Summary
Strategies to effectively treat people with CKD have been identified by conventional clinical research. Despite this evidence, awareness, screening, detection, diagnosis, risk factor control, treatment, and outcomes remain substandard. Translating clinical evidence into actionable measures that reduce the burden of CKD is a pressing need. Expansion from a “bench-to-bedside” paradigm (conventional type 1 translation) to research that encompasses “clinic and community” is the core concept of type 2 translation. Specifically, this is the discipline of identifying factors and using strategies that lead to adoption, maintenance, and sustainability of science-based interventions in practice. This review identifies key elements of type 2 translational research and highlights the current scope of this type of research for CKD. For type 2 translation to achieve the goals of providing high-quality care and better health outcomes, key facilitators (e.g., theory-based frameworks, adaptable interventions, and inclusion of sustainability and evaluation metrics) and essential elements (e.g., multidisciplinary team care, health information technology, and stakeholder engagement) must be integrated. The National Institute of Diabetes and Digestive and Kidney Diseases recently funded five proposals that aim to improve outcomes for people with CKD, focusing on diverse components of the healthcare continuum: patient safety and transitions; delivery of high-quality, evidence-based CKD care; and elimination of disparities. The need for type 2 translational research in CKD is urgent because of preventable human suffering and unsustainable costs of providing care. Focus on the theory, framework, and approaches we have suggested may help us meet that challenge.
Introduction
Clinical research has identified strategies that are effective and safe for treatment of people with CKD. Despite the available evidence, awareness, screening, detection, diagnosis, risk factor control, treatment, and outcomes for CKD remain substandard in the United States (1,2). Indeed, it has been argued that many research findings are of limited use to clinicians and largely are ineffective at influencing real-world practice or public health (3). The often lengthy gap between generating clinical research and implementing findings into practice has been construed as “lost in translation” (4). These issues are particularly germane to racial, ethnic, and lower-socioeconomic groups that are disproportionately encumbered by CKD. The need is pressing to translate scientific evidence into actionable measures that reduce this burden.
Creating linkage between interventions shown to improve outcomes in clinical trials (efficacy) and their actual implementation into clinical care (effectiveness) is essential. At a time when the United States health care system is fragmented with variability in access, quality, and outcomes, interventions that aim to close these gaps will play an important role in mitigating morbidity and mortality of patients with CKD and ESRD. The current climate of health care of reform in the United States provides a platform for advancing this field: The Affordable Care Act and Health Information Technology for Economic and Clinical Health Act are introducing the idea of the medical home and medical neighborhood; the Patient-Centered Outcomes Research Institute is emphasizing the importance of translating medical breakthroughs into enhanced patient experiences and funding ground-breaking research in this area; and the American Board of Internal Medicine Foundation is helping providers become better stewards of finite health care resources with their “Choosing Wisely” campaign.
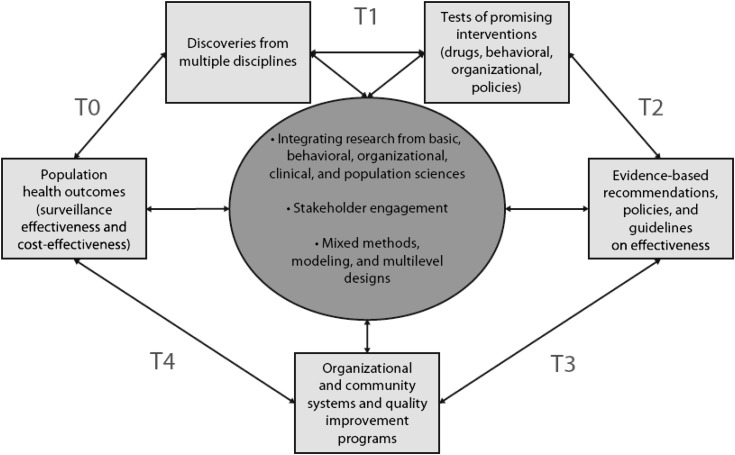
A series of phases of translational research grew from the National Institutes of Health Roadmap initiative, which emphasized moving scientific discoveries to practical applications (Figure 1) (5,6). Expansion from a “bench-to-bedside” paradigm (conventional type 1 translation) to research that encompasses “clinic and community” is a core concept of type 2 translation. Specifically, this is the discipline of identifying factors and using strategies that lead to adoption, maintenance, and sustainability of science-based interventions in practice. Following type 2, types 3 and 4 translational research are extensions into organizational implementation (e.g., quality improvement) and to policies and programs concerned with large-scale public health impact (e.g., surveillance and cost-effectiveness), respectively. Feedback from these latter phases can identify high-impact areas for focus in earlier phases via type 0 investigations (e.g., population health indicators). This review focuses on fundamental elements of type 2 translational research and highlights its current scope in CKD. With the exception of type 1, other phases of translational research are largely undeveloped for CKD, but the field will ultimately be challenged to move in these directions to improve overall health of people with, or at high risk for, kidney disease.
