Abstract
In this study, we examined the therapeutic effects of an immune-stimulating peptide, WKYMVm, in ulcerative colitis. The administration of WKYMVm to dextran sodium sulfate (DSS)-treated mice reversed decreases in body weight, bleeding score and stool score in addition to reversing DSS-induced mucosa destruction and shortened colon. The WKYMVm-induced therapeutic effect against ulcerative colitis was strongly inhibited by a formyl peptide receptor (FPR) 2 antagonist, WRWWWW, indicating the crucial role of FPR2 in this effect. Mechanistically, WKYMVm effectively decreases intestinal permeability by stimulating colon epithelial cell proliferation. WKYMVm also strongly decreases interleukin-23 and transforming growth factor-β production in the colon of DSS-treated mice. We suggest that the potent immune-modulating peptide WKYMVm and its receptor FPR2 may be useful in the development of efficient therapeutic agents against chronic intestinal inflammatory diseases.
Keywords: therapeutics, ulcerative colitis, WKYMVm
Introduction
Inflammatory bowel diseases (IBDs) such as Crohn's disease or ulcerative colitis are chronic diseases that cause inflammation of the intestine.1, 2 Although the incidence of IBD varies in different countries, a gradual increase has been noted recently, and the disease is a major health problem worldwide.2, 3 Although the search for the cause of IBD has been a hot issue for several decades, it is still not clear what causes IBD. Currently, various factors, including environment, diet and genetic makeup, have been suggested to be associated with IBD pathogenesis.1, 2, 3 Among these, a genetic defect that affects the response of the human immune system to offending agents, such as bacteria, viruses or proteins in food, has been associated with IBD.1, 2, 3
The host immune system is closely associated with the pathogenesis and progress of IBD.4 Both innate and adaptive immune systems are critically involved in the response to intestinal microbiota.4, 5 Pattern recognition receptors, such as the toll-like receptor, have roles in sensing conserved microbial molecules in the intestinal environment.4 Despite the need for efficient therapeutic molecules to treat human IBD, no cure has yet been developed. Clinically, some immunosuppressive agents that target tumor necrosis factor (TNF) are currently used to treat IBD.6
WKYMVm is a synthetic peptide that was identified by screening a peptide library.7, 8 The peptide binds to at least three formyl peptide receptors (FPRs): FPR1, FPR2 and FPR3.9, 10, 11 WKYMVm stimulates the chemotactic migration of leukocytes such as neutrophils, monocytes, dendritic cells and natural killer cells.12, 13, 14, 15 It also stimulates superoxide anion production in phagocytes including neutrophils and monocytes.12, 16 Recently, we demonstrated that WKYMVm administered to a polymicrobial sepsis model had potent therapeutic activity in cecal ligation and puncture mice.17 The peptide was shown to inhibit the production of inflammatory cytokines, such as TNF-α and interleukin (IL)-1β, and augment the production of Th1 cytokines (IFN-γ (interferon-γ) and IL-12) to achieve this therapeutic effect against sepsis.17 Here, we investigate the effects of WKYMVm on dextran sodium sulfate (DSS)-induced ulcerative colitis, including its effects on cytokine production.
Materials and methods
Animals and DSS-treated ulcerative colitis model
Six-week-old C57BL/6 mice were obtained from Orient Bio Inc. (Seongnam, Korea). After adapting for 1 week following arrival, the mice were given drinking water containing 3% DSS (m.w. 36 000–50 000; Sigma-Aldrich, St Louis, MO, USA) w/v for 5 days followed by fresh water until the end of the experiment on the 8th day. WKYMVm (Anygen, Gwangju, Korea) or vehicle (phosphate-buffered saline) was subcutaneously injected into DSS mice six times (at 0, 12, 24, 36, 48 and 60 h after DSS treatment). Body weight, rectal bleeding and stool score (stool consistency or diarrhea) were measured daily according to a previous report.18
Histology
The mice were subjected to DSS treatment and were administered with phosphate-buffered saline or WKYMVm at a dose of 8 mg kg−1. The mice were euthanized 8 days after DSS treatment, and the intestines were fixed, sectioned and stained with hematoxylin and eosin for morphological analysis.
