Abstract
Radiation and drug resistance remain the major challenges and causes of mortality in the treatment of locally advanced, recurrent and metastatic breast cancer. Dysregulation of phospholipase D (PLD) has been found in several human cancers and is associated with resistance to anticancer drugs. In the present study, we evaluated the effects of PLD inhibition on cell survival, cell death and DNA damage after exposure to ionizing radiation (IR). Combined IR treatment and PLD inhibition led to an increase in the radiation-induced apoptosis of MDA-MB-231 metastatic breast cancer cells. The selective inhibition of PLD1 and PLD2 led to a significant decrease in the IR-induced colony formation of breast cancer cells. Moreover, PLD inhibition suppressed the radiation-induced activation of extracellular signal-regulated kinase and enhanced the radiation-stimulated phosphorylation of the mitogen-activated protein kinases p38 and c-Jun N-terminal kinase. Furthermore, PLD inhibition, in combination with radiation, was very effective at inducing DNA damage, when compared with radiation alone. Taken together, these results suggest that PLD may be a useful target molecule for the enhancement of the radiotherapy effect.
Keywords: breast cancer, phospholipase D, phospholipase D inhibitor, radiosensitivity
Introduction
Chemotherapy and radiotherapy (RT) are common treatments used to decrease the tumor burden and to ameliorate tumor-related symptoms. However, the current regimes are not curative in most cases and, in general, cancer mortality rates have not decreased significantly in recent years.1 This situation has prompted many researchers and companies to develop novel compounds that possess higher and more selective antitumor activities. Radiation therapy plays a critical role in the local and regional control of malignant tumors. Recent attempts to enhance the efficacy of radiation therapy have focused on using conventional chemotherapeutic agents as biological response modifiers.2
Phospholipase D (PLD) catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid, which consequently activates a signaling cascade leading to cell growth, survival and angiogenesis.3 Two mammalian isoforms of phosphatidylcholine-specific PLD (that is, PLD1 and PLD2) have been identified. PLD is overexpressed in a wide variety of solid human tumors, including breast cancer.4 PLD isoform-selective inhibitors have been reported to block tumor invasion in a metastatic breast cancer model.5 However, the mechanism underlying the antitumor effects associated with PLD inhibitors has not yet been clearly defined. The therapeutic efficacy of RT can be enhanced by pharmacological compounds that target specific pathways involved in cell survival.
Because the effect of combining PLD inhibition with IR is currently unknown, the aim of this study was to evaluate the influence of selective PLD1 or PLD2 inhibition on tumor radiosensitivity. Here, we show for the first time that PLD inhibitors potentiate the effect of RT in the MDA-MB-231 metastatic breast cancer cell line through the promotion of apoptosis, the induction of DNA damage and the suppression of colony formation.
Materials and methods
Cell culture and chemicals
The human breast cancer cell line MDA-MB-231 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and was cultured in complete Dulbecco's modified Eagle's medium (Hyclone, Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum (Hyclone) and 1% antibiotic solution (Gibco, Carlsbad, CA, USA). Cells were maintained in a 37 °C humidified incubator with 5% CO2. The PLD1-selective inhibitor (VU0155069) and the PLD2-selective inhibitor (VU0285655-1) were purchased from Cayman Chemical (Ann Arbor, MI, USA).
Cell viability assay
To determine cell viability, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed. The absorbance at 570 nm was measured using an ELISA reader (Berthold, Bad Wildbad, Germany), and the percentage of viable cells was indicated relative to the control.
Radiation exposure
MDA-MB-231 cells were irradiated at room temperature using γ-rays from a Cs-137 blood irradiator (Eckert & Ziegler, Berlin, Germany) at a dose rate of 1.3 Gy per min. Non-irradiated controls were handled identically to the irradiated cells with the exception of the radiation exposure. After irradiation, cultures were kept at 37 °C in an incubator with 5% CO2.
Clonogenic assay
MDA-MB-231 cells were cultured in six-well plates. Once cells were attached, they were exposed to either the vehicle control (dimethyl sulfoxide) or 10 μℳ PLD inhibitor for 4 h and were then irradiated with γ-radiation using the blood irradiator. After 48 h of incubation, the cells were washed and then maintained in a 37 °C, 5% CO2 incubator for 10 days to allow colony formation. Individual colonies were fixed with formaldehyde and stained with 2% crystal violet in methanol. The number of colonies was then counted, and the surviving fraction of cells was normalized to the surviving fraction of the corresponding control. The survival fraction was calculated as the mean number of colonies/(cells seeded × plating efficiency). The plating efficiency represents the percentage of cells that grow into colonies under a specified culture condition. In the radiation survival curve, the different conditions were normalized to the control.
