Abstract
Hepatic GK (glucokinase) plays a key role in maintaining glucose homoeostasis. Many stimuli regulate GK activity by controlling its gene transcription. We hypothesized that endogenous lipophilic molecules modulate hepatic Gck expression. Lipophilic molecules were extracted from rat livers, saponified and re-constituted as an LE (lipophilic extract). LE synergized with insulin to induce primary hepatocyte, but not β-cell, Gck expression in an SREBP-1c (sterol-regulatory-element-binding protein-1c)-independent manner. The dramatic induction of Gck mRNA resulted in a significant increase in GK activity. Subsequently, the active molecules were identified as retinol and retinal by MS after the purification of the active LE fractions. Retinoids synergized with insulin to induce Gck expression by the activation of both RAR [RA (retinoic acid) receptor] and RXR (retinoid X receptor). Inhibition of RAR activation completely abolished the effect of retinal. The hepatic GK specific activity and Gck mRNA levels of Zucker lean rats fed with a VAD [VA (vitamin A)-deficient] diet were significantly lower than those of rats fed with VAS (VA-sufficient) diet. Additionally, the hepatic Gck mRNA expression of Sprague–Dawley rats fed with a VAD diet was lower than that of rats fed with VA-marginal, -adequate or -supplemented diets. The reduced expression of Gck mRNA was increased after an intraperitoneal dose of RA in VAD rats. Furthermore, an intravenous injection of RA rapidly raised hepatic Gck expression in rats fed with a VAS control diet. Understanding the underlying mechanism that mediates the synergy may be helpful for developing a treatment strategy for patients with diabetes.
Keywords: hepatic glucokinase gene expression, insulin, primary hepatocyte, retinoid, synergistic effect, vitamin A deficiency
INTRODUCTION
Glucose must first be phosphorylated before being utilized by cells. This reaction is catalysed by a family of enzymes called hexokinases, which are found in different organisms ranging from bacteria to humans [1]. Mammalian hexokinase IV (D), also known as GK (glucokinase) (ATP:D-hexose 6-phosphotransferase; EC 2.7.1.1), plays a key role in maintaining glucose homoeostasis [2]. GK mutations have been associated with maturity onset diabetes of the young [3]. Whole-body or tissue-specific deletion of Gck in rodents demonstrated that either pancreatic β-cell or hepatic GK activity is essential for glucose homoeostasis [4,5].
As an essential micronutrient, VA (vitamin A; retinol) plays crucial roles in the general health of an individual [6]. Therefore retinol homoeostasis must be delicately maintained to meet optimal physiological requirements. This homoeostasis is achieved by a network of enzymes and proteins involved in the transport, production and catabolism of retinoids [7]. The regulation of this system can be attributed to the control of the expression of some of these enzymes by the active metabolite of retinol, RA (retinoic acid) [8]. RA exists in multiple isomeric forms, such as all-trans RA and 9-cis-RA, and RA regulates gene expression through activation of two families of nuclear receptors, RARs (RA receptors; RARα, β and γ ) activated by all-trans RA and RXRs (retinoid X receptors; RXRα, β and γ ) activated only by 9-cis-RA [9]. Elevation of hepatic VA contents in patients with diabetes was observed more than 70 years ago (in 1937) [10]. Depletion of hepatic glycogen content in VAD (VA-deficient) rats was reported in 1957 [11].
Long-term regulation of hepatic GK activity is controlled by its mRNA level. Transcription of Gck is regulated differentially by an upstream promoter in pancreatic β-cells and a downstream promoter in hepatocytes [12,13]. Activation of either one of them leads to the generation of a Gck mRNA with distinct 5′ sequences derived from the tissue-specific first exon. In rat liver, Gck mRNA is induced by insulin and suppressed by glucagon, a counter regulatory hormone to the actions of insulin [14,15]. It has been reported that RA induced Gck expression in rat hepatocytes without any additive effects on insulin-mediated induction [16,17].
Insulin resistance, diabetes and other metabolic abnormalities are associated with profound changes in hepatic lipid and glucose metabolism. These can be attributed to the altered expression of insulin-responsive genes [18]. Insulin-responsive elements in the Srebp-1c promoter have been identified as two LXR (liver X receptor)-binding sites and one sterol-regulatory element [19]. This implies that insulin could regulate the expression of its responsive genes by stimulation of the synthesis of endogenous agonists for nuclear receptor activation. We hypothesized that endogenous lipophilic molecules may play a role in the expression of genes involved in glucose metabolism. Herein, we report that an LE from rat liver synergized with insulin to induce hepatocyte Gck expression in an SREBP-1c (sterol-regulatory-element-binding protein-1c)-independent manner. The active molecules were subsequently identified as retinol and retinal. Retinoids synergized with insulin to induce Gck expression both in vitro and in vivo.
MATERIALS AND METHODS
Reagents
The reagents for primary hepatocyte isolation and culture have been published earlier [20]. GK activity assay reagents were obtained from Fisher Scientific (Pittsburgh, PA, U.S.A.). Ro41-5253 was obtained from Biomol (Plymouth Meeting, PA, U.S.A.). T0101317 was from J-Star Research (South Plainfield, NJ, U.S.A.). LG268 was synthesized in the core facility at the University of Texas Southwestern Medical Center at Dallas. All other compounds were purchased from Sigma (St. Louis, MO, U.S.A.) unless described otherwise.
Animals
Sprague–Dawley rats (for hepatocytes) and Wistar rats (for islets) were purchased from Harlan Breeders (Indianapolis, IN, U.S.A.). Zucker lean rats (fa/+, for diet study) were bred at UTK. Rats were housed in colony cages, and fed with a standard rodent diet. All procedures were approved by the Institutional Animal Care and Use Committee at the UTK, Pennsylvania State University or Duke University Medical Center.
Pancreatic islets, INS-1 cells and primary hepatocytes
Islets were harvested using Liberase R1 enzyme (Roche Diagnostics, Indianapolis, IN, U.S.A.) as described in [21]. Approximately 100 islets per treatment were incubated in 2 ml of RPMI 1640 medium containing 10 % (v/v) fetal calf serum in the absence or presence of LE (80 μg/ml) for 6 h. INS-1 cells were maintained as described previously [22]. Cells at 2 × 106 per 60 mm plate were treated without or with LE (80 μg/ml) in 2 ml of serum-free RPMI 1640 for 6 h. Primary hepatocytes were isolated and they were cultured as previously described [20]. All the tissue culture procedures were performed under yellow light.