Measurement of intestine permeability
Food was withdrawn from the mice for 6 h, and the animals were gavaged with fluorescein isothiocyanate–dextran (10 mg per head; Sigma-Aldrich). Serum was collected retro-orbitally 4 h after the gavage. Fluorescein isothiocyanate–fluorescence was measured using the Gemini XPS fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell proliferation assay
Caco-2 human epithelial colorectal adenocarcinoma cells were cultured with Dulbecco's modified Eagle's medium containing 20% fetal bovine serum (Life Technologies, Grand Island, NY, USA). Healthy cultured cells were seeded at 4 × 103 cells per well. After 24 h, WKYMVm was added at several concentrations (0, 10, 100 and 1000 nM) in the absence or presence of cyclosporine H (CsH, 1 μM) or WRWWWW (WRW4, 10 μM) for 24 h. Quantification of cell proliferation was performed using the Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., Rockville, MD, USA).
Wound healing assay
Caco-2 human epithelial colorectal adenocarcinoma cells were cultured with Dulbecco's modified Eagle's medium containing 20% fetal bovine serum. Vehicle or WKYMVm (1 μM) was added into a scratched Caco-2 cell layer for 0, 12 or 24 h. Images were obtained using a digital camera attached to a light microscope.
Measurement of colon cytokines
To measure DSS treatment-induced cytokine production, mice were administered with WKYMVm at 0, 12, 24, 36, 48 and 60 h after DSS treatment. Intestines were collected at day 7 after DSS treatment and sliced into 1-cm segments. Each segment was incubated in Roswell Park Memorial Institute 1640 medium for 24 h, and cytokines secreted into the supernatant were measured using enzyme-linked immunosorbent assay (BD Biosciences, San Jose, CA, USA or eBioscience Inc., San Diego, CA, USA) according to the manufacturer's instructions.
Statistical analysis
All data were evaluated using the t-test, and statistical significance was set at P<0.05.
Results
Administration of WKYMVm ameliorates DSS-induced ulcerative colitis
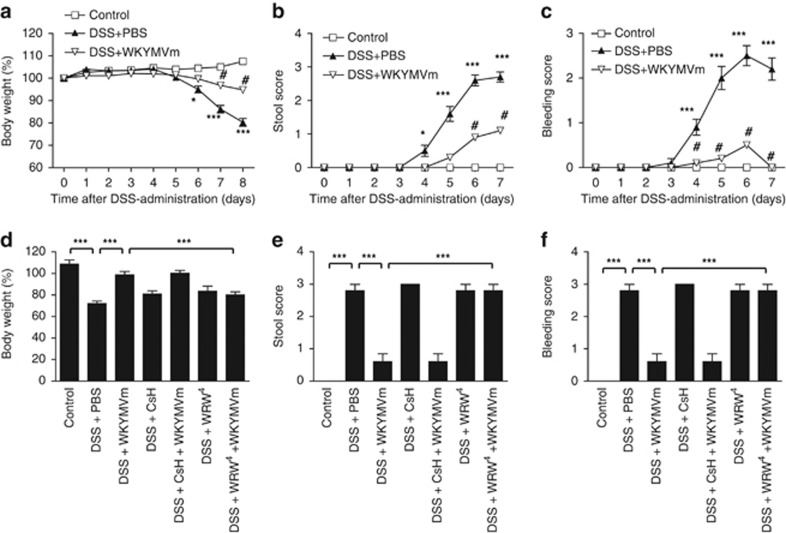
To examine whether WKYMVm has a therapeutic effect on ulcerative colitis, we used the DSS model. DSS-treated C57BL/6 mice showed a decreased body weight over to time compared with control mice (Figure 1a). WKYMVm administration to DSS-treated mice reversed this decrease in body weight (Figure 1a). Bleeding and stool scores were also monitored after DSS treatment. WKYMVm administration effectively attenuated the DSS-induced increase in the bleeding score and the stool score (Figures 1b and c). As WKYMVm binds to both FPR1 and FPR29, 10, 11, we also examined the effect of an FPR1 antagonist (CsH)19 and an FPR2 antagonist (WRW4)20 on the WKYMVm-induced therapeutic effect on ulcerative colitis. The WKYMVm-induced therapeutic effect on ulcerative colitis was strongly inhibited by WRW4, but not by CsH, indicating that WKYMVm exerts its effects via FPR2 (Figures 1d–f).