Western blot
Cells were lysed for 30 min at 4 °C in lysis buffer (10 mℳ Tris-HCl, pH 7.4; 150 mℳ NaCl; 1% Triton X-100; 1% deoxycholate; 0.1% SDS; 5 mℳ EDTA). The protein concentration was then measured using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions. Equal amounts of protein were electrophoresed on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidine fluoride membranes. After blocking for 30 min, the membranes were incubated overnight at 4 °C with the following primary antibodies: cleaved caspase-3, extracellular signal-regulated kinase (ERK), phosphorylated (p)-ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK (all antibodies at 1:1000, Cell Signaling Technology, Danvers, MA, USA), poly adenosine diphosphate ribose polymerase (PARP; 1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and γ-H2AX (1:1000, Abcam, London, UK). The membranes were washed 5 times and incubated with a horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse immunoglobulin for 1 h in a 1:5000 dilution. Immunoreactive bands were examined using an enhanced chemiluminescence detection system.
DAPI staining
MDA-MB-231 cells were treated with 10 μℳ PLD inhibitor for 4 h and were exposed to 5 Gy. The cells were then incubated for 48 h after ionizing radiation (IR), and were washed twice with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (PBS-B); the cells were then fixed with 70% ethanol containing 0.5% Tween-20 at 4 °C for 30 min. The fixed cells were washed with PBS-B and stained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min at room temperature. The stained cells were washed twice with PBS-B and observed using a Zeiss Axiophot microscope (Göttingen, Germany) at × 400 magnification.
Annexin V staining
Annexin V staining was carried out using a phycoerythrin (PE) Annexin V Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA). Briefly, 5 × 105 cells were added to 25 cm2 flasks for each data point. After 24 h, the cells were treated with 10 μℳ PLD inhibitor or vehicle control (dimethyl sulfoxide) for 4 h and then irradiated with 5 Gy radiation. After 48 h of incubation, the cells were washed with PBS and then carefully trypsinized. Following centrifugation at 1000 × g for 3 min, the cells were counted using a hematocytometer and then resuspended in 1 × binding buffer at a concentration of 1 × 106 cells per ml. Next, 100 μl of the cell suspension were added to 5 μl PE Annexin V and 7-amino-actinomycin. The samples were then incubated at room temperature for 15 min in the dark. Finally, 400 μl binding buffer were added, and the cells were suspended and subjected to flow cytometry analysis (BD FACSAria, BD Biosciences).
DNA damage assay
A DNA damage assay was carried out using an OxiSelect Comet Assay kit (Cell Biolabs, San Diego, CA, USA). Briefly, cells were seeded in a six-well plate, treated with the PLD inhibitor for 4 h, and were then exposed to IR. After 48 h, the cells were harvested and washed with PBS. The cell suspension was then mixed with low melting agarose in a 1:10 ratio, and 75 μl of the cell suspension was pipetted onto the comet slide. The slides were incubated at 4 °C for 30 min and subsequently immersed in lysis buffer for 30 min; the slides were then electrophoresed with TAE buffer at 25 V for 20 min. Finally, the slides were dried and stained with DNA dye, and the comet tails were imaged using a fluorescent microscope (Nikon, Tokyo, Japan).
Statistical analysis
The results are expressed as the mean±s.d. of the number of experiments indicated. Differences among the groups were determined using analysis of variance with P-value <0.05 or 0.01 indicating statistical significance.