RNA extraction and quantitative real-time PCR
Methods for preparation and analysis of RNA were described previously [20]. The real-time PCR primer sets for detecting Gck [23], Cyp26a1 [24] and Ubc [25] cDNAs have been reported previously. The primer sequences for Srebp-1c, 5′-GGAGCCA-TGGATTGCACATT-3′ (forward) and 5′-AGGCCAGGGAAGT-CACTGTCT-3′ (reverse), were designed using Primer Express software (Applied Biosystems). The gene expression level was normalized to that of the control housekeeping gene 36B4 unless described otherwise. Results are presented as fold induction for which the control group is arbitrarily assigned a value of 1, or the difference of the Ct (threshold cycle value) between the experimental gene and the control gene (36B4).
GK specific activity assay
For hepatocytes, cells at 3 × 106 per 60 mm plate were incubated in a medium containing the vehicle control, LE (80 μg/ml), insulin (1 nM) and insulin + LE for 9 h. Plates were washed once with cold PBS, frozen in liquid nitrogen and stored at −80 °C before GK activity assay [26]. For liver tissue of rats fed with VAD and VAS (VA-sufficient) diets, GK activity was measured by a discontinuous assay as described in [27]. Glucose-phosphorylating activity was measured spectrophotometrically in the presence of 0.5 mM glucose (considered as hexokinase activity) and 30 mM glucose (total activity). The activity obtained by subtracting the activity measured at 0.5 mM glucose from the total activity measured at 30 mM glucose was considered the GK activity. One unit is defined as activity that phosphorylates 1 μmol of D-glucose to D-glucose 6-phosphate per min. GK specific activity is presented as m-units/mg of protein of cell or tissue lysate.
Separation and extraction of LE components for activity assay
The LE preparation method has been published earlier [20]. Approx. 0.4 mg of LE was spotted on 1/8 of a silica gel TLC plate. The rest of the plate was spotted with 8 mg of LE in a continuous line. The plate was developed with ethyl ether/toluene (1:2, v/v) for 1 h. The 1/8 portion was cut and stained in iodine vapour. The lipid bands were marked, and the whole TLC plate was divided into 12 fractions from bottom to top. The corresponding bands were scraped from the plate and extracted twice with 10 ml of 15 % acetyl acetate in hexane. Then 1 ml of each fraction was combined and named the ‘mixed fraction’, which theoretically contains 5 % of the input. All fractions were dried under nitrogen, weighed and reconstituted in 150 μl of ethanol, except for the mixed fraction, which was reconstituted in 20 μl of ethanol, a 2-fold dilution compared with the input. The purity of the reconstituted fractions was confirmed using TLC and the same development condition. After development, the plate was visualized with iodine vapour and the image was scanned with an HP ScanJet5300C scanner. The fractions and the input were tested for their effects on Gck expression using real-time PCR.
MS analysis
Fractions with LE activity, and standards of retinol, retinal and cholesterol were dissolved in anhydrous ethanol and applied directly on to a Jeol AccuTOF-DART™ mass spectrometer in the MS core facility at the UTK. Positive mode was used. To identify each peak, natural abundance of isotope was assumed and used to deduce the molecular formula of each monoisotopic peak. The possible molecular formula of each peak was predicted by the software of the MS system with an allowed m/z error of less than 2 mmu (milli mass units). The spectral patterns of fragment ions derived from retinol, retinal and cholesterol were predicted by the software with the MS system, and confirmed using commercially available standards run under the same conditions.
Dietary studies and RA treatments in vivo
For dietary studies, female Sprague–Dawley rats (Charles River, Wilmington, MA, U.S.A.) were fed with a semisynthetic diet (AIN-93G diet, prepared for us by Research Diets, New Brunswick, NJ, U.S.A.), containing one of four levels of VA. The diets, designated VAD, VAM (VA-marginal), VA-Ad (VA-adequate) and VA-Sup (VA-supplemented) (0, 0.4, 4 and 100 mg of retinol equivalents per kg of diet respectively) were fed continuously from weaning to 8 weeks of age [28]. For the four diet groups, there were no differences in body weight, and body condition was indistinguishable. The VAD groups had significantly lower plasma retinol, 0.09 μM, compared with 0.9–1.4 μM in the other three groups.
For liver GK activity study, male Zucker lean rats (fa/+, four rats per group) were fed with a VAD purified diet (no. 5822; 0 IU (international unit)/g VA; TestDiet, Richmond, IN, U.S.A.) or a basal diet (no. 5755; 22.1 IU/g VA; TestDiet). The diets were fed continuously from weaning to 9 weeks of age. At this time, the body weight gain of rats fed with VAD diet stopped due to VA deficiency [29]. Animals were fasted for 6 h before they were killed for analysis of hepatic Gck expression and GK activity.
Two studies of the acute response to RA were conducted. In the first study, a 16 h time course in VAD rats, rats fed with the VAD diet as described above, were treated once with 100 μg of RA, delivered intraperitoneally for rapid uptake [24]. After 0 h (placebo treatment) and 3, 6, 10 and 16 h of RA treatment, the rats were individually killed and liver was collected for analysis. A second, short-term 90 min experiment was conducted in which rats of the same strain and sex, purchased at 6 weeks of age, were fed with a stock rodent diet until 8 weeks of age, at which time they received a single injection of albumin-bound RA administered into the common iliac vein, leading to rapid hepatic uptake [30]. The RA dose equalled ~ 25 μg per rat (10 μg/100 g body weight), and liver was collected after 0 h (vehicle injection), and 0.5, 1 and 1.5 h after injection of RA, as described above.
Statistics
Results are presented as means ± S.D. The number of experiments represents the independent experiments using hepatocytes isolated from different animals on different days. A Levene’s test was used to determined homogeneity of variance among groups using SPSS 16.0 statistical software, and where necessary, natural log transformation was performed before analysis. An independent-samples Student’s t test was used to compare two conditions. Multiple comparisons were analysed by one-way ANOVA using LSD (least significant difference) or Tukey’s test (for Figures 8 and 9 only due to unequal sample numbers in different groups) when equal variance was assumed, and the Games–Howell test was used when equal variance was not assumed. Differences were considered statistically significant at P < 0.05.