Figure 1.
The therapeutic effects of WKYMVm in an experimental ulcerative colitis model. Established colitis was induced using 3% dextran sodium sulfate (DSS) in water. WKYMVm (8 mg kg−1) was subcutaneously administered six times (at 0, 12, 24, 36, 48 and 60 h) after establishing the DSS model in the absence (a–c) or presence of cyclosporine H (CsH) (4 mg kg−1) or WRWWWW (WRW4) (4 mg kg−1) (d–f). Body weights (a, d), stool scores (b, e) and bleeding scores (c, f) were measured daily. The data obtained at day 7 are shown in (d–f). The data are expressed as the mean±s.e.m. **P<0.01, ***P<0.001 compared with control. #P<0.05 compared with DSS alone. Sample size: n=8 mice per group (a–c) or n=5 mice per group (d–f).
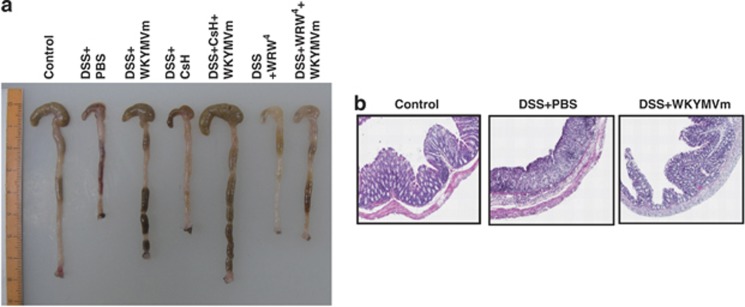
Colon shortening is associated with the progression of ulcerative colitis. As shown in Figure 2a, DSS treatment strongly elicited colon shortening, which was efficiently reversed by WKYMVm administration. The WKYMVm-induced reversal of colon shortening was inhibited by WRW4, but not by CsH, supporting the hypothesis that the peptide exerts its effects via FPR2 but not FPR1 (Figure 2a). The histological data indicate that DSS treatment caused destruction of the mucosa structure, which was dramatically inhibited by WKYMVm administration (Figure 2b).
Figure 2.
WKYMVm protects dextran sodium sulfate (DSS)-induced experimental ulcerative colitis. Established colitis was induced using 3% DSS in water. WKYMVm (8 mg kg−1) was subcutaneously administered six times (at 0, 12, 24, 36, 48 and 60 h) after establishing the DSS model in the absence or presence of cyclosporine H (CsH) (4 mg kg−1) or WRWWWW (WRW4) (4 mg kg−1). Colons were isolated from DSS mice at day 7. (a) Macroscopic examination of colons from DSS mice. (b) Colon cross-sections from DSS mice were stained with hematoxylin and eosin. The data are representative of five (a) or eight (b) mice per group.