Results
PLD inhibition in combination with radiation inhibits cell growth in breast cancer cells
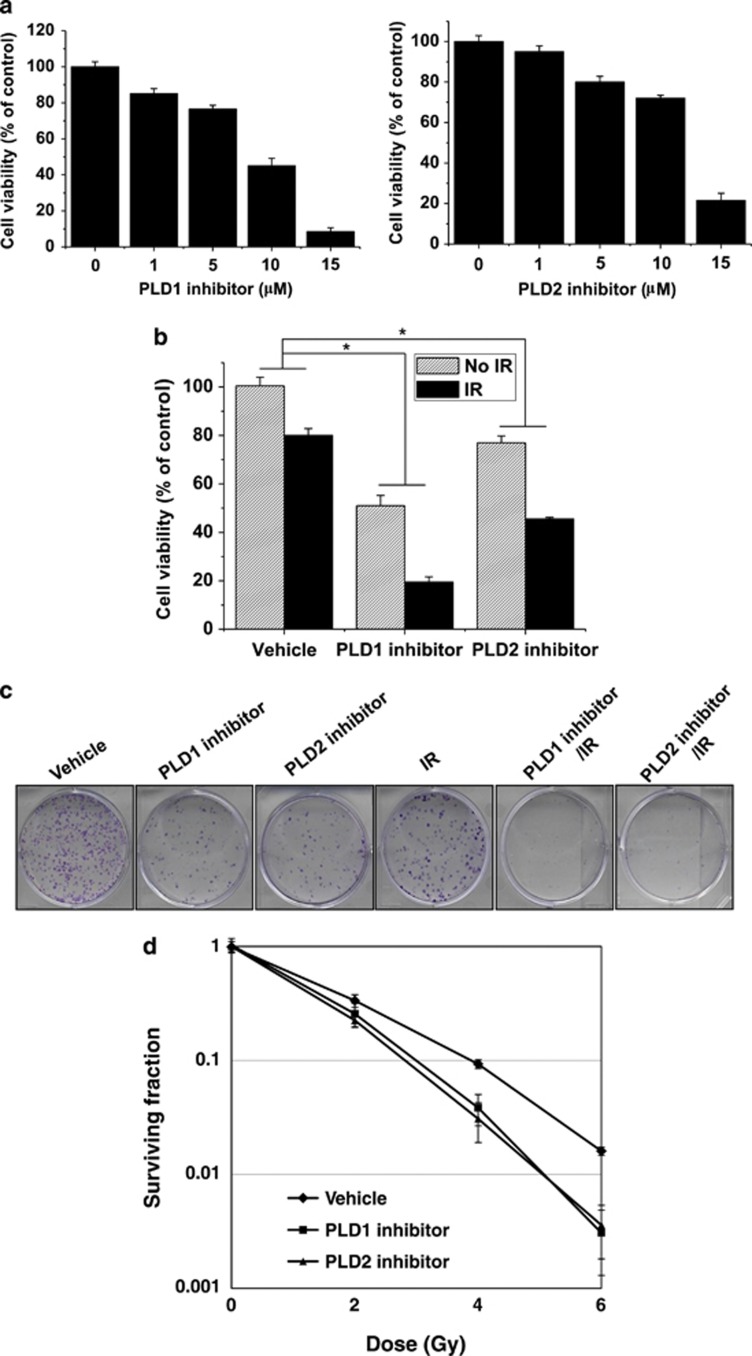
Both the PLD1- and the PLD2-selective inhibitors induced cytotoxicity in a dose-dependent manner in MBA-MB-231 cells when treated for 72 h (Figure 1a). Combining 5 Gy radiation with 10 μℳ of the PLD1 or PLD2 inhibitor suppressed cell viability more effectively than either radiation or the PLD inhibitor alone (Figure 1b). In addition, combining the radiation treatment and the PLD inhibitor resulted in a synergistic radiosensitizing effect. In further clonogenic assays, both the PLD1 and PLD2 inhibitors were effective in blocking the colony formation of MDA-MB-231 cells, although treatment with 5 Gy radiation also inhibited colony formation (Figure 1c). Pretreatment with either the PLD1 inhibitor or the PLD2 inhibitor greatly enhanced the effect that radiation had on the cell's clonogenic ability.
Figure 1.
Phospholipase D (PLD) inhibitors in combination with radiation augment cell growth inhibition in breast cancer cells. (a) MDA-MB-231 cells were treated with different concentrations of the PLD1 or PLD2 inhibitor for 72 h, and cell viability was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. (b) MDA-MB-231 cells were treated with the indicated PLD inhibitor (10 μℳ) for 4 h and were then exposed to 5 Gy radiation. After 72 h, the cell viability was measured using an MTT assay. *P<0.05. (c) The cells were treated with 10 μℳ of the PLD1 or PLD2 inhibitor and/or exposed to 5 Gy ionizing radiation (IR) for 4 h. After 10 days, colonies were fixed with formaldehyde and stained with 2% crystal violet. (d) The number of colonies was counted, and the surviving fraction was normalized to the surviving fraction of the corresponding control. Data are presented as the mean±s.d. of three independent experiments.
We further examined the survival curves of the vehicle-treated and the PLD inhibitor-treated cells after various doses of radiation. The slopes of the survival curves of the MDA-MB-231 cells treated with a combination of radiation and either the PLD1 or the PLD2 inhibitor were greater than those of the cells treated with radiation alone, indicating that the combined treatment increased the cell's sensitivity to radiation, resulting in a decrease in the surviving fraction of cells (Figure 1d). Taken together, these results indicated that both the PLD1 and the PLD2 inhibitor potentiated the radiosensitivity of MDA-MB-231 breast cancer cells.