Figure 8. The hepatic Gck mRNA (A) and GK enzyme activity (B) in livers of Zucker lean rats fed with VAS and VAD diets and the Gck mRNA in livers of Sprague–Dawley rats in steady state (C) and after treatment of VAD rats with RA administered intraperitoneally (D).
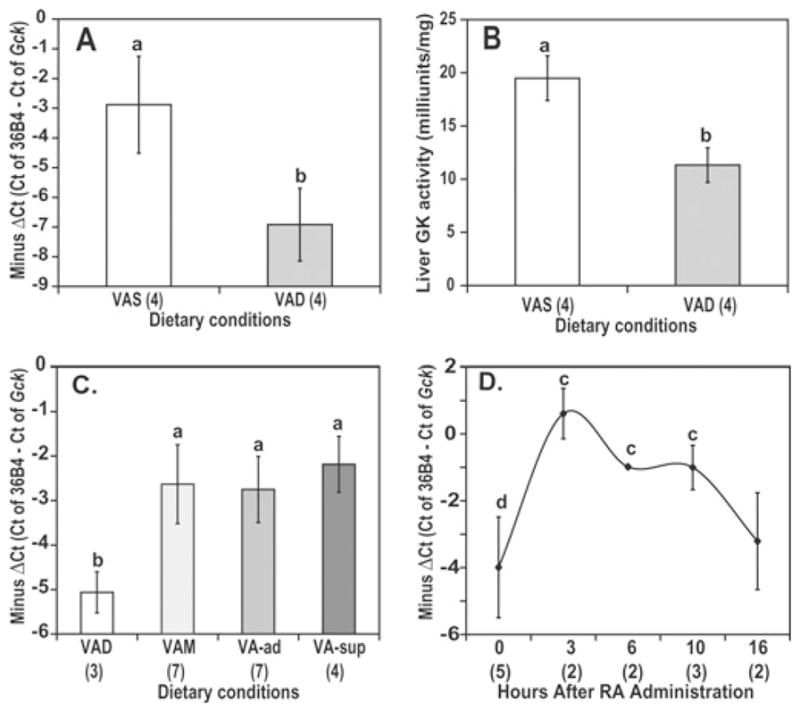
(A, B) Zucker lean rats were fed with VAD and VAS diet for 9 weeks. (C) Different categories of VA status were produced by feeding rats with one of four diets: VAD, VAM, VA-Ad and VA-Sup. (D) VAD rats received a single intraperitoneal administration of 100 μg of RA. Individual VAD rat livers were collected for analysis at the indicated time points. The results are presented as − ΔCt [−ΔCt = (Ct of 36B4) − (Ct of Gck )] for mRNA and m-units/mg of GK enzyme activity. Total RNA was isolated from individual rat livers (a > b; c > e, f; and d > f, all P < 0.02). Animal number, n, per group is shown in parentheses.
Figure 9. Expression levels of Ubc mRNA (A), Cyp26a1 mRNA (B) and Gck mRNA (C) in the liver of VAS control-fed rats after a single intravenous injection of 25 μg of RA.

Total RNA was isolated from the liver of individual rats at the indicated time points. The results are presented as − ΔC t [− ΔCt = (Ct of 36B4) − (Ct of indicated genes)]. Animal numbers are 2, 2, 3 and 3 for 0, 0.5, 1 and 1.5 h after RA injection respectively (a > c > d; b > d; e > f > g; all P < 0.03).
RESULTS
LE synergized with insulin to induce Gck expression in primary hepatocytes
LE and insulin alone increased the expression of Gck mRNA by 7.6 ± 5.9- and 19.3 ± 12.4-fold respectively (Figure 1A). LE + insulin induced Gck expression by 155 ± 72-fold, which is much higher than the sum of the individual effects of LE and insulin (7.6 + 19.3 = 26.9), demonstrating a synergy between LE and insulin. The induction occurred at a physiological concentration of glucose (5 mM in the culture medium), suggesting the physiological relevance of the observation.
Figure 1. LE synergizes with insulin to induce Gck expression in rat liver hepatocytes.
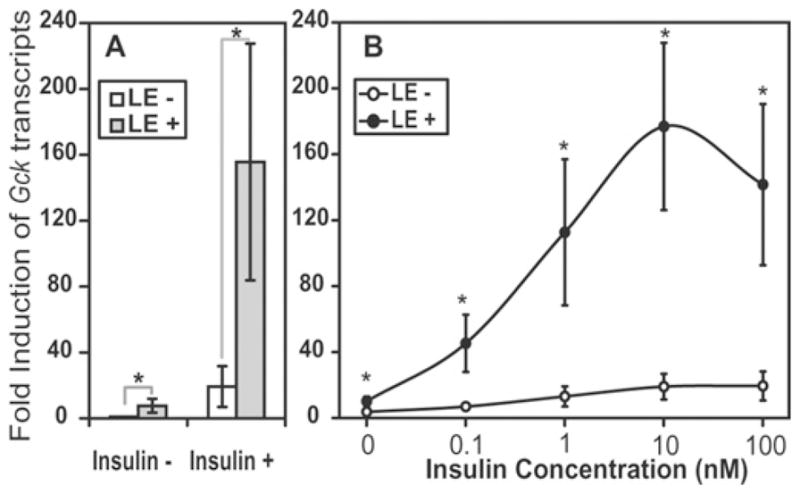
(A) The synergistic effect at 1 nM insulin (mean ± S.D., n = 7 with four batches of LE preparation, all *P < 0.001). (B) The synergistic effect of LE at increasing concentrations of insulin (means ± S.D., n = 3, all *P < 0.02 for comparing the control group with the LE group at the corresponding insulin concentration). Hepatocytes were treated with indicated concentrations of insulin in the absence or presence of LE for 6 h. The Gck mRNA expression level in the vehicle control group was arbitrarily assigned a value of 1.