Figure 1.
Phases of translational research as a context for moving scientific discovery to practical applications in the clinic and community (6).
Facilitators and Elements of Type 2 Translational Research
For type 2 translation to achieve the goals of providing high-quality care and better health outcomes for patients with CKD, key facilitators (e.g., theory-based frameworks, adaptable interventions, and inclusion of sustainability and evaluation metrics) and essential elements (e.g., multidisciplinary team care, health information technology, and stakeholder engagement) must be integrated as a whole.
Theory-based Interventions
Theories are sets of interrelated concepts and definitions that organize, predict, and explain phenomena, events, and behavior (7). They are inherently abstract and can be broadly applied to various clinical content or topic areas. Strong behavioral, social, and organizational theories applied in health care delivery, such as individual behavior change, social marketing, health communication, social/behavioral economics, and diffusion of innovations, challenge researchers to articulate targets of interventions, identify relationships among variables, predict mechanisms and directionality of effect, and specify necessary resources for project implementation. In so doing, they provide insight into how and why individual components of complex projects are successful and are useful for the planning and execution of interventions. Additionally, behavioral, social, and organizational theories provide a universal language and context to researchers, health care providers, and policymakers, enabling them to compare and optimize programs that are otherwise heterogeneous to enhance the uptake of research findings into daily medical practice (8).
Evaluation and Sustainability Frameworks
As theoretical frameworks allow for better planning and execution of interventions, evaluation and sustainability frameworks facilitate translation. They focus on effectiveness, whether programs have achieved their objectives, and whether programs can be adapted and widely adopted in different settings. This knowledge is essential to understanding which programs can achieve and sustain a desired effect, over what time period, and at what cost, particularly in settings with limited resources. Many evaluation frameworks exist, each with different advantages, but almost all examine dimensions of reach (participation rate, representativeness of participants), adoption (representativeness of settings and staff essential to program implementation), and sustainability (cost, resources, and reproducibility) (9,10). Evaluation at the individual level (e.g., patients, staff, and health care providers) and organizational level (e.g., hospital, clinic, integrated healthcare system) must be incorporated into type 2 translational research protocols. Tracking data necessary for analyses at this level are worth the upfront effort because they provide policymakers, health care leaders, and researchers the ability to select and tailor interventions before adopting a program that might interrupt clinical practice.
Adaptability
Although set protocols have been the mainstay of rigorous research and ensure reproducibility and efficacy across different settings and patient populations, it is becoming increasingly evident that type 2 translational research programs need to be flexible to adapt to the community in which they aim to translate evidence into practice (11–13). A prime example is the need to account for differing levels of health literacy, given the diversity of individuals affected by CKD and findings that low health literacy is associated with vital outcomes, such as increased mortality and decreased access to transplantation, among adults with ESRD (14,15). Implementing evidence-based “best practices” is facilitated by clear communication among patients, family members, and the health care team (16). Use of common language and reducing medical jargon is essential to connect with a wide spectrum of health care consumers. Recognition of diversity, including levels of health literacy, and use of culturally appropriate language and materials are of considerable value (17). To that end, projects make a meaningful impact among high-risk groups with CKD only if they adapt protocols, materials, and outreach efforts to varying levels of health literacy and cultural backgrounds.
Health Information Technology
Eliminating the quality gap between evidence and practice requires greater identification of CKD, enhanced provider adherence to guidelines, and heightened patient engagement in health. Type 2 translational research projects that target these areas can be most successful when they use novel health information technology. For example, electronic health records with automated estimated GFR reporting can help primary care providers identify patients with CKD and ensure timely referral to nephrologists, while point-of-care decision support may increase adherence to evidence-based guidelines for clinical practice (18,19). Electronic registries identify cohorts and allow for population-level management of patients, thereby enhancing the application of guidelines through patient outreach (20). Registries also provide data for local, regional, and national surveillance of trends among patients with CKD. In regards to engagement, the ubiquitous nature of mobile phones allows for outreach to patients of varied backgrounds and socioeconomic status. Texting and phone-based self-management programs have been used to increase engagement and improve health outcomes among patients with other chronic diseases (21).
Multidisciplinary Team Care
Type 2 translational research inherently requires a team. Ideally, professionals from various disciplines (e.g., medicine, nursing, pharmacy, nutrition/dietetics, and social work) and domains of expertise (e.g., primary care, diabetology, and nephrology) collaborate to identify factors that lead to adoption, maintenance, and sustainability of science-based interventions in practice (22,23). In addition to physicians, nurse practitioners and physician’s assistants on the team enable achievement of patient-centered goals and provide oversight of chronic care (24). Consideration may be given to others with expertise complementary to health care professionals. For example, in the Diabetes Prevention Program (DPP), community health workers facilitated strategies for individual behavior change (25).
