance between the antioxidant activity of free pCA and degradation media (p < 0.05).
Results and Discussion
Polymer Synthesis and Characterization
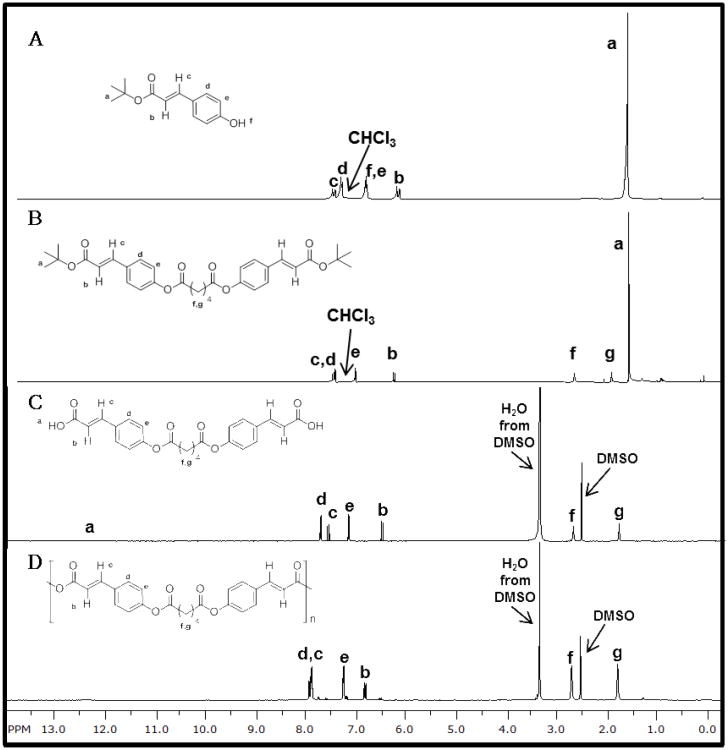
To achieve controlled pCA release, the bioactive was chemically incorporated into a polymer backbone as outlined in Scheme 1. Similar to the ferulic acid-containing polymers,[10] a one-pot Knoevenagel condensation reaction was utilized by reacting t-butanol with Meldrum's acid to form a malonic acid monoester, which was immediately reacted with 4-hydroxybenzaldehyde in the presence of pyridine and piper idine as catalysts to yield t-butyl pCA (1) (Figure 1A). This product was subsequently coupled with adipoyl chloride (1:2 ratio of adipoyl chloride:pCA) to yield the intermediate, 2 (Figure 1B). Diacid (3) was formed after removing the t-butyl ester from 2 with TFA and structure confirmed using 1H NMR (Figure 1C), 13C NMR, and FTIR spectra. DSC and MS were utilized for melting point and molecular weight determination, respectively.
Figure 1.
1H NMR spectra of t-butyl pCA, 1 (A), t-butyl pCA-based diacid intermediate, 2 (B), pCA-based diacid, 3 (C), and pCA-based polymer, 4 (D) are illustrated above.
Polymer 4 was synthesized via solution polymerization using 3 in the presence of triethylamine and triphosgene. As indicated by 1H NMR (Figure 1D) and FTIR spectroscopies, pCA was successfully incorporated without noticeable alterations in structural integrity. The bioactive was preserved after polymerization as observed in the obtained spectra. By chemically incorporating pCA (5) into the backbone of a poly(anhydride-ester) (4), a high drug loading was obtained (78 % by weight). The polymer exhibited a Mw and polydispersity index of 26,700 Da and 1.9, respectively with a decomposition temperature of 302 °C and Tg at 57 °C.