It is possible that LE lowers the concentration required to reach a maximal fold induction by insulin. To test this, hepatocytes were treated without or with LE in the presence of increasing concentrations of insulin (from 0 to 100 nM). Figure 1(B) shows that the Gck expression increased from 1 (0 nM insulin) to 16-fold without LE and increased from 7- to 139-fold with LE. The induction by insulin reached the maximum (16.3-fold) at 10 nM insulin. The synergy between LE and insulin was evident at all concentrations of insulin tested. The fold induction by LE + insulin was beyond that which could be achieved by insulin alone. This result indicated that LE synergizes with insulin, rather than sensitizing the cells to an insulin-mediated transcriptional response.
LE had no effects on Gck expression in pancreatic β-cells, whereas LE + insulin induced GK activity in primary hepatocytes
Alternative promoters are used for tissue-specific Gck expression in hepatocytes and pancreatic β-cells [13]. As the primer set for real-time PCR detects both β-cell and hepatocyte Gck cDNA, the Gck expression in rat islets and two clones of INS-1 cells were examined. There was no significant induction of Gck mRNA in islets, 833/15 and 833/117 INS-1 cells after LE treatment (Figure 2A). These results indicate that the LE effect is specific to hepatocytes.
Figure 2. Effects of LE on the expression of Gck mRNA in rat islets and pancreatic β-cells and on GK activity in primary hepatocytes.

(A) Gck transcript levels in insulin-secreting cells. The level of Gck mRNA for each cell type in the vehicle control group was arbitrarily assigned a value of 1 (mean ± S.D., n = 3). (B) GK activity in primary hepatocytes treated without or with LE in the absence or presence of 1 nM insulin for 9 h. GK specific activity was measured as described in the Materials and methods section (mean ± S.D., n = 3, all *P < 0.02 for comparing LE + insulin with the vehicle control, LE or insulin group respectively).
GK activity in hepatocyte cell lysates was measured (Figure 2B). LE alone did not change GK activity, whereas GK activity in the LE + insulin group was significantly higher than that in the vehicle, insulin alone and LE alone groups. Thus, although the increase in GK activity was less than that for Gck mRNA, there was a significant induction of GK activity after LE + insulin treatment.
LE did not affect the decay of Gck mRNA in primary hepatocytes
Experiments were designed to measure the effect of LE on the decay of Gck mRNA (Figure 3A). Hepatocytes were treated with vehicle or LE in the absence or presence of 1 nM insulin for 6 h. A set of dishes treated with LE + insulin for 6 h was washed once with PBS and then incubated in a medium containing 3 μM α-amanitin, a specific inhibitor of RNA polymerase II complex [31], without or with LE. Levels of 36B4 and Gck transcripts at the indicated time points were determined by real-time PCR analysis. LE synergized with insulin to increase Gck mRNA, as anticipated (Figure 3B). The decay of 36B4 mRNA (an invariable control gene) was not affected by LE treatment (Figure 3C). The estimated half-life of 36B4 mRNA was more than 6 h for either vehicle or LE group. The decay rate of Gck mRNA was similar in the absence or presence of LE (Figure 3D). The levels of Gck mRNA decreased rapidly after the removal of insulin and the initiation of transcription inhibition by α-amanitin. The estimated half-life of Gck mRNA for either group was approx. 45 min, which agrees well with published results [14]. These results demonstrate that LE does not affect the rate of decay of Gck mRNA.
Figure 3. LE does not regulate the decay of Gck mRNA in rat liver hepatocytes.

(A) Schematic diagram of the experimental design. Hepatocytes were treated with or without 80 μg/ml of LE in the absence or presence of 1 nM insulin for 6 h. One set of dishes was treated with LE + insulin and washed once with PBS and then incubated in a fresh medium containing 3 μM α-amanitin in the absence and presence of LE. Total RNA was isolated at 0.5, 1, 2, 4 and 6 h after the treatment and subjected to real-time PCR analysis. (B) Synergism of LE with insulin on Gck expression (all *P < 0.002). (C) Stability of 36B4 transcripts. (D) Stability of Gck transcripts. The level of each transcript at zero time was arbitrarily assigned a value of 100 % (mean ± S.D., n = 4).
The induction of Gck expression by LE + insulin occurred in an Srebp-1c induction-independent manner
Since SREBP-1c, an insulin-induced transcription factor, has been suggested to be the main mediator of insulin-induced Gck expression [32], an induction of Srebp-1c expression would be anticipated if it also mediates the action of LE. The expression of Gck (Figure 4A) and Srebp-1c (Figure 4B) was monitored over a 24 h period of time after the indicated treatments. The Gck mRNA levels in the control group (a value of 1 at zero time) decreased significantly to 0.2 ± 0.04 in 3 h and further declined up to 24 h. The Gck mRNA levels in the LE group were significantly higher than those in the control group at each of the corresponding time points. The reduction in Gck mRNA occurred in both groups and stabilized after 6 h of incubation. Treatment with insulin alone resulted in only a small increase in Gck mRNA at 3 h, compared with its level at zero time. The Gck mRNA levels in the LE + insulin group were significantly higher than those in the insulin group at each of the 3, 6, 9 and 12 h time points. The synergy between insulin and LE was evident as early as 3 h. Thus the presence of LE maintained hepatocyte Gck mRNA at a significantly higher level, regardless of the presence or absence of insulin.
Figure 4. Comparison of the expression levels of Gck (A) and Srebp-1c (B) mRNA over time and the effect of LXR activation on Gck (C) and Srebp-1c (D) expression in hepatocytes.

(A, B) Hepatocytes were treated with or without LE in the absence or presence of 1 nM insulin. The expression level of indicated transcripts at zero time was assigned a value of 1 (mean ± S.D., n = 3, all *P < 0.02 for comparing the vehicle control and LE groups at 3, 6, 9 and 12 h respectively; all **P < 0.02 for comparing insulin with the LE + insulin groups at 3, 6, 9 and 12 h respectively; all ***P < 0.02 for comparing insulin with the LE + insulin groups at 3 and 6 h respectively). (C, D) Hepatocytes were treated without or with 1 μM T0901317 in the absence or presence of 1 nM insulin for 6 h. The level of indicated transcripts in the vehicle control group was arbitrarily assigned a value of 1 (mean ± S.D., n = 3, all *P < 0.001).