Stakeholder Engagement
Successful translation of research into improved population health requires not only a multidisciplinary team but also stakeholder engagement and involvement at many points along the research continuum. Diverse stakeholders may include members of the lay community, nonprofit organizations, patients, health care providers, health care delivery system managers, and public health officials not directly involved in the research team. As advisors, they provide enhanced understanding of barriers and facilitators of participation and the environmental and social contexts in which the research will take place. Their knowledge favorably influences the agenda, setting, validity, and feasibility of the research design from the informed consent process to data collection and outcome measures (26). From a pragmatic perspective, participatory research is essential. From a dissemination perspective, stakeholder involvement ensures external validity and identifies how study results may be applied to different real-world settings.
Diabetes as an Example and Opportunity
Type 2 translational research has been largely nascent in the discipline of nephrology. Hence, examples from related fields are useful to illustrate its worth and necessity. As a successful model, the DPP evolved from a clinical trial into community-based intervention (27). In the original clinical trial sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), study participants were randomly assigned to lifestyle intervention, treatment with the drug metformin, or a “placebo” control group (28). For the lifestyle intervention, a curriculum addressing diet, physical activity, and behavior change was taught to participants one-on-one by case managers. The main goal of the intervention was 5%–7% weight reduction achieved by a healthy diet low in fat and calories, along with 30 minutes of physical activity 5 days per week. The clinical trial ended 1 year earlier than planned because the results were so clear: Lifestyle intervention reduced the relative risk of developing type 2 diabetes by 58% over 4 years. The group receiving metformin also benefited, but the results were less impressive with a 31% relative risk reduction over the same period.
The DPP Outcomes Study (DPPOS) is a follow-up study designed to assess long-term effects of DPP treatments on development of type 2 diabetes and complications (29). After 10 years, the lifestyle intervention sustained a reduction in the rate of new-onset type 2 diabetes by 34%, including a 49% reduction in those older than age 60 years, and delayed onset of diabetes by approximately 4 years. Again, beneficial but lesser effects of metformin treatment included an 18% reduction in the diagnosis of new-onset type 2 diabetes and a delay in diabetes onset by about 2 years. Of note, attainment of normoglycemia reduced the incidence of diabetes by 56% no matter how this reversion was achieved or however transiently (30). With regard to complications, the DPPOS reported diabetic retinopathy in 8% of participants with prediabetes and 13% of those who developed diabetes (31). Such additional insights from the long-term follow-up study suggest that people with prediabetes or newly diagnosed type 2 diabetes be screened for retinopathy.
To move this new knowledge into action for nearly 80 million adults in the United States with prediabetes, the DPP was used to model a public health initiative, the National Diabetes Prevention Program sponsored by the Centers for Disease Control and Prevention (32). This initiative was authorized by the U.S. Congress in March 2010 to establish a network of DPP-type lifestyle intervention programs for people at high risk of developing type 2 diabetes. To widely implement the intervention in a cost-effective manner, sites were selected on the basis of their multidisciplinary teams and capabilities to train lifestyle coaches and deliver services adapted to their community. Support is provided for research, evaluation, monitoring, and technical assistance, all based on existing theoretical and evaluation frameworks. Fidelity to the science is assured by standards consistent with the DPP as requirements for sites. Lifestyle coaches teach groups of participants about healthy eating habits and physical activity during weekly sessions. After the initial 16 sessions, groups continue to meet monthly for ongoing support. Data are collected to validate that the lifestyle intervention has been delivered as intended and to assess whether the DPP/DPPOS results actually translate into real-world settings. Inaugural partners include the YMCA programs (intervention sites) and United Health Group (a health insurance payer).
On the other hand, the Chronic Disease Self-Management Program (CDSMP) is an example of an intervention that, despite strong evidence of effectiveness, has not been widely translated to clinical practice. The CDSMP is a lay person–led workshop designed to improve confidence and engagement in self-management behaviors among people with chronic health conditions (33–35). Lay leaders deliver the 6-week program to groups of participants in 2.5-hour weekly sessions in the community. Using tenets of social cognitive theory and self-efficacy, participants learn coping strategies, such as action planning and feedback, behavior modeling, problem-solving, positive self-talk, and decision-making (36). They also learn to manage symptoms by various strategies: relaxation, healthy eating, exercise, rest, medication, and communicating with health care professionals.
Clinical research has shown the effectiveness of the CDSMP. Across studies, participants had improvements in pain, disability/functional impairments, fatigue, psychological well-being, self-rated health, distress, aerobic capacity, cognitive symptom management, self-efficacy, and ability to communicate with health care professionals after program completion (37). Benefits of participating in the CDSMP may extend up to 2 years (34). However, with notable exceptions (e.g., the Kaiser Permanente health plan), it is not routinely incorporated into the United States health care system. This may be related to the fragmented method of funding health care. Even though the cost of CDSMP is very low, responsibility for payment remains problematic. In addition, although study results were published in high-quality journals, these data may not have been widely disseminated to primary care providers. In contrast to the United States, the United Kingdom and Denmark have fully integrated CDSMP into their health care systems and people attend community-based workshops free of charge.