WKYMVm administration protects intestinal permeability
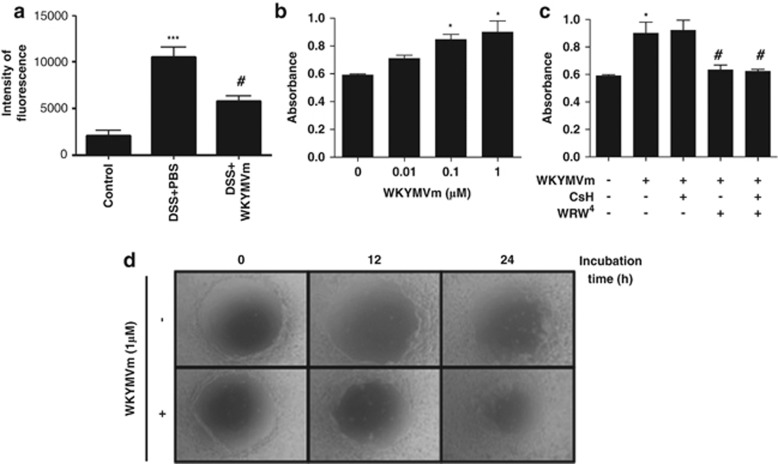
Intestinal permeability is increased during the pathogenesis of ulcerative colitis induced by DSS treatment owing to the destruction of the intestinal epithelial barrier.21 We also observed that DSS treatment increased intestinal permeability in mice, whereas WKYMVm administration effectively decreased the intestinal permeability in DSS-treated mice (Figure 3a). Changes in intestinal permeability are associated with the proliferation and migration of intestinal epithelial cells.22 As we observed that WKYMVm inhibits intestinal permeability, we hypothesized that WKYMVm may affect the proliferation of intestinal epithelial cells. Stimulation of Caco-2 cells (the intestinal epithelial cells) with WKYMVm induced cell proliferation (Figure 3b). To examine whether FPR1 and FPR2 have roles in WKYMVm-induced Caco-2 cell proliferation, the cells were pretreated with CsH (an FPR1 antagonist)19 or WRW4 (an FPR2 antagonist)20 before WKYMVm treatment. Inhibition of FPR2, but not FPR1, caused inhibition of WKYMVm-induced cell proliferation (Figure 3c). We also examined the effects of WKYMVm treatment on Caco-2 cell migration. WKYMVm addition into a scratched Caco-2 cell layer strongly induced wound healing (Figure 3d).
Figure 3.
Protective effects of WKYMVm on the intestinal barrier in colitis. C57BL/6 mice were treated with vehicle, 3% dextran sodium sulfate (DSS) or 3% DSS plus WKYMVm (8 mg kg−1, six subcutaneous administrations at 12-h intervals) for 5 days, and fresh water was provided for an additional 2 days. On day 7, fluorescein isothiocyanate (FITC)–dextran was administered by gavage and serum was collected 4 h later. The amount of FITC–dextran in the serum was measured (a). Caco-2 cells were stimulated with several concentrations (0, 10, 100 and 1000 nM) of WKYMVm (b), or with 1 μM of WKYMVm in the absence or presence of cyclosporine H (CsH; 1 μM) or WRWWWW (WRW4) (10 μM; c) for 24 h. Cell proliferation was measured using the Cell Counting Kit-8 (b, c). The data are expressed as the mean±s.e.m. (n=7 mice per group). ***P<0.001 compared with control. #P<0.05 compared with DSS alone (a). *P<0.05 compared with vehicle. #P<0.05 compared with WKYMVm alone (b, c). Vehicle or WKYMVm (1 μM) was added to a scratched Caco-2 cell layer for 0, 12 or 24 h. Images were obtained with a digital camera attached to a light microscope. The data are representative of four independent experiments (d).