In the same study, Srebp-1c mRNA in both the control group and the LE group decreased at the same rate and stabilized after 6 h without insulin (Figure 4B). A comparison of Figures 4(B) and 4(A) shows that the induction pattern of Srebp-1c mRNA does not match that of Gck mRNA. First, LE only transiently synergized with insulin to induce the expression of Srebp-1c mRNA at 3 and 6 h. Secondly, the peaks in Gck mRNA induced by insulin and LE + insulin treatments preceded those of Srebp-1c mRNA induced by the same treatments. Moreover, Gck mRNA in the LE + insulin group had already started to decline when the Srebp-1c mRNA began to reach a peak at 9–12 h. These results suggest that the effect of LE could be SREBP-1c independent, which was further examined next.
The expression of Srebp-1c can be specifically induced by treatment with T0901317, a non-sterol agonist for LXR activation [33]. Treatments with T0901317, insulin and T0901317 + insulin induced Gck mRNA levels by 1.4 ± 0.3-, 12 ± 2.5- and 18 ± 1.6-fold respectively, whereas T0901317 alone did not significantly induce Gck expression (Figure 4C). Although the induction by T0901317 + insulin was significantly higher than that produced by insulin alone (P < 0.05), the difference between them (1.5-fold, 18 versus 12) was not comparable with the difference between LE + insulin and LE treatments (8-fold, 155 versus 19, shown previously in Figure 1A). This comparison demonstrates that T0901317 and insulin did not synergize to increase Gck expression. However, T0901317, insulin and T091317 + insulin all induced Srebp-1c mRNA significantly (Figure 4D). Moreover, T0901317 synergized with insulin, demonstrating a robust response of Srebp-1c expression to LXR activation. These results thus indicate that the massive induction of Srebp-1c expression by T0901317 or T0901317 + insulin is not sufficient to mimic the LE or LE + insulin effects on Gck expression.
Retinol and retinal were identified as the active molecules present in LE
To identify the active molecules present in LE, it was first separated on a TLC plate into eight detectable lipid bands (Figure 5A), after which the whole TLC plate from the origin to solvent front was divided into 12 fractions and each was extracted to obtain the components of them. Figure 5(B) shows an image of the TLC plate after running authentic standards of ROL (all-trans-retinol; 28.7 μg), RAL (all-trans-retinal; 28.4 μg), all-trans-RA (Figure 5B, ‘RA’; 15 μg), along with LE (200 μg) and 1/15 of each of the reconstituted fractions. Fraction F5 had the same mobility as retinol in this TLC system. The effects of each fraction on Gck mRNA expression were measured (Figure 5C). When the Gck expression level in hepatocytes treated with insulin alone was assigned a value of 1 (control), the input, F5, F6, F7 and the mixed fraction with equivalent amounts to the input resulted in a further induction of Gck expression by 5.3-, 2.3-, 1.3-, 1.7-and 2.1-fold respectively. Although part of the LE activity in the input was apparently lost, as the fold induction by each active fraction was lower than the fold induction by the un-separated input, nevertheless the fold induction of Gck mRNA by the mixed fraction was the same as for fraction F5. This demonstrated that there were no additive effects when the components of all the fractions were combined. Since F5 appeared as a single band, it was subjected to MS analysis for identification. Figure 5(D) shows the positive m/z spectrum of F5. The m/z values of the indicated monoisotopic peaks matched the theoretical m/z values of the fragment ions for retinol, retinal and cholesterol, with errors of less than 2 mmu. The existence of these monoisotopic peaks was confirmed.
Figure 5. Fractionation and characterization of the components of LE.
(A) Separation of LE on a silica gel TLC plate. The detailed procedure is described in the Materials and methods section. The boundaries of fractions F1–12 are marked. (B) Separation of retinoid standards, LE and reconstituted fractions on a TLC plate, from left to right: lane 1, ROL (28.7 μg); lane 2, RAL (28.4 μg); lane 3, RA (15 μg); lane 4, LE (200 μg); lanes 5–16, 1:15 (v/v) of each fraction from fractions F1–F12. (C) The effects of each fraction on Gck expression. Hepatocytes were treated with the vehicle control, LE input, equivalent amounts of fractions F1–F12 or the mixed fraction in the presence of 1 nM insulin for 6 h. The level of Gck mRNA in the control group was arbitrarily assigned a value of 1. (D) Positive m/z spectrum of fraction F5 obtained by a Jeol AccuTOF-DARTTM mass spectrometer. The allowed m/z error was less than 2 mmu. Natural abundance of isotope was assumed and used for deducing the molecular formula of each monoisotopic peak (* indicates unknown and ** indicates cholesterol).
Since cholesterol has no effect on Gck expression (results not shown), we analysed the effects of ROL, RAL and RA (Figure 6). Without insulin, a significant induction of Gck expression was detected at 2 μM of ROL, 0.2 μM of RAL and 0.002 μM of RA. When insulin was present there was a 14.2 ± 1.7-fold increase with insulin alone, and the synergy of insulin plus retinoids began to be evident at the same concentrations at which the retinoids alone were effective. The overall sequence of potency, from the weakest to the strongest inducer, was ROL < RAL < RA. Thus, although all three retinoids induced Gck expression and synergized with insulin at 2 μM or higher, only RA synergized with insulin at concentrations as low as 0.002 μM. These results demonstrated that although several retinoids added to hepatocytes synergize with insulin to induce Gck expression, RA is the most potent by several orders of magnitude.
Figure 6. Retinoids synergize with insulin to induce Gck mRNA expression in rat primary hepatocytes.

Hepatocytes were treated with the vehicle control or increasing concentrations of ROL, RAL and RA in the absence or presence of 1 nM insulin for 6 h. Gck mRNA level in the vehicle control group was assigned a value of 1 (mean ± S.D., n = 3, a < b, c < d, all P < 0.05).
Activation of both RAR and RXR mimicked and inhibition of RARα activation abolished the synergy between retinoids and insulin on Gck expression
As retinoids are known to activate RAR and RXR, primary hepatocytes were treated with TTNPB {(E)-4-[2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthyl-2-enyl)propen-1-yl]benzoic acid} (1 μM), a specific panagonist of RARs, and LG268 (1 μM), an RXR-specific agonist. Activation of RAR alone or RXR alone was sufficient to significantly induce Gck expression without or with insulin (Figure 7A). Additionally, a robust synergy with insulin was evident for both agonists. The fold induction were comparable with those induced by 20 μM of RAL or RA (comparing Figures 7A with Figure 6), indicating that retinoids synergize with insulin by activation of both RAR and RXR.