Because diabetes is such a serious and common chronic condition, the DPP and CDSMP interventions present considerable opportunity for type 2 translation. Data from DPP/DPPOS have not yet been reported for diabetic kidney disease (DKD) but are eagerly anticipated. For example, preventing diabetes is generally believed to prevent kidney disease, but this presumption remains to be confirmed. DKD occurs in about 40% of people with type 2 diabetes overall, with even higher rates among disproportionately affected ethnic and racial minorities (38). More than half of incident cases of ESRD are currently attributable to diabetes in the United States (1). However, this number underestimates its true burden in that kidney disease amplifies risk such that death is more likely than is progression to ESRD (39). In other words, the ESRD population with diabetes can be viewed as a “survivor cohort.” The United States Renal Data System has reported that incidence of ESRD attributed to diabetes has stabilized or improved since 2000 (1). Although this observation has been widely hailed as a success of better care, not all people are similarly benefiting. For example, blacks and American Indians in the 30- to 39-year-old age groups have shown steadily increasing incidence rates of ESRD due to diabetes over the same time period (1). These high-risk groups represent important populations for evaluation of strategies to reduce risk, a prototype for type 2 translational research. Occurrence of type 2 diabetes is now rampant in nonwhite youth (ages 10–19 years) and exceeds the frequency of type 1 diabetes (40). Notably, among Pima Indians, youth-onset type 2 diabetes is associated with higher rates of both ESRD and death by midlife compared with type 2 diabetes diagnosed in adults (41). Moreover, prevalence of DKD has increased steadily in the United States since the 1980s, largely attributable to the progressive climb in the total number of people developing diabetes (42). Although there are no published data about the CDSMP for kidney disease, it has been tested in a multitude of people with chronic conditions, including diabetes. Therefore, it is likely that many had CKD and that the skills learned in CDSMP are applicable.
In sum, kidney disease is a great tragedy of diabetes. Treatments are available but underused, a problem especially evident in racial and ethnic minorities that are disproportionately laden with DKD. If preventing diabetes proves to avert kidney disease, then programs such as the Centers for Disease Control and Prevention–sponsored National Diabetes Prevention Program and CDSMP are essential tools to reduce DKD’s effect on risky comorbid conditions, ESRD, and death.
Initial Approaches to Type 2 Translational Research for CKD
Since its inception, the National Kidney Disease Education Program has documented the public health burden of CKD and the existing gap between science and delivery of care. Although some processes have been implemented to improve CKD care, their advances are lost or not achieved if programs are not sustained or adequately supported. For example, a point-of-care decision support tool in a primary care practice led to noticeable improvements in detection of CKD, anemia, and measures of bone and mineral metabolism disorders after a 1-year quality improvement project. However, the gains diminished 2 years after the program ceased, perhaps because it did not leverage health information technology (43). At a United States Veterans Affairs primary care clinic, provider education and an electronic CKD registry increased performance of various process measures, but corresponding outcomes (e.g., BP control or use of renin-angiotensin system inhibitors) did not improve, perhaps because of lack of system redesign (e.g., protected time, ancillary support, and multidisciplinary team) (44). Such examples of shortcomings reveal the necessity of sustainability and redesign of health care delivery systems to implement lasting changes in CKD care.
The NIDDK responded to these unmet needs by sponsoring a conference followed by a request for applications for type 2 translational research projects with the explicit goal of improving health outcomes for people with CKD (45). Five proposals that focus on diverse components of the health care continuum were funded; topics include patient safety and transitions; delivery of high-quality, evidence-based early and late CKD care, including preparation for renal replacement therapy (RRT) care; and elimination of disparities (Table 1). Each of these projects uses key facilitators and essential elements of type 2 translational research.
Table 1.