WKYMVm administration affects cytokine profiles in the DSS colitis model
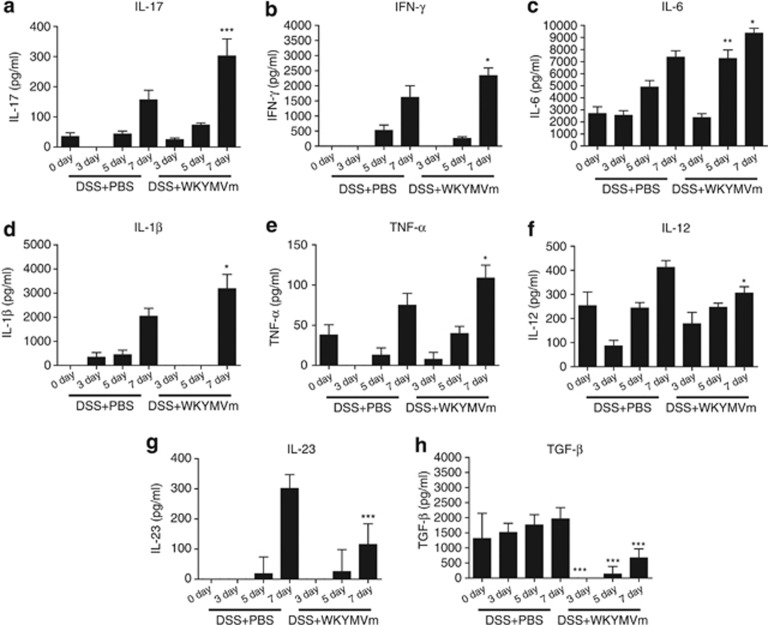
We measured cytokine profiles in the DSS model and found that DSS treatment increased IL-17, IFN-γ, IL-6, IL-1β and TNF-α levels (Figure 4a–e). WKYMVm administration augmented the production of these cytokines in the intestine of DSS-treated mice (Figure 4a–e). However, IL-12, IL-23 and transforming growth factor (TGF)-β were significantly decreased by WKYMVm administration (Figure 4f–h). No significant changes in IL-22 levels were observed in the intestine of DSS-treated mice that were administered with WKYMVm (data not shown).
Figure 4.
Effect of WKYMVm on cytokine production in the colon of dextran sodium sulfate (DSS)-treated mice. Mice were administered with WKYMVm or phosphate-buffered saline (PBS) at 0, 12, 24, 36, 48 and 60 h after DSS treatment. The intestine was collected on day 7 from untreated, DSS-treated and DSS+WKYMVm-treated mice, and was sliced into 1-cm segments. Each segment was incubated in Roswell Park Memorial Institute (RPMI) 1640 medium for 24 h, and cytokines secreted into the supernatant were measured using enzyme-linked immunosorbent assay (ELISA). (a) Interleukin (IL)-17; (b) interferon-γ (IFN-γ); (c) IL-6; (d) IL-1β (e) tumor necrosis factor (TNF)-α (f) IL-12; (g) IL-23; (h) transforming growth factor (TGF)-β. The data are expressed as the mean±s.e.m. (n=7 for a–h). *P<0.05, **P<0.01, ***P<0.001 compared with DSS alone.
Discussion
As the intestine has a unique microbial environment, the intestinal innate immune system has an important role in the maintenance of intestinal homeostasis. The intestinal barrier, which impedes penetrating intact bacteria or other macromolecules, is crucial for maintaining the integrity of a normal intestine. Ulcerative colitis can be initiated by destroying the integrity of the mucosal barrier that protects against microbes, particularly commensal flora.1, 2 Thus, identification of molecules that can stimulate innate immunity against microbes and maintain the intestinal epithelial barrier may contribute to the development of therapeutic agents against ulcerative colitis.
In this study, we demonstrated that WKYMVm has therapeutic activity against colitis in DSS-treated mice. The therapeutic effect of WKYMVm in this colitis model was markedly inhibited by an FPR2 antagonist (WRW4), but not by an FPR1 antagonist (CsH), indicating that FPR2 is critically involved in this process (Figures 1 and 2). Very recently, Wang and colleagues also demonstrated that FPR2-deficient mice showed defects in commensal bacterium-dependent homeostasis in response to DSS challenge and exhibited delayed mucosal restoration after injury, suggesting that FPR2 is important in mediating homeostasis, inflammation and the epithelial repair process in the colon.23 These results support our hypothesis that the receptor for WKYMVm, FPR2, can be regarded as a target molecule for treatment of ulcerative colitis.
The WKYMVm peptide was shown to stimulate the bactericidal activity of phagocytic cells by enhancing the production of reactive oxygen species such as superoxide anions.12, 17 In DSS-treated mice, the WKYMVm peptide increased the proliferation and migration of intestinal epithelial cells, resulting in the inhibition of intestinal permeability. Maintenance of intestinal permeability is important for maintaining the intestinal homeostasis by inhibiting harmful actions of microorganisms in the intestinal microenvironment. As intestinal epithelial cells express three FPRs (FPR1, FPR2 and FPR3)24, 25 and WKYMVm stimulated Caco-2 cell proliferation and migration, which were inhibited by WRW4 but not by CsH, it is reasonable to assume that WKYMVm stimulates FPR2, resulting in the proliferation and migration of intestinal epithelial cells and the subsequent recovery of DSS-induced intestinal permeability in mice.