PLD inhibition promotes IR-induced cell death
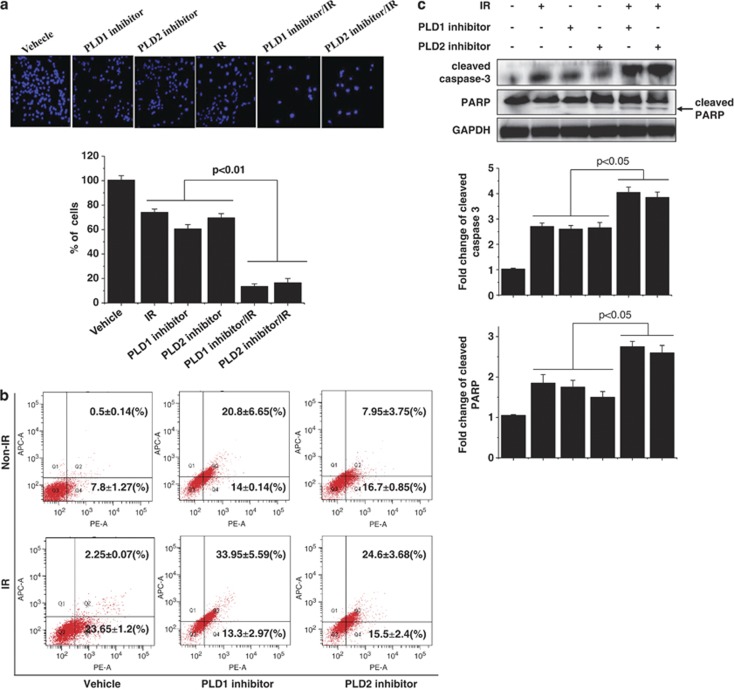
Next, we investigated the radiosensitizing effect of the PLD inhibitors on cell death. MDA-MB-231 cells were pretreated with either the PLD1 or PLD2 inhibitor for 4 h, and then IR was administered. After 48 h, the cells were stained with DAPI to determine which cells were still alive. As shown in Figure 2a, a combination of 5 Gy radiation with 10 μℳ PLD inhibitor significantly enhanced the amount of cell death, compared with that of either single treatment.
Figure 2.
Phospholipase D (PLD) inhibition promotes ionizing radiation (IR)-induced cell death. (a) MDA-MB-231 cells were treated with 10 μℳ of PLD inhibitor for 4 h and/or exposed to 5 Gy radiation. After 48 h, the cells were stained with 4',6-diamidino-2-phenylindole (DAPI) and observed by fluorescence microscopy. The live cells were quantitated. (b) The cells were treated with 10 μℳ PLD inhibitor for 4 h and/or exposed to 5 Gy radiation. After 48 h, the cells were stained with Annexin V and 7-amino-actinomycin (7-AAD) and analyzed by flow cytometry. Data are expressed as the mean±s.d. for three experiments. (c) The cells were treated with PLD inhibitor and/or 5 Gy radiation. The cell lysates were analyzed by western blot using the indicated antibodies. The band intensity was quantified. These blots are representative of the results obtained from three experiments.
Treatment with a PLD inhibitor in the presence of IR markedly reduced the early apoptotic cell population and increased the late apoptotic or necroptotic cell population, relative to either treatment alone, as analyzed using Annexin V/7-amino-actinomycin staining (Figure 2b). Thus, it seems that the type of cell death experienced by cells treated with both IR and a PLD inhibitor is a mixture of apoptosis and necrosis. To further confirm the effects of radiation and PLD inhibition on apoptosis, we examined the activation of caspase-3 and PARP cleavage using western blot analysis. Treatment with either 5 Gy radiation or the PLD inhibitor alone increased the levels of active caspase-3 and cleaved PARP protein. However, treating cells with both IR and a PLD inhibitor resulted in significantly enhanced protein levels of active caspase-3 and cleaved PARP compared with treating cells with either the radiation alone or the inhibitor alone (Figure 2c). Taken together, these results suggested that the radiosensitizing effect of the PLD inhibitors is through the induction of apoptosis in breast cancer cells.