National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Type 2 Translational Research Projects Designed to Test Effectiveness of Interventions for Prevention, Treatment, and Management of CKD
| PI | Title | Focus within Health Care System | Goal | Interventions | Key Facilitators | Essential Elements |
|---|---|---|---|---|---|---|
| Tuttle and Corbett | Medication Intervention in Translational Care to Optimize CKD Outcomes and Costs (CKD-MIT) | Post–hospital discharge | Ensure patient safety through care transitions | Pharmacy-led medication reconciliation and patient motivation/self-management | Self-management model | Team care: pharmacists, primary care providers, nurses Health information technology: electronic data collection system Adaptable: accounts for patient health literacy |
| Nally | Navigating the Challenges of CKD | Nephrology clinic; primary care provider offices | Educate patients and improve CKD care coordination | CKD patient navigators coordinate care, eliminate system-barriers, and educate/motivate patients through navigators plus CKD personal health records | Motivational interviewing Patient-enhanced CKD personal health record | Team care: navigators, primary care providers, nephrologists, patients Health information technology: registry, CKD personal health record, mobile applications |
| Boulware | Decision Support Interventions to Improve Renal Replacement Therapy | Primary care and predialysis specialty clinics | Enhance informed decision-making about RRT | Practice-level improvement of primary care and specialty collaboration on RRT preparation | Implementation science | Collaborative care between primary care providers and specialists |
| Health care providers | Enhanced preparation for RRT | Specialty provider training to improve skills in engaging in shared and informed RRT decisions | Shared decision-making | Patient engagement in decisions about care | ||
| Patients | Patient decision-support through provider of educational decision-aids (audiovisual and written) | Addresses educational and literacy needs of patients | ||||
| Powe | KARE: Kidney Awareness Registry and Education | Primary care | Mitigate disparities through improved delivery of CKD care | PCP decision support through a point-of-care electronic registry Patient-directed education and self-management program | Social cognitive theory Chronic care model RE-AIM evaluation framework | Health information technology: CKD registry; telephone self-management system Team care: health coaches, medical assistants, primary care providers Adaptable: accounts for patient health literacy, clinic culture Stakeholders: primary care providers, public health officials |
| Vazquez | Improving CKD Detection and Care in the High-Risk and Underserved | Primary care–specialty care interface | Mitigate disparities through improved delivery of CKD care | Provider decision support Detection, outreach, patient and provider education | Chronic care model | Health information technology: Comprehensive registry: updated Stakeholders: primary care clinics |
The intent is that interventions will have a high likelihood of being widely adopted and sustained in diverse health care settings and by individuals and communities at highest risk. PI, principal investigator; RRT, renal replacement therapy; RE-AIM, Reach Effectiveness Adoption Implementation Maintenance.
Patient Safety and Transitions in Care
Transitions are risky experiences for patients, and, therefore, these are prime targets for type 2 translation research. For example, risks of adverse outcomes are extraordinarily high when people with CKD move from an inpatient hospitalization to the outpatient setting. The CKD population has more comorbid conditions, is hospitalized more often and for longer lengths of stay, and incurs greater health care costs than patients with other chronic diseases (46–48). Moreover, patients with CKD are less likely to receive evidence-based therapies when hospitalized for serious illnesses, such as myocardial infarction, and commonly have complex drug regimens and adverse events that contribute to poor outcomes (49,50). Interventions to improve medication management in the transition from hospital-to-home reduce readmissions and declining health in the general population of hospitalized patients (51,52). Unknown is the effect enhanced transitional care can have on health outcomes, costs, and probability of readmission for a very high-risk group, such as the CKD population.
In one of the NIDDK-funded projects, Tuttle and Corbett and colleagues are studying the effectiveness of a transitional care strategy focused on improving medication information transfer. The rationale that underlies this strategy is that careful attention to medication management is foundational to caring for CKD and reducing complications. Therefore, for hospitalized patients with CKD who are transitioning to home, accurate and comprehensive medication information transfer is essential. The hypothesis is that medication discrepancies after hospital discharge are widespread and that their resolution, through improved transfer of medication information during transitional care, will reduce acute care utilization and costs. This strategy is also hypothesized to improve management of CKD and related risk factors, leading to fewer complications that result in repeated episodes of hospitalization.
Patients with CKD are recruited while hospitalized for reasons other than psychiatric or maternity admissions. Study participants are then randomly assigned to control or intervention groups. Participants assigned to the intervention group receive a home visit by a pharmacist within 5 days of discharge. The “5 As” self-management model (Assessment, Advice, Agreement, Assistance, and Arrangements) is used to implement the intervention to promote accurate and comprehensive transfer of medication information (53,54). System and self-management barriers are overcome by identifying and resolving hospital-to-home medication discrepancies and providing patients with skills for ongoing medication information transfer (e.g., updating medication lists, sharing with providers). On the basis of previous pilot studies, this pharmacist-led medication intervention is expected to substantially reduce acute care utilization and improve evidence-based management for people with CKD (51,52).
Delivery of High-quality Evidence-based CKD Care
Poor CKD awareness and inadequate coordination among patients and health care providers prevent widespread implementation of evidence-based treatments and self-management regimens that are effective in slowing progression of CKD to ESRD. As such, Nally and colleagues have targeted their research project to the health care system to improve delivery of high-quality chronic CKD care. Specifically, they have developed a novel multidisciplinary CKD Patient Navigator program, based on successful navigator programs in the oncology field, to promote adherence to CKD care regimens (55). By engaging patients and guiding them through complex health care delivery systems, navigators use motivational interviewing and other behavior change frameworks to help patients overcome personal barriers to receiving evidence-based CKD care and reduce delays in accessing the continuum of CKD services, from initial CKD diagnosis to preparation for RRT.
Advance preparation for initiating RRT in patients with progressive CKD is an ideal example of evidence-based CKD care associated with improved mortality and morbidity (56). However, previous studies have demonstrated poor RRT preparation, including inadequate management of CKD-related comorbid conditions (e.g., hypertension and anemia), use of temporary catheter vascular access, and urgent dialysis initiation (57,58). Evidence suggests that early, shared, and informed decision-making among patients, families, and health care providers about preparation for RRT initiation may improve patient outcomes, including management of BP and anemia, use of permanent vascular access at RRT initiation, and selection of RRT modalities consistent with patients' values (59–62).