We also examined changes in levels of several additional cytokines upon WKYMVm administration to DSS-treated mice. IL-23 was demonstrated to mediate inflammation in the colon.26 Mechanistically, IL-23 can induce the recruitment of inflammatory leukocytes into the inflamed intestine.27 Here, we have shown that WKYMVm administration strongly inhibited IL-23 production in the intestine of DSS-treated mice (Figure 4g). This suggests that WKYMVm blocks the pathological progress of ulcerative colitis by inhibiting IL-23 production. TGF-β is known to inhibit inflammatory responses. Knockout of the TGF-β1 gene causes inflammation in multiple organs in mice including the gut.28, 29 Blocking type II TGF-β or disruption of Smad3 (a mediator of TGF-β signaling) can induce colitis or intestinal inflammation in experimental animals.30, 31 However, colonic biopsies from areas of active inflammation in patients with ulcerative colitis exhibit increased levels of TGF-β.32 In this study, we observed that WKYMVm administration inhibits DSS-induced TGF-β production (Figure 4h). This suggests that the paradoxical upregulation of TGF-β in colonic biopsies of ulcerative patients may contribute to the pathological progression of ulcerative colitis. IL-17 also has an important role in the progression of the chronic inflammatory response, including ulcerative colitis.33 IL-17 can mediate an inflammatory response in the inflamed colon.33 However, other reports have demonstrated that IL-17 has protective activity against chronic intestinal inflammation.34, 35 The transfer of IL-17A-deficient T lymphocytes into RAG1-null mice induced severe colitis.35 Previously, we also demonstrated that stimulation of macrophages with IL-17 decreased lipopolysaccharide-induced TNF-α production.17 In this study, we found that the administration of WKYMVm increased IL-17 levels in the colon of DSS-treated mice (Figure 4a). Taken together, our results suggest that WKYMVm-induced IL-17 production may be involved in the therapeutic effect against ulcerative colitis. We previously demonstrated that the administration of WKYMVm in a cecal ligation and puncture sepsis model markedly decreased the production of TNF-α and IL-1β.17 However, WKYMVm administration in a DSS-induced ulcerative colitis model significantly augmented the levels of these two proinflammatory cytokines in the intestine (Figure 4d,e). It is not clear why WKYMVm exerted differential effects on TNF-α and IL-1β production in a sepsis model versus an ulcerative colitis model. As the same dose was used in both studies (8 mg kg−1), the in vivo effects of WKYMVm may therefore depend on the sampling time (∼7 days versus ∼72 h) or the sampled tissue (the intestine slice versus the peritoneal fluid). In ulcerative colitis, WKYMVm likely exerts its effects by dampening local levels of IL-23, TGF-β or IL-17.
In conclusion, the potent immune-modulating peptide WKYMVm had a therapeutic effect on ulcerative colitis by inhibiting the epithelial permeability and modulating the cytokine profile. We suggest that WKYMVm is a potent agonist of FPRs that may be a potential therapeutic agent in the management of chronic intestinal inflammatory diseases.
Acknowledgments
This research was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MEST; No. 35B-2011-1-E00012, 2013 041811, 2012R1A2A2A01007751) and by a grant from Next generation of BioGreen 21 project (PJ008196) from Rural Development Administration, Republic of Korea.
SK and YB have pending patent applications. The remaining authors declare no conflict of interest.