The effects of radiation and PLD inhibition on MAPK, JNK and p38 MAPK activation
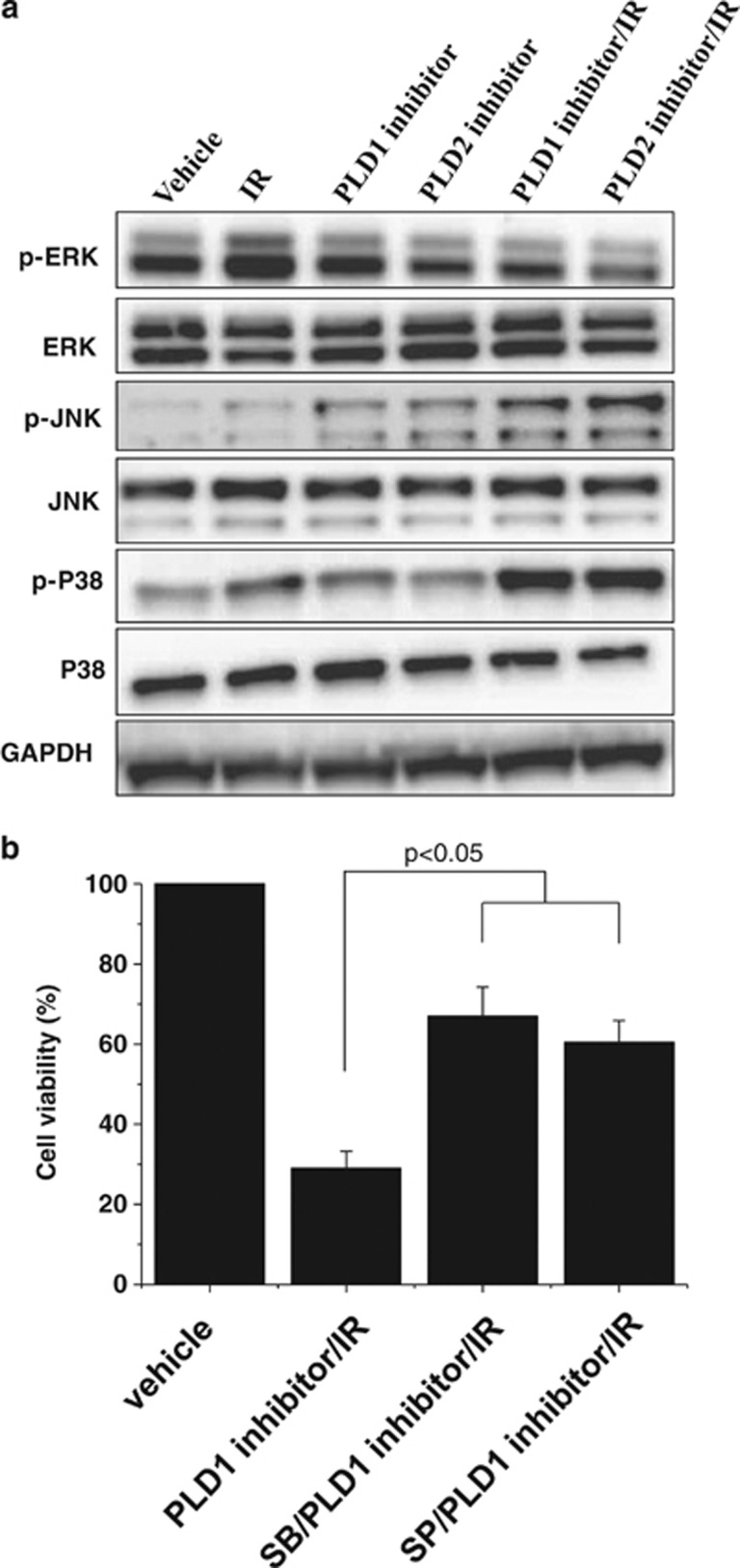
One of the relevant downstream targets of mitogenic PLD signaling is the MAPK pathway. Cells treated with 5 Gy radiation had increased levels of phosphorylated (active) MAPK, whereas PLD1 or PLD2 inhibition had only a marginal effect on the phosphorylation of MAPK. The combined treatment decreased the amounts of IR-induced phosphorylation of MAPK (Figure 3). Phosphorylated JNK and p38 MAPK have been implicated in the induction of apoptosis in response to environmental stimuli.6 Radiation increased the amount of phosphorylated p38 MAPK and phosphorylated JNK. Treatment with a PLD inhibitor enhanced the radiation-induced phosphorylation of p38 MAPK and JNK (Figure 3a).
Figure 3.
The effects of radiation and phospholipase D (PLD) inhibition on the activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK). (a) MDA-MB-231 cells were treated with 10 μℳ PLD inhibitor and/or exposed to 5 Gy radiation. After 1 h, the lysates were analyzed by western blot using the indicated antibodies. These blots are representative of the results obtained from three experiments. (b) MDA-MB-231 cells were pretreated with 10 μℳ of SP600125 (SP) or SB203580 (SB), and treated with 10 μℳ PLD inhibitor and exposed to 5 Gy radiation. The cell viability was measured as described in the MATERIALS AND METHODS section.
We further examined whether p38 MAPK or JNK is involved in the PLD1 inhibitor-induced augmentation of cell death in the presence of IR. Pretreating cells with either a p38 MAPK inhibitor (SB203580) or a JNK inhibitor (SP600125) suppressed the PLD1 inhibitor/IR-induced cell death, as determined using cell viability assay (Figure 3b). Thus, it is suggested that p38 MAPK or JNK is involved in the PLD1 inhibitor-induced augmentation of cell death in the presence of IR.