Boulware and colleagues will examine the effect of a series of system, provider, and patient interventions to improve early shared decision-making about RRT among diverse patients with CKD. By increasing communication between primary care and nephrology practitioners, enhancing provider skills in engaging in shared decision-making conversations with patients, and providing patient decision support through language-concordant, literacy-appropriate audiovisual and written materials, this project aims to minimize the translational gap between evidence and practice as it pertains to RRT preparation.
Health Care Disparities
Disparities in CKD care have been documented for decades, but progress toward reducing them has been slow (63). The gap between research and practice is particularly wide in public health care settings, which disproportionately care for those most vulnerable, such as the uninsured and underinsured, racial and ethnic minorities, and people with limited health literacy (64,65). Low levels of CKD awareness among primary care providers, a prerequisite for delivery of optimal CKD care, and low patient understanding of disease and self-efficacy, both necessary for optimal adherence to medical regimens, are key barriers to better health outcomes (66,67). Moreover, because low CKD awareness in patients is associated with lesser health literacy and poverty, addressing this barrier may play a key role in reducing disparities in health outcomes among patients with CKD (68,69).
The Kidney Awareness Registry and Education (KARE) study will use an adapted social cognitive theory framework to promote individual behavior change and minimize disparities in CKD care. KARE consists of two separate interventions directed toward primary care providers and patients with CKD in the Community Health Network, the integrated delivery system serving uninsured and publically insured residents in San Francisco, California. Using the Chronic Care Model as a framework for the delivery of high-quality ambulatory care, Powe and colleagues will study the effect on provision of CKD care with an electronic registry to identify patients with CKD, highlight evidence-based guidelines, provide point-of-care decision support, and deliver actionable feedback in a timely manner to multidisciplinary health care teams. The registry includes data on race and ethnicity, language, and gender, to increase transparency of disparities (70,71). Additionally, KARE examines the effect of a telephone self-management program, culturally and linguistically tailored to ethnically diverse patients, on BP control and CKD awareness in primary care settings.
Poor coordination among providers in fragmented public health systems contributes to health care disparities. Vazquez and colleagues, in another NIDDK-sponsored project titled “Improving CKD Detection and Care in a High-Risk Underserved Population,” aim to reduce disparities through a model of joint primary and nephrology care. This novel system relies on a robust, fully operational health information technology–enabled program, the Parkland Intelligent e-Coordination and Evaluation System. This program harnesses the electronic medical record to implement, coordinate, and monitor delivery of evidence-based practice. As such, this technology allows collaboration among providers to slow CKD progression, minimize complications, and ensure optimal RRT preparation among people with CKD in the high-risk, underserved population of Dallas County, Texas.
Conclusions
Translation of scientific evidence into actionable measures that reduce the burden of chronic diseases is a top priority in health care. Type 2 translational research is a key strategy for identifying pathways to improve care. The need is particularly urgent in CKD because of the preventable human suffering and unsustainable costs of providing care. Several opportunities have been identified as vital areas for type 2 translational research in CKD, including patient safety and transitions in care, delivery of evidence-based care, and elimination of disparities. Discovering better ways to ensure that patients receive the CKD care they need is a formidable challenge. A focus on type 2 translation that uses the theory, framework, and approaches we have suggested may help us rise to that challenge.
Disclosures
K.R.T. has provided consulting services to Eli Lilly and Company regarding novel therapies for diabetic kidney disease. This work is not related to the present paper. None of the other authors have disclosures to report.
Acknowledgment
This work was supported in part by grants R34 DK094016 (Dr. Tuttle, Dr. Corbett), K23 DK094850 (Dr. Tuot) and R34DK093992 (Dr. Powe) from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.U S Renal Data System : USRDS 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 2.Shah A, Fried LF, Chen SC, Qiu Y, Li S, Cavanaugh KL, Norris KC, Whaley-Connell AT, McCullough PA, Mehrotra R, KEEP Investigators : Associations between access to care and awareness of CKD. Am J Kidney Dis 59[Suppl 2]: S16–S23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler R, Glasgow RE: A proposal to speed translation of healthcare research into practice: Dramatic change is needed. Am J Prev Med 40: 637–644, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Lenfant C: Shattuck lecture—clinical research to clinical practice—lost in translation? N Engl J Med 349: 868–874, 2003 [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Medical Research Scholars Program: Translational research. Available at: http://commonfund.nih.gov/clinicalresearch/overview-translational.aspx Accessed March 6, 2013.