Figure 7. Effects of RAR/RXR activation (A) or inhibition of RARα (B) on the expression of Gck mRNA.

(A) Hepatocytes were incubated in media containing the vehicle control, TTNPB (RAR pan-agonist; 1 μM), LG268 (RXR agonist; 1 μM) or TTNPB + LG268 in the absence and presence of 1 nM insulin (mean ± S.D., n = 3, a < b < c < d, e < f < g, all P < 0.03). (B) Hepatocytes were treated with indicated concentrations of Ro41-5253, an RAR antagonist, without or with 2 μM RAL in the presence or absence of 1 nM insulin (mean ± S.D., n = 3, a′ < b′, c′ < d′ < e′, all P < 0.03). The expression level of Gck mRNA in the vehicle control group was assigned a value of 1.
If this conclusion is true, it may be possible to blunt the synergy using an antagonist of RAR activation. Figure 7(B) shows Gck mRNA levels in hepatocytes treated with RAL and insulin in the presence of increasing concentrations of Ro41-5253, a specific antagonist of RARα activation [34]. When RAL was not added, Ro41-5253 did not cause any significant reduction in Gck expression in the absence or presence of insulin. When insulin was absent, the fold induction of Gck expression by 2 μM RAL (3.7 ± 1.2-fold) was completely abolished (1.1 ± 0.3-fold) by 10 μM Ro41-5253. The synergy between 2 μM RAL and insulin (46.1 ± 11.8-fold) was completely abolished (9.4 ± 3.4-fold) by 10 μM Ro41-5253. These results imply that activation of RARα is required for the synergistic effects of retinoids and insulin.
VA deficiency resulted in a reduction in hepatic Gck expression and GK activity
To examine the physiological significance of the observation obtained with hepatocytes, GK specific activity and Gck mRNA levels in the livers of Zucker lean rats fed with VAD and VAS diets were measured. Expression of Gck mRNA was assessed by real-time PCR analysis and expressed as the negative difference of the Ct [− ΔCt = (Ct of 36B4) − (Ct of Gck)]. The larger the − ΔCt, the higher the expression level of the indicated transcripts. As shown in Figure 8(A), the Gck mRNA levels in the livers of rats fed with a VAD diet were significantly lower than those in the livers of rats fed with a VAS diet. This reduction of Gck mRNA correlates with a significant decrease in GK specific activity in livers of VAD rats (Figure 8B). The hepatic GK activity of VAD rats is significantly lower than that of VAS rats (11.3 ± 1.6 versus 19.5 ± 2.1 m-units/mg respectively).
To further confirm the observation, we also measured Gck expression in livers of Sprague–Dawley rats fed with four diets with different levels of VA, denoted by VAD, VAM, VA-Ad and VA-Sup diets. The levels of Gck mRNA in the liver of VAD rats were significantly lower than those in VAM, VA-Ad or VA-Sup diet groups (Figure 8C). These results demonstrated that a lack of retinoids due to VA deficiency results in a reduction in hepatic Gck expression.
RA treatment induced Gck expression in livers of VAD rats
Since RA was more potent in inducing hepatocyte Gck expression, and is the transcriptionally active metabolite of dietary VA [35], we next examined its effect on hepatic Gck expression in VAD rats. The Gck mRNA (Figure 8D) was measured at 0, 3, 6, 10 and 16 h after a single intraperitoneal dose of 100 μg of RA. The Gck expression was significantly induced as early as 3 h and remained significantly higher for at least 10 h before returning to the basal level 16 h later. These kinetics agreed with the effects of LE on Gck expression in hepatocytes (Figure 3A), in which Gck mRNA reached a peak at 3 h and fell back to basal level at 24 h after LE. This in vivo experiment thus indicated that RA is sufficient for inducing Gck expression in the liver of VAD rats. The results also suggest that RA, which has a half-life of less than 1 h in rat plasma in vivo [36], must be present nearly continuously to maintain normal levels of hepatic Gck expression.
Acute RA treatment rapidly induced Gck expression in livers of VAS rats
Next, we examined whether Gck expression responds acutely to an increase in the circulating level of RA in the VAS state. For this experiment, rats fed with a standard rodent diet were treated with a single 25 μg dose of RA administered intravenously, and liver tissue was collected after 0 h (vehicle treatment) and 0.5, 1 and 1.5 h. For comparison, the expression of Cyp26a1, an RA-4-hydroxylase, rapidly up-regulated by RA in rat liver [24] was measured (Figure 9B) along with Ubc (Figure 9A) and Gck mRNAs (Figure 9C). The hepatic expression of Ubc mRNA was not affected by the RA treatment. Hepatic Cyp26a1 mRNA was induced rapidly and dramatically after RA injection, demonstrating the success of RA injection. Hepatic Gck mRNA was induced concomitantly, and nearly as dramatically, at 0.5, 1 and 1.5 h after injection respectively. All these results demonstrated that hepatic Gck expression level can be regulated directly by retinoids, and especially by RA, in vivo. These results imply that circulating RA may play an important role in controlling physiological responses of Gck expression to nutritional and hormonal stimuli.
DISCUSSION
Liver is well known to contain numerous lipids that release sterols and retinoids after saponification. Activation of the oxysterol receptor, LXR, results in the induction of Gck expression in mouse liver [37]. The results shown here (Figure 4) and presented by others [38] indicated that this induction may be indirect as no direct induction of Gck mRNA by T0901317 could be observed despite robust induction of Srebp-1c mRNA in rat hepatocytes, suggesting an SREBP-1c-independent mechanism mediating LE + insulin effects. The lack of involvement of SREBP-1c in mediating insulin-induced Gck expression has been shown in a time course study similar to ours and a knockdown study using siRNA (small interfering RNA) methodology [39]. This conclusion is supported by results obtained from mice bearing Srebp-1c deletion. Their liver Gck expression responded normally to the cycle of fasting and re-feeding [33].