PLD inhibition increases IR-induced DNA damage
Several recent studies have focused on radiosensitizers that may have the ability to increase the induction of DNA damage by IR.7, 8 An increase in DNA double-strand breaks (DSBs) and an impaired DNA damage repair system have been shown to be related to the synergistic effects between IR and anticancer drugs. The ability to recover from DNA damage is the factor that is most commonly believed to influence a cell's sensitivity to IR.9 Thus, we assessed whether PLD inhibitors induced the cell's radiosensitization because of an increase in DNA damage.
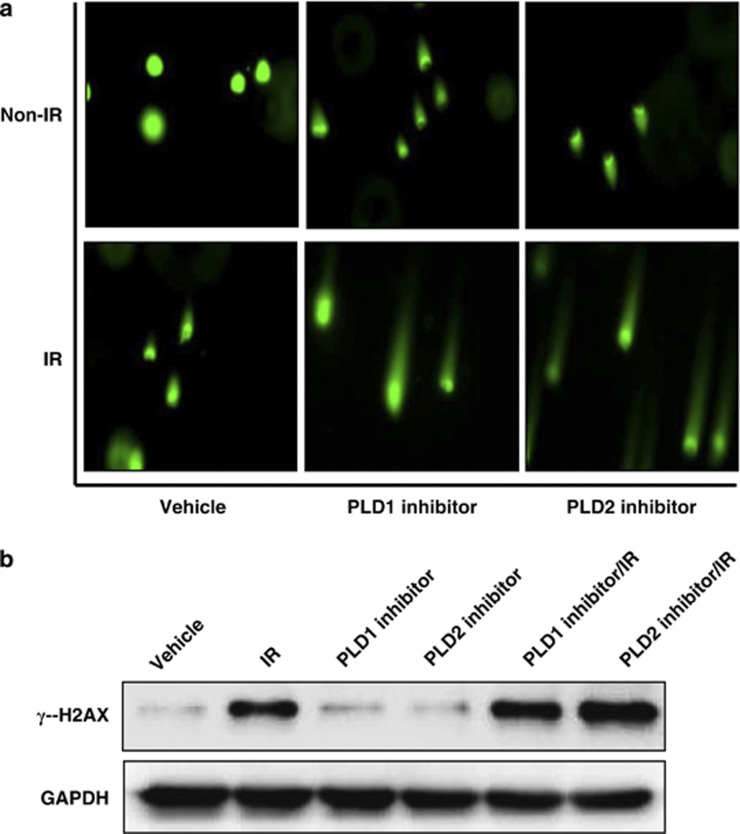
To accomplish this, the olive tail moment was measured using an alkaline comet assay. The comet assay is a sensitive method for detecting DNA strand breaks and measuring the repair kinetics within a single cell. Cells containing damaged DNA have the appearance of a comet, with a bright head and tail, whereas undamaged DNA appears as an intact nucleus with no tail. MDA-MB-231 cells were treated with IR and/or a PLD inhibitor, and a comet assay was performed 48 h after IR treatment. As shown in Figure 4a, treatment with 5 Gy radiation or treatment with either the PLD1 or the PLD2 inhibitor showed a slight increase in the number of cells with comet tails, whereas the combined treatment greatly increased the comet tail length, compared with cells treated with IR or PLD inhibitor alone.
Figure 4.
Phospholipase D (PLD) inhibition increases ionizing radiation (IR)-induced DNA damage. (a) MDA-MB-231 cells were treated with 10 μℳ PLD inhibitor for 4 h, after which they were exposed to 5 Gy IR. At 48 h, an alkaline comet assay was performed, and the comet tails were observed by fluorescence microscopy. (b) The cells were treated with PLD inhibitor for 4 h and/or exposed to IR. After 1 h, the lysates were analyzed by western blot using the indicated antibody. These blots are representative of the results obtained from three experiments.
We further investigated the involvement of DNA damage in the PLD inhibitor-induced radiosensitization by monitoring the formation of γ-H2AX in response to DNA DSBs; this marker provides the basis for a sensitive assay of DNA damage.10, 11 H2AX is a variant of the core histone H2A family, and the phosphorylated form of H2AX is referred to as γ-H2AX. The expression of phosphorylated H2AX (γ-H2AX) indicates an increase in the accumulation of IR-induced DSBs. Therefore, we examined the expression level of γ-H2AX in MDA-MB-231 cells treated with either radiation alone, a PLD inhibitor alone or a combination of the two. Treatment with either IR alone or the PLD1 or PLD2 inhibitor alone increased the expression of γ-H2AX. Combining the PLD inhibitor with IR resulted in a greater increase in the expression of γ-H2AX than cells treated with either treatment alone, suggesting that the combined treatment of a PLD inhibitor and IR results in DNA strand breaks and delays DNA damage repair, thereby inducing apoptosis in MDA-MB-231 breast cancer cells. Taken together, these results suggest that PLD inhibition sensitizes the cell to IR-induced DNA damage.