- 6.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C: National Institutes of Health approaches to dissemination and implementation science: Current and future directions. Am J Public Health 102: 1274–1281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glanz K, Rimer BK, Viswanath K: Health behavior and health education: theory, research, and practice, 4th Ed., San Francisco, CA, Jossey-Bass, 2008 [Google Scholar]
- 8.Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N: Changing the behavior of healthcare professionals: The use of theory in promoting the uptake of research findings. J Clin Epidemiol 58: 107–112, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Green LW, Kreuter MW: Health promotion planning: an educational and ecological approach, 3rd Ed., Mountain View, CA, Mayfield Pub. Co., 1999 [Google Scholar]
- 10.Dearing JW: Evolution of diffusion and dissemination theory. J Public Health Manag Pract 14: 99–108, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Glasgow RE, Nelson CC, Strycker LA, King DK: Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med 30: 67–73, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Glasgow RE: RE-AIMing research for application: Ways to improve evidence for family medicine. J Am Board Fam Med 19: 11–19, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Handley MA, Hammer H, Schillinger D: Navigating the terrain between research and practice: A Collaborative Research Network (CRN) case study in diabetes research. J Am Board Fam Med 19: 85–92, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh KL, Wingard RL, Hakim RM, Eden S, Shintani A, Wallston KA, Huizinga MM, Elasy TA, Rothman RL, Ikizler TA: Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol 21: 1979–1985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu CY: Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol 4: 195–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright Nunes JA, Wallston KA, Eden SK, Shintani AK, Ikizler TA, Cavanaugh KL: Associations among perceived and objective disease knowledge and satisfaction with physician communication in patients with chronic kidney disease. Kidney Int 80: 1344–1351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lora CM, Gordon EJ, Sharp LK, Fischer MJ, Gerber BS, Lash JP: Progression of CKD in Hispanics: Potential roles of health literacy, acculturation, and social support. Am J Kidney Dis 58: 282–290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain A, Hemmelgarn BR: Impact of estimated glomerular filtration rate reporting on nephrology referrals: A review of the literature. Curr Opin Nephrol Hypertens 20: 218–223, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Fox CH, Swanson A, Kahn LS, Glaser K, Murray BM: Improving chronic kidney disease care in primary care practices: An upstate New York practice-based research network (UNYNET) study. J Am Board Fam Med 21: 522–530, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jr, Jain A, Nally JV, Jr: Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol 6: 40–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schillinger D, Handley M, Wang F, Hammer H: Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: A three-arm practical clinical trial. Diabetes Care 32: 559–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow RE, Chambers D: Developing robust, sustainable, implementation systems using rigorous, rapid and relevant science. Clin Transl Sci 5: 48–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damschroder LJ, Goodrich DE, Robinson CH, Fletcher CE, Lowery JC: A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: A mixed methods study. BMC Health Serv Res 11: 248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohman-Strickland PA, Orzano AJ, Hudson SV, Solberg LI, DiCiccio-Bloom B, O’Malley D, Tallia AF, Balasubramanian BA, Crabtree BF: Quality of diabetes care in family medicine practices: influence of nurse-practitioners and physician’s assistants. Ann Fam Med 6: 14–22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG: Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 35: 357–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callard F, Rose D, Wykes T: Close to the bench as well as at the bedside: Involving service users in all phases of translational research. Health Expect 15: 389–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garfield SA, Malozowski S, Chin MH, Narayan KMV, Glasgow RE, Green LW, Hiss RG, Krumholz HM, Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group : Considerations for diabetes translational research in real-world settings. Diabetes Care 26: 2670–2674, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group : Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM, Diabetes Prevention Program Research Group : 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374: 1677–1686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group : Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet 379: 2243–2251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan DM, Chew E, Christophi CA, Davis MD, Fowlers S, Goldstein BJ, Hamman RF, Hubbard DL, Knowler WC, Molitch ME, Diabetes Prevention Program Research Group : The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 24: 137–144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention: National Diabetes Prevention Program. Available at: cdc.gov/diabetes/prevention/index.htm Accessed July 13, 2012.
- 33.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, Gonzalez VM, Laurent DD, Holman HR: Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care 37: 5–14, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Lorig KR, Ritter PL, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, Gonzalez VM, Laurent DD, Holman HR: Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 39: 1217–1223, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M: Effect of a self-management program on patients with chronic disease. Eff Clin Pract 4: 256–262, 2001 [PubMed] [Google Scholar]
- 36.Bandura A: Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev 84: 191–215, 1977 [DOI] [PubMed] [Google Scholar]
- 37.Foster G, Taylor SJ, Eldridge SJ, Ramsay J, Griffths CJ: Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev CD005108, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tuttle KR, Nelson RG; For the Kidney Disease Outcomes Quality Initiative (KDOQI) Work Group. National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kid Dis 49: S1–S179, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP : Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention: Diabetes Public Health Resource. 2001 National Diabetes Fact Sheet. Available at: cdc.gov/diabetes/pubs/factsheet11.htm Accessed July 13, 2012.