RA has been shown to induce Gck expression without any synergistic effect with insulin [16]. Our studies clearly demonstrated that retinoids synergized with insulin to induce Gck expression. This discrepancy may be caused by technical differences, such as culture plates, the medium and the lower concentration of dexamethasone used. The contribution of these factors to the discrepancy is worth investigating. In addition, RA induced Gck expression in islets after 24 and 48 h incubation [40]. The lack of induction in our study may be caused by the short-term (6 h) incubation, which did not allow us to observe the induction at later time points. Another explanation is that islets or INS-1 cells may lack the enzymes required to synthesize RA from retinol or retinal in LE. Further studies are necessary to determine the mechanism that causes the difference.
Elevation of hepatic VA contents in patients with diabetes was observed in 1937 [10]. In 1957, it was noted that hepatic glycogen storage was depleted in VAD rats [11]. The authors concluded that the depletion of glycogen was caused by the reduction of glycogenesis from trioses, rather than directly from glucose. Hepatic glycogen content was dramatically increased in rats fed with excess VA for only 2 days [41]. Recently, an elevation of plasma retinol-binding protein 4, a transport protein for retinol, has been observed in insulin-resistant humans and animals [42], suggesting the involvement of VA metabolism in glucose metabolism and insulin resistance. It is possible that the effects of VA status on the hepatic Gck expression may also play a role in these observations.
Retinol is reversibly converted into retinal, and retinal is irreversibly converted into RA [35]. Rat liver microsomes and cytosol contain enzymes that convert retinol into retinal and retinal into RA. The production of retinal was observed when the retinol concentration was 10 μM or higher. The production of RA from retinol only occurred at concentrations of 50 μM or higher [43]. Our results demonstrated that retinol was effective at 2 μM or higher. Retinal at 2 μM or higher, which exceeds normally low levels present in liver, was almost equivalent to RA in its ability to synergize with insulin. However, RA was effective, and synergized with insulin, at 0.002 μM. These findings imply that retinol and retinal most likely play a regulatory role in hepatic Gck expression when they are converted into the active metabolite, RA, in situ.
Normally, homoeostasis of plasma and tissue retinol levels is delicately maintained through a network of enzymes and proteins involved in the production, storage, transport and catabolism of retinoids [7,35]. Therefore circulating RA plays a role in signalling the body’s retinoid status [8]. Indeed, RA produced in the intestine and circulating through the portal system to the liver has been observed [6,44]. Our results have demonstrated that a single treatment with RA, intraperitoneally or intravenously, is sufficient to induce Gck expression in the livers of VAD and VA-Ad rats. It seems that RA either synthesized in hepatocytes or delivered through the circulation can induce Gck expression. It remains to be determined whether synthesis of RA in hepatocytes, or extrahepatic organs, followed by export to liver, varies in response to physiological changes, such as during the cycle of fasting and re-feeding, and in diabetes.
In recent years, efforts have been devoted to developing allosteric activators for increasing intracellular GK activity [45]. Our results demonstrate that the pathway mediating the synergy between retinoids and insulin is a potential drug target. Since GK is an inducible enzyme at the transcriptional level, manipulation of its mRNA level is probably more achievable under normal physiological conditions. In addition, because LE (mainly retinol) induces Gck expression specifically in hepatocytes, it becomes possible to induce GK activity in liver without perturbing its level in pancreatic β-cells. An improved understanding of the whole pathway may be beneficial for developing treatment strategies for patients with both types of diabetes.
Acknowledgments
We thank Ms Bella Yao and Dr Liguo Song at the MS core facility of the Chemistry Department of UTK for their technical support.
FUNDING
This work was supported by start-up funds from the University of Tennessee at Knoxville (to G. C.) and by the National Institutes of Health [grant number CA90214 (to A. C. R.)].
Abbreviations used
- Ct
threshold cycle value
- GK
glucokinase
- IU
international unit
- LE
lipophilic extract
- LXR
liver X receptor
- mmu
milli mass unit
- RA
retinoic acid
- RAL
all-trans-retinal
- RAR
RA receptor
- ROL
all-trans-retinol
- RXR
retinoid X receptor
- SREBP-1c
sterol-regulatory-element-binding protein-1c
- TTNPB
{(E)-4-[2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthyl-2-enyl)propen-1-yl]benzoic acid}
- VA
vitamin A
- VA-Ad
VA-adequate
- VAD diet
VA-deficient diet
- VAM
VA-marginal
- VAS diet
VA-sufficient diet
- VA-Sup
VA-supplemented
References
- 1.Cardenas ML, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401:242–264. doi: 10.1016/s0167-4889(97)00150-x. [DOI] [PubMed] [Google Scholar]
- 2.Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Han Y, Taub R, Grimsby J. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12. [PubMed] [Google Scholar]
- 3.Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P, et al. Familial hyperglycemia due to mutations in glucokinase – definition of a subtype of diabetes mellitus. N Engl J Med. 1993;328:697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- 4.Grupe A, Hultgren B, Ryan A, Ma YH, Bauer M, Stewart TA. Transgenic knockouts reveal a critical requirement for pancreatic [beta] cell glucokinase in maintaining glucose homeostasis. Cell. 1995;83:69–78. doi: 10.1016/0092-8674(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 5.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 6.Sporn M, Robers A, Goodman D. The Retinoids, Biology, Chemistry, and Medicine. Raven Press; New York: 1994. [Google Scholar]
- 7.Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–4458. doi: 10.1021/bi7003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 9.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 10.Moore T. Vitamin A and carotene: the vitamin A reserve of the adult human being in health and disease. Biochem J. 1937;31:155–164. doi: 10.1042/bj0310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf G, Lane MD, Johnson BC. Studies on the function of vitamin A in metabolism. J Biol Chem. 1957;225:995–1008. [PubMed] [Google Scholar]