Discussion
In this study, our results demonstrate that both PLD1 and PLD2 inhibitors are potent radiosensitizers of human breast cancer cells. PLD has been implicated in playing a role in the growth and survival of cells by the observation that PLD activity increases in response to mitogenic signals. PLD protein levels and activity are upregulated in a variety of cancers, including breast, colon, gastric, kidney and thyroid cancers.12 PLD1 tends to be overexpressed in tumors that express high levels of cytokeratins 5 and 17, which are markers of basal-like tumors and are frequently associated with poor prognosis.13 Moreover, a polymorphism in the PLD2 gene is associated with an increased risk of colorectal cancer.14 PLD2 point mutations have also been identified in breast cancer cells,15 and a change from glutamine to alanine in PLD2 (Q163A) results in higher enzymatic activity and invasiveness in breast cancer cells compared with the wild-type PLD2 (Young Hoon Jang, unpublished observation).
These studies provide compelling evidence that the elevated activity and expression of PLD observed in cancer are functionally linked with oncogenic signals and tumorigenesis. Reducing the levels of PA could be a strategy to repress the survival signal that in turn suppresses apoptosis.16 Considering the role of PLD in tumor progression, PLD inhibitors have emerged as potential anticancer drugs. Isoform-selective PLD inhibitors have recently been developed and characterized.5 PLD inhibitors have been shown to reduce invasiveness and anchorage-independent growth in metastatic breast and colorectal cancer models.5, 16
RT has been used to remove cancer cells that remain after surgery or to reduce the volume of an advanced tumor before surgery. However, the RT dose is limited by the total dose that the patient can be exposed to without complications. One method to solve this problem is to identify anticancer drugs that target specific intracellular signaling pathways to sensitize the tumor cells to IR or to select pharmacological compounds that can act as potential radiosensitizers. Therefore, this study was the first conducted to examine the radiosensitizing effects of PLD inhibition in breast cancer cells.
Cellular radiosensitivity is determined by a number of fundamental processes, such as DNA damage, DNA repair capacity, cell cycle progression and apoptosis. Treating MDA-MB-231 cells with a PLD inhibitor and IR resulted in much more cell death than either treatment alone. Based on the results of the radiation survival assay, the combined treatment also led to significantly fewer and smaller colonies than either treatment alone, suggesting that the PLD inhibitor enhanced the radiosensitization of the MDA-MB-231 breast cancer cells.
It has been reported that radiation stimulates PLD activity in human squamous carcinoma cells.17 In the present study, radiation-induced PLD activation might play an antiapoptotic role as a compensatory mechanism for radiation-induced apoptosis. The ERK pathway plays a major role in regulating cell growth and differentiation, and is highly induced in response to growth factors, cytokines, phorbol esters and oxidant injury. Our results indicate that the PLD inhibitor suppressed the radiation-induced ERK activation and promoted the IR-induced activation of p38 MAPK and JNK. When compared with individual treatment with IR or a PLD inhibitor, the combined treatment was markedly more potent in causing apoptosis, as analyzed by Annexin V staining, cleavage of caspase-3 and cleavage of PARP. Genetic insults, such as IR, induce DNA DSBs, which are repaired by interchromosomal and intrachromosomal homologous recombination and end joining.18, 19 The nonhomologous end joining pathway is essential to repairing IR-induced DSBs, which are responsible for the loss of radiation survival.20 In the present study, treatment with IR or a PLD inhibitor alone increased the comet tail length when compared with the vehicle control, whereas the combined treatment led to a greater increase in the tail length than either treatment alone.
γ-H2AX expression has been shown to be a sensitive indicator of DSBs induced by clinically relevant doses of IR. At sites of IR-induced DNA DSBs, the histone H2AX becomes phosphorylated. Although the specific role of γ-H2AX in the repair of DSBs has not been clarified, recent reports indicate that the dephosphorylation of γ-H2AX in cells exposed to IR is correlated with the repair of the DSBs21, 22 and cellular radiosensitivity.23, 24 Treatment with a PLD inhibitor promoted the IR-induced γ-H2AX expression in breast cancer cells.
In conclusion, we demonstrated for the first time that a PLD inhibitor can radiosensitize human breast cancer cells to promote apoptosis and DNA damage. Although the effects of IR and PLD inhibition should be considered in normal breast cells, PLD inhibition may effectively improve the therapeutic efficacy of radiation therapy against breast cancer cells.