References
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- Economou M, Pappas G. New global map of Crohn's disease: genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008;14:709–720. doi: 10.1002/ibd.20352. [DOI] [PubMed] [Google Scholar]
- Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Desreumaux P, Sandborn WJ, Colombel JF. Crohn's disease: beyond antagonists of tumour necrosis factor. Lancet. 2008;372:67–81. doi: 10.1016/S0140-6736(08)60995-2. [DOI] [PubMed] [Google Scholar]
- Baek SH, Seo JK, Chae CB, Suh PG, Ryu SH. Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries. J Biol Chem. 1996;271:8170–8175. doi: 10.1074/jbc.271.14.8170. [DOI] [PubMed] [Google Scholar]
- Seo JK, Choi SY, Kim Y, Baek SH, Kim KT, Chae CB, et al. A peptide with unique receptor specificity: stimulation of phosphoinositide hydrolysis and induction of superoxide generation in human neutrophils. J Immunol. 1997;158:1895–1901. [PubMed] [Google Scholar]
- Le Y, Gong W, Li B, Dunlop NM, Shen W, Su SB, et al. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- He R, Tan L, Browning DD, Wang JM, Ye RD. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. J Immunol. 2000;165:4598–4605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- Bae YS, Ju SA, Kim JY, Seo JK, Baek SH, Kwak JY, et al. Trp-Lys-Tyr-Met-Val-D-Met stimulates superoxide generation and killing of Staphylococcus aureus via phospholipase D activation in human monocytes. J Leukoc Biol. 1999;65:241–248. doi: 10.1002/jlb.65.2.241. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kim Y, Kim Y, Kim JH, Suh PG, Ryu SH. Trp-Lys-Tyr-Met-Val-D-Met is a chemoattractant for human phagocytic cells. J Leukoc Biol. 1999;66:915–922. doi: 10.1002/jlb.66.6.915. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kang HK, Jo EJ, Kim JI, Lee YN, Lee SH, et al. Trp-Lys-Tyr-Met-Val-Met stimulates phagocytosis via phospho-lipase D-dependent signaling in mouse dendritic cells. Exp Mol Med. 2004;36:135–144. doi: 10.1038/emm.2004.20. [DOI] [PubMed] [Google Scholar]
- Kim SD, Kim JM, Jo SH, Lee HY, Lee SY, Shim JW, et al. Functional expression of formyl peptide receptor family in human NK cells. J Immunol. 2009;183:5511–5517. doi: 10.4049/jimmunol.0802986. [DOI] [PubMed] [Google Scholar]
- Bae YS, Song JY, Kim Y, He R, Ye RD, Kwak JY, et al. Differential activation of formyl peptide receptor signaling by peptide ligands. Mol Pharmacol. 2003;64:841–847. doi: 10.1124/mol.64.4.841. [DOI] [PubMed] [Google Scholar]
- Kim SD, Kim YK, Lee HY, Kim YS, Jeon SG, Baek SH, et al. The agonists of formyl peptide receptors prevent development of severe sepsis after microbial infection. J Immunol. 2010;185:4302–4310. doi: 10.4049/jimmunol.1001310. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Grünbaum L, Seifert R. Differential inhibition of human neutrophil activation by cyclosporins A, D, and H. Cyclosporin H is a potent and effective inhibitor of formyl peptide-induced superoxide formation. J Immunol. 1991;147:1940–1946. [PubMed] [Google Scholar]
- Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD, et al. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J Immunol. 2004;173:607–614. doi: 10.4049/jimmunol.173.1.607. [DOI] [PubMed] [Google Scholar]
- Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn's disease pathogenesis. Ann NY Acad Sci. 2012;1258:159–165. doi: 10.1111/j.1749-6632.2012.06612.x. [DOI] [PubMed] [Google Scholar]
- Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- Chen K, Liu M, Liu Y, Yoshimura T, Shen W, Le Y, et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest. 2013;123:1694–1704. doi: 10.1172/JCI65569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol. 2007;179:8112–8121. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4's anti-inflammatory actions on intestinal epithelium. Am J Physiol Cell Physiol. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Pallone F, Monteleone G. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, et al. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995;146:264–275. [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- Geremia A, Jewell DP. The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2012;6:223–237. doi: 10.1586/egh.11.107. [DOI] [PubMed] [Google Scholar]
- O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]