- 41.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG: Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 296: 421–426, 2006 [DOI] [PubMed] [Google Scholar]
- 42.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wentworth AL, Fox CH, Kahn LS, Glaser K, Cadzow R: Two years after a quality improvement intervention for chronic kidney disease care in a primary care office. Am J Med Qual 26: 200–205, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Drawz PE, Miller RT, Singh S, Watts B, Kern E: Impact of a chronic kidney disease registry and provider education on guideline adherence—a cluster randomized controlled trial. BMC Med Inform Decis Mak 12: 62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Institute for Diabetes and Digestive and Kidney Diseases. Conference summary for Workshop on Translating CKD Research into Improved Clinical Outcomes, October 18 and 19, 2010. Available at: http://www3.niddk.nih.gov/fund/other/ckdt2workshop/index.html Accessed July 13, 2012
- 46.Daratha KB, Short RA, Corbett CF, Ring ME, Alicic R, Choka R, Tuttle KR: Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol 7: 409–416, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Nitsch D, Nonyane BA, Smeeth L, Bulpitt CJ, Roderick PJ, Fletcher A: CKD and hospitalization in the elderly: A community-based cohort study in the United Kingdom. Am J Kidney Dis 57: 664–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider KM, O’Donnell BE, Dean D: Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Outcomes 7: 82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD, Acute Coronary Treatment and Intervention Outcomes Network registry : Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 121: 357–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassan Y, Al-Ramahi RJ, Aziz NA, Ghazali R: Adverse drug events in hospitalized patients with chronic kidney disease. Int J Clin Pharmacol Ther 48: 571–576, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Corbett CF, Setter SM, Daratha KB, Neumiller JJ, Wood LD: Nurse identified hospital to home medication discrepancies: Implications for improving transitional care. Geriatr Nurs 31: 188–196, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Setter SM, Corbett CF, Neumiller JJ, Gates BJ, Sclar DA, Sonnett TE: Effectiveness of a pharmacist-nurse intervention on resolving medication discrepancies for patients transitioning from hospital to home health care. Am J Health Syst Pharm 66: 2027–2031, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Glasgow RE, Emont S, Miller DC: Assessing delivery of the five ‘As’ for patient-centered counseling. Health Promot Int 21: 245–255, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Hung DY, Shelley DR: Multilevel analysis of the chronic care model and 5A services for treating tobacco use in urban primary care clinics. Health Serv Res 44: 103–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paskett ED, Harrop JP, Wells KJ: Patient navigation: An update on the state of the science. CA Cancer J Clin 61: 237–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metcalfe W, Khan IH, Prescott GJ, Simpson K, MacLeod AM: Can we improve early mortality in patients receiving renal replacement therapy? Kidney Int 57: 2539–2545, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Avram MM, Blaustein D, Fein PA, Goel N, Chattopadhyay J, Mittman N: Hemoglobin predicts long-term survival in dialysis patients: A 15-year single-center longitudinal study and a correlation trend between prealbumin and hemoglobin. Kidney Int Suppl 87: S6–S11, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Cueto-Manzano AM, Martínez-Ramírez HR, Cortés-Sanabria L: Management of chronic kidney disease: primary health-care setting, self-care and multidisciplinary approach. Clin Nephrol 74[Suppl 1]: S99–S104, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Thilly N, Boini S, Kessler M, Briançon S, Frimat L: Chronic kidney disease: Appropriateness of therapeutic management and associated factors in the AVENIR study. J Eval Clin Pract 15: 121–128, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Lenz O, Sadhu S, Fornoni A, Asif A: Overutilization of central venous catheters in incident hemodialysis patients: Reasons and potential resolution strategies. Semin Dial 19: 543–550, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Flesher M, Woo P, Chiu A, Charlebois A, Warburton DE, Leslie B: Self-management and biomedical outcomes of a cooking, and exercise program for patients with chronic kidney disease. J Ren Nutr 21: 188–195, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Powe NR: Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 19: 1271–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Eakin EG, Bull SS, Glasgow RE, Mason M: Reaching those most in need: A review of diabetes self-management interventions in disadvantaged populations. Diabetes Metab Res Rev 18: 26–35, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plantinga LC, Tuot DS, Powe NR: Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis 17: 225–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bodenheimer T, Lorig K, Holman H, Grumbach K: Patient self-management of chronic disease in primary care. JAMA 288: 2469–2475, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Wright JA, Wallston KA, Elasy TA, Ikizler TA, Cavanaugh KL: Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis 57: 387–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuot DS, Plantinga LC, Hsu CY, Jordan R, Burrows NR, Hedgeman E, Yee J, Saran R, Powe NR, Centers for Disease Control Chronic Kidney Disease Surveillance Team : Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol 6: 1838–1844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wagner JH, Justice AC, Chesney M, Sinclair G, Weissman S, Rodriguez-Barradas M, VACS 3 Project Team : Patient- and provider-reported adherence: Toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol 54[Suppl 1]: S91–S98, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Chin MH, Walters AE, Cook SC, Huang ES: Interventions to reduce racial and ethnic disparities in health care. Med Care Res Rev 64[Suppl]: 7S–28S, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]