- 12.Iynedjian PB, Pilot PR, Nouspikel T, Milburn JL, Quaade C, Hughes S, Ucla C, Newgard CB. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci USA. 1989;86:7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnuson MA, Shelton KD. An alternate promoter in the glucokinase gene is active in the pancreatic beta cell. J Biol Chem. 1989;264:15936–15942. [PubMed] [Google Scholar]
- 14.Iynedjian PB, Jotterand D, Nouspikel T, Asfari M, Pilot PR. Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon–cAMP system. J Biol Chem. 1989;264:21824–21829. [PubMed] [Google Scholar]
- 15.Sibrowski W, Seitz HJ. Rapid action of insulin and cyclic AMP in the regulation of functional messenger RNA coding for glucokinase in rat liver. J Biol Chem. 1984;259:343–346. [PubMed] [Google Scholar]
- 16.Cabrera-Valladares G, Matschinsky FM, Wang J, Fernandez-Mejia C. Effect of retinoic acid on glucokinase activity and gene expression in neonatal and adult cultured hepatocytes. Life Sci. 2001;68:2813–2824. doi: 10.1016/s0024-3205(01)01065-7. [DOI] [PubMed] [Google Scholar]
- 17.Decaux JF, Juanes M, Bossard P, Girard J. Effects of triiodothyronine and retinoic acid on glucokinase gene expression in neonatal rat hepatocytes. Mol Cell Endocrinol. 1997;130:61–67. doi: 10.1016/s0303-7207(97)00074-9. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G. Liver lipid molecules induce PEPCK-C gene transcription and attenuate insulin action. Biochem Biophys Res Commun. 2007;361:805–810. doi: 10.1016/j.bbrc.2007.07.108. [DOI] [PubMed] [Google Scholar]
- 21.Schisler JC, Jensen PB, Taylor DG, Becker TC, Knop FK, Takekawa S, German M, Weir GC, Lu D, Mirmira RG, Newgard CB. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci USA. 2005;102:7297–7302. doi: 10.1073/pnas.0502168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Hohmeier HE, Gasa R, Tran VV, Newgard CB. Selection of insulinoma cell lines with resistance to interleukin-1beta- and gamma-interferon-induced cytotoxicity. Diabetes. 2000;49:562–570. doi: 10.2337/diabetes.49.4.562. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Tsatsos NG, Towle HC. Direct role of ChREBP · Mlx in regulating hepatic glucose-responsive genes. J Biol Chem. 2005;280:12019–12027. doi: 10.1074/jbc.M413063200. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zolfaghari R, Ross AC. Cloning of rat cytochrome P450RAI (CYP26) cDNA and regulation of its gene expression by all-trans-retinoic acid in vivo. Arch Biochem Biophys. 2002;401:235–243. doi: 10.1016/S0003-9861(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 25.Rioja I, Clayton C, Graham S, Life P, Dickson M. Gene expression profiles in the rat streptococcal cell wall-induced arthritis model identified using microarray analysis. Arthritis Res Ther. 2005;7:R101–R117. doi: 10.1186/ar1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seoane J, Barbera A, Telemaque-Potts S, Newgard CB, Guinovart JJ. Glucokinase overexpression restores glucose utilization and storage in cultured hepatocytes from male Zucker diabetic fatty rats. J Biol Chem. 1999;274:31833–31838. doi: 10.1074/jbc.274.45.31833. [DOI] [PubMed] [Google Scholar]
- 27.Davidson AL, Arion WJ. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987;253:156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- 28.Cifelli CJ, Ross AC. Chronic vitamin A status and acute repletion with retinyl palmitate are determinants of the distribution and catabolism of all-trans-retinoic acid in rats. J Nutr. 2007;137:63–70. doi: 10.1093/jn/137.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCollum EV, Davis M. The necessity of certain lipins in the diet during growth. J Biol Chem. 1913;15:167–175. [Google Scholar]
- 30.Cifelli CJ, Ross AC. all-trans-retinoic acid distribution and metabolism in vitamin A-marginal rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G195–G202. doi: 10.1152/ajpgi.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bushnell DA, Cramer P, Kornberg RD. Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 Å resolution. Proc Natl Acad Sci USA. 2002;99:1218–1222. doi: 10.1073/pnas.251664698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein 1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 34.Keidel S, LeMotte P, Apfel C. Different agonist- and antagonist-induced conformational changes in retinoic acid receptors analyzed by protease mapping. Mol Cell Biol. 1994;14:287–298. doi: 10.1128/mcb.14.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross AC, Harrison EH. In: Handbook of Vitamins. McCormick DE, Rucker RR, Suttie JW, Zempleni J, editors. Dekker/CRC Press; Boca Raton, FL: 2006. pp. 1–39. [Google Scholar]
- 36.El Mansouri S, Tod M, Leclerq M, Petitjean O, Perret G, Porthault M. Time- and dose-dependent kinetics of all-trans-retinoic acid in rats after oral or intravenous administration(s) Drug Metab Dispos. 1995;23:227–231. [PubMed] [Google Scholar]
- 37.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansmannel F, Mordier S, Iynedjian PB. Insulin induction of glucokinase and fatty acid synthase in hepatocytes: analysis of the roles of sterol-regulatory-element-binding protein-1c and liver X receptor. Biochem J. 2006;399:275–283. doi: 10.1042/BJ20060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregori C, Guillet-Deniau I, Girard J, Decaux JF, Pichard AL. Insulin regulation of glucokinase gene expression: evidence against a role for sterol regulatory element binding protein 1 in primary hepatocytes. FEBS Lett. 2006;580:410–414. doi: 10.1016/j.febslet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Cabrera-Valladares G, German MS, Matschinsky FM, Wang J, Fernandez-Mejia C. Effect of retinoic acid on glucokinase activity and gene expression and on insulin secretion in primary cultures of pancreatic islets. Endocrinology. 1999;140:3091–3096. doi: 10.1210/endo.140.7.6765. [DOI] [PubMed] [Google Scholar]
- 41.Singh M, Singh VN, Venkitasubramanian TA. Early effects of feeding excess vitamin A: hepatic glycogen, blood lactic acid, plasma NEFA and glucose tolerance in rats. Life Sci. 1968;7:239–247. doi: 10.1016/0024-3205(68)90197-5. [DOI] [PubMed] [Google Scholar]
- 42.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 43.Napoli JL, Race KR. Microsomes convert retinol and retinal into retinoic acid and interfere in the conversions catalyzed by cytosol. Biochim Biophys Acta. 1990;1034:228–232. doi: 10.1016/0304-4165(90)90081-7. [DOI] [PubMed] [Google Scholar]
- 44.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 45.Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]