In Vitro pCA Release from Polymer
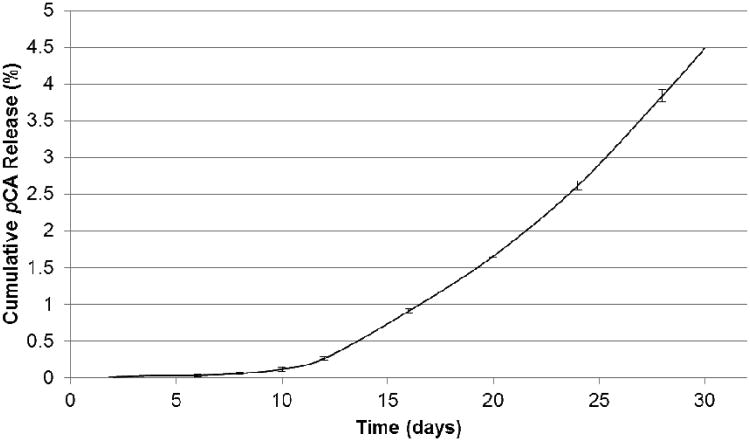
The in vitro degradation of the polymer to yield 5 and adipic acid (not detected by HPLC) was used to determine the pCA release rate. Polymer 4 was pressed into discs and incubated in PBS pH 7.4 at 37 °C to mimic physiological conditions. Polymer degradation is measured by the appearance of pCA in degradation media via HPLC. pCA release is dictated by the hydrolysis of anhydride and ester bonds; previous studies (using salicylic acid as bioactive) indicated more rapid anhydride bond hydrolysis than the ester bonds.[11] Within this study, however, diacid has limited solubility (≪ 0.002 mg/mL) relative to pCA under the conditions used and minimal diacid absorbance was observed at the specified wavelength. Although diacid could be present (retention time 7.26 min) following anhydride bond hydrolysis, it was not observed in the degradation media. Over the 30 day timeframe, pCA was detected at a 3.82 min retention time, indicating hydrolysis of both anhydride and ester bonds.
Initially, the polymer displayed an 8 day lag time (period where minimal to no pCA release occurs), commonly observed in polyanhydrides due to its surface eroding behavior.[8, 12] After 30 days, 4.5 % pCA is released from the polymer (Figure 2) indicating complete degradation is > 30 days. This system is expected to exhibit a linear release profile, as other poly(anhydride-esters) have demonstrated,[13]. By extrapolating the curve, degradation should be complete in ∼14 months (i.e., 100% pCA release).
Figure 2.
In vitro cumulative pCA (5) release from pCA-based poly(anhydride-ester) (4) (pCA ± standard deviation).
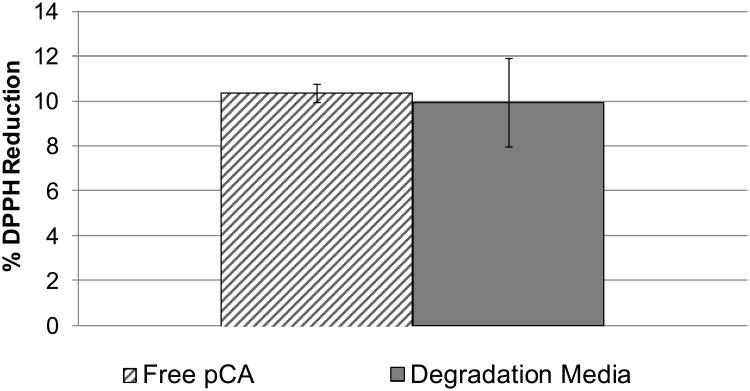
Antioxidant Assay.[14]
To ensure polymer synthesis did not affect pCA bioactivity, an antioxidant, radical quenching assay was used. The degradation media (from day-10) was compared to the corresponding free pCA solution (Figure 3). Student's t-tests were performed demonstrating that the observed antioxidant activity showed no statistical differences between degradation media and free pCA solutions (p < 0.05). Given that the polymer degradation products retain antioxidant activity similar to free pCA, we conclude that the pCA activity has not been diminished by the synthetic methodology.
Figure 3.
Radical quenching assay comparing free pCA to polymer degradation media (mean percent reduction ± standard deviation).
Conclusions
A pCA-based poly(anhydride-ester) was synthesized using solution polymerization method. These methods rendered a biodegradable polymer with high drug loading (78 %) that releases pCA over time via hydrolysis of anhydride and ester bonds. Favorable thermal properties including a Tg well above physiological temperature indicate the potential for in vivo use. The controlled, sustained release of the bioactive may be beneficial for maintaining pCA concentrations at biologically relevant levels necessary to combat gastrointestinal diseases. Additionally, the synthetic method presented herein allows for altering the pCA release rate by changing the structure of the linker molecule.