Acknowledgments
This work was supported by a grant from the Translational Research Center for Protein Function Control, NSF (2009-0092960), South Korea, by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST; no. 2012002009) and by the National R&D program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea Grant 0920050.
The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Candelaria M, Garcia-Arias A, Cetina L, Duenas-Gonzalez A. Radiosensitizers in cervical cancer: cisplatin and beyond. Radiat Oncol. 2006;1:15–31. doi: 10.1186/1748-717X-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvy PE, Lavieri RR, Lindsley CW, Brown HA. Phospholipase D: enzymology, functionality, and chemical modulation. Chem Rev. 2011;111:6064–6119. doi: 10.1021/cr200296t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Frohman MA. The potential for phospholipase D as a new therapeutic target. Expert Opin Ther Targets. 2007;11:707–716. doi: 10.1517/14728222.11.5.707. [DOI] [PubMed] [Google Scholar]
- Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, et al. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yang ES, Kim SY, Shin SW, Park JW. Regulation of heat shock-induced apoptosis by sensitive to apoptosis gene protein. Free Radic Bio Med. 2008;45:167–176. doi: 10.1016/j.freeradbiomed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Haykal J, Geara F, Haddadin MJ, Smith A, Gail-Muhtasib H. The radiosensitizer 2-benzoyl-3-phenyl-6,7-dichloro-quinoxaline 1,4-dioxide induces DNA damage in EMT-6 mammary carcinoma cells. Radiat Oncol. 2009;4:25–34. doi: 10.1186/1748-717X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supiot S, Gouard S, Charrier J, Apostolidis C, Charal JF, Barber J, et al. Mechanisms of cell sensitization to alpha radioimmunotherapy by doxorubicin or paclitaxel in multiple myeloma cell lines. Clin Cancer Res. 2005;11:7047s–7052s. doi: 10.1158/1078-0432.CCR-1004-0021. [DOI] [PubMed] [Google Scholar]
- Hu Q, Hill RP. Radiosensitivity, apoptosis and repair of DNA double-strand breaks in radiation-sensitive Chinese hamster ovary cell mutants treated at different dose rates. Radiat Res. 1996;146:636–645. [PubMed] [Google Scholar]
- Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012;327:123–133. doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Su W, Chen Q, Frohman MA. Targeting phospholipase D with small-molecule inhibitors as a potential therapeutic approach for cancer metastasis. Future Oncol. 2009;9:1477–1486. doi: 10.2217/fon.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozgit JM, Pentecost BT, Marconi SA, Ricketts-Loriaux RS, Otis CN, Arcaro KF. PLD1 is overexpressed in an ER-negative MCF-7 cell line variant and a subset of phosphor-Akt-negative breast carcinomas. Br J Cancer. 2007;97:809–817. doi: 10.1038/sj.bjc.6603926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Banno Y, Yoshida H, Kikuchi R, Akao Y, Murate T, et al. Catalytic inactivation of human phospholipase D2 by a naturally occurring Gly901Asp mutation. Arch Med Res. 2006;37:696–699. doi: 10.1016/j.arcmed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Kang DW, Lee SH, Yoon JW, Park WS, Choi KY, Min DS. Phospholipase D1 drives a positive feedback loop to reinforce the Wnt/β-catenin/TCF signaling axis. Cancer Res. 2010;70:4233–4242. doi: 10.1158/0008-5472.CAN-09-3470. [DOI] [PubMed] [Google Scholar]
- Avila MA, Otero G, Cansado J, Dritschilo A, Velasco JA, Notario V. Activation of phospholipase D participates in signal transduction pathways responsive to gamma-radiation. Cancer Res. 1993;1:4474–4476. [PubMed] [Google Scholar]
- Haber JE. Partners and pathways repairing a double-strand break. Trends Genet. 2000;16:259–264. doi: 10.1016/s0168-9525(00)02022-9. [DOI] [PubMed] [Google Scholar]
- Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- Olive PL. The DNA single and double strand breaks in cell killing by ionizing radiation. Radiat Res. 1998;150:S42–S51. [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov IB, Smirnova AN, Krutilina RI, Svetlova MP, Solovjeva LV, Nikiforov AA, et al. Dephosphorylation of histone γ-H2AX during repair of DNA double strand break repair during the mammalian cells and its inhibition by calyculin A. Radiat Res. 2003;160:309–317. doi: 10.1667/rr3043. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Taneja N, Davis M, Choy JS, Beckett MA, Singh R, Kron SJ, et al. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem. 2004;279:2273–2280. doi: 10.1074/jbc.M310030200. [DOI] [PubMed] [Google Scholar]