Acknowledgments
This work was supported by the National Institutes of Health (NIH 5 R01DE0132070-09 and NIH 1 R01DE019926-01) and the U.S. Department of Education Graduate Assistance in Areas of National Need (GAANN) Fellowship (MAO). Dr. Bryan Langowski (Rutgers, Department of Chemistry & Chemical Biology) is thanked for intellectual discussions.
References
- 1.[a] Herrmann K, Nagel CW. CRC Cr Rev Food Sci. 1989;28:315. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]; [b] El-Seedi HR, El-Said AMA, Khalifa SAM, Göransson U, Bohlin L, Borg-Karlson AK, Verpoorte R. J Agr Food Chem. 2012;60:10877. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 2.[a] Shahidi F, Chandrasekara A. Phytochem Rev. 2009;9:147. [Google Scholar]; [b] Ferguson LR, Zhu ST, Harris PJ. Mol Nutr Food Res. 2005;49:585. doi: 10.1002/mnfr.200500014. [DOI] [PubMed] [Google Scholar]; [c] Luceri C, Giannini L, Lodovici M, Antonucci E, Abbate R, Masini E, Dolara P. Br J Nutr. 2007;97:458. doi: 10.1017/S0007114507657882. [DOI] [PubMed] [Google Scholar]
- 3.[a] Luceri C, Guglielmi F, Lodovici M, Giannini L, Messerini L, Dolara P. Scand J Gastroentero. 2004;39:1128. doi: 10.1080/00365520410007908. [DOI] [PubMed] [Google Scholar]; [b] Guglielmi F, Luceri C, Giovannelli L, Dolara P, Lodovici M. Brit J Nutr. 2007;89:581. doi: 10.1079/BJN2003849. [DOI] [PubMed] [Google Scholar]
- 4.Konishi Y, Hitomi Y, Yoshioka E. J Agric Food Chem. 2004;52:2527. doi: 10.1021/jf035366k. [DOI] [PubMed] [Google Scholar]
- 5.Biswick T, Park DH, Shul YG, Choy JH. J Phys Chem Solids. 2010;71:647. [Google Scholar]
- 6.[a] Kaneko T, Matsusaki M, Hang TT, Akashi M. Macromol Rapid Commun. 2004;25:673. [Google Scholar]; [b] Kimura K, Inoue H, Kohama SI, Yamashita Y, Sakaguchi Y. Macromolecules. 2003;36:7721. [Google Scholar]
- 7.[a] Kaneko T, Thi TH, Shi DJ, Akashi M. Nature Materials. 2006;5:966. doi: 10.1038/nmat1778. [DOI] [PubMed] [Google Scholar]; b Nagata M, Hizakae S. Macromol Biosci. 2003;3:412. [Google Scholar]; [c] Jin XM, Carfagna C, Nicolais L, Lanzetta R. Macromolecules. 1995;28:4785. [Google Scholar]; [d] Spiliopoulos IK, Mikroyannidis JA. J Polym Sci, Part A: Polym Chem. 1996;34:2799. [Google Scholar]; [e] Kaneko T, Kaneko D, Wang S. Plant Biotechnol. 2010;27:243. [Google Scholar]
- 8.Göpferich A, Tessmar J. Adv Drug Deliv Rev. 2002;54:911. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 9.Nair LS, Laurencin CT. Prog Polym Sci. 2007;32:762. [Google Scholar]
- 10.Ouimet MA, Griffin J, Carbone-Howell AL, Wu WH, Stebbins ND, Di R, Uhrich KE. Biomacromolecules. 2013;14:854. doi: 10.1021/bm3018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdmann L, Uhrich KE. Biomaterials. 2000;21:1941. doi: 10.1016/s0142-9612(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker-Brothers K, Uhrich K. J Biomed Mater Res Part A. 2004;70A:309. doi: 10.1002/jbm.a.30083. [DOI] [PubMed] [Google Scholar]
- 13.Whitaker-Brothers K, Uhrich K. J Biomed Mater Res Part A. 2006;76A:470. doi: 10.1002/jbm.a.30356. [DOI] [PubMed] [Google Scholar]
- 14.Scherer R, Godoy H. Food Chemistry. 2009;112:654. [Google Scholar]