Summary
Background and objectives
Although AKI is common among hospitalized children, comprehensive epidemiologic data are lacking. This study characterizes pediatric AKI across the United States and identifies AKI risk factors using high-content/high-throughput analytic techniques.
Design, setting, participants, & measurements
For the cross-sectional analysis of the 2009 Kids Inpatient Database, AKI events were identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes. Demographics, incident rates, and outcome data were analyzed and reported for the entire AKI cohort as well as AKI subsets. Statistical learning methods were applied to the highly imbalanced dataset to derive AKI-related risk factors.
Results
Of 2,644,263 children, 10,322 children developed AKI (3.9/1000 admissions). Although 19% of the AKI cohort was ≤1 month old, the highest incidence was seen in children 15–18 years old (6.6/1000 admissions); 49% of the AKI cohort was white, but AKI incidence was higher among African Americans (4.5 versus 3.8/1000 admissions). In-hospital mortality among patients with AKI was 15.3% but higher among children ≤1 month old (31.3% versus 10.1%, P<0.001) and children requiring critical care (32.8% versus 9.4%, P<0.001) or dialysis (27.1% versus 14.2%, P<0.001). Shock (odds ratio, 2.15; 95% confidence interval, 1.95 to 2.36), septicemia (odds ratio, 1.37; 95% confidence interval, 1.32 to 1.43), intubation/mechanical ventilation (odds ratio, 1.2; 95% confidence interval, 1.16 to 1.25), circulatory disease (odds ratio, 1.47; 95% confidence interval, 1.32 to 1.65), cardiac congenital anomalies (odds ratio, 1.2; 95% confidence interval, 1.13 to 1.23), and extracorporeal support (odds ratio, 2.58; 95% confidence interval, 2.04 to 3.26) were associated with AKI.
Conclusions
AKI occurs in 3.9/1000 at-risk US pediatric hospitalizations. Mortality is highest among neonates and children requiring critical care or dialysis. Identified risk factors suggest that AKI occurs in association with systemic/multiorgan disease more commonly than primary renal disease.
Introduction
AKI, defined as an abrupt decline in renal function, is a common complication among hospitalized patients with a rising incidence (1–3). Higher AKI rates have striking implications, because AKI is associated with increased mortality and greater morbidity (4–6). In adults, a creatinine increase as modest as 0.3 mg/dl is associated with a fourfold increase in mortality (4); critically ill pediatric patients with AKI severe enough to require dialysis experience mortality rates of 30%–50% (7,8). Children with AKI experience longer hospitalizations, prolonged intensive care unit (ICU) stays, and greater need for mechanical ventilation (6,9). Additionally, children who survive AKI are likely to experience residual renal abnormalities; proteinuria, hypertension, and reduced GFRs persist in up to 60% of AKI survivors (10–12).
Although adult AKI epidemiologic data exist (13), the epidemiology of pediatric AKI remains inadequately described. Most pediatric studies have been from single centers and are limited by small sample size and a propensity to focus only on critical care populations or children who require dialysis (2,14–22). Studies have suggested that the epidemiology of pediatric AKI has changed dramatically over the past few decades, noting that sepsis, congenital heart disease, and oncologic illness have replaced hemolytic uremic syndrome, GN, and primary renal diseases as the most common causes of AKI in hospitalized children (14,23). However, this assertion has been based primarily on anecdotal evidence and analysis of small AKI subsets (2,20,21).
In this study, we extensively analyzed a national pediatric hospitalization database, which included large and small hospitals, primary and tertiary care centers, rural and urban settings, and general and critical care populations, to characterize the epidemiology of pediatric AKI across the United States. Additionally, we applied the high-content, high-throughput data analytic techniques usually reserved for genomic/proteomic analysis to identify factors associated with AKI in hospitalized children.
Materials and Methods
Data Sources
This study used the 2009 Kids’ Inpatient Database (KID) (24), an all-payer, inpatient care database for children in the United States that contains information included in a typical discharge abstract. Each patient record contains up to 25 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes and 15 ICD-9-CM procedure codes. The KID also contains all patient-refined, diagnosis-related group data, which allow for patients across similar diagnostic groups to be compared from a case complexity standpoint (25). The 2009 KID contains data on 3,391,934 discharges from 4121 hospitals in 44 states that occurred between January 1, 2009 and December 31, 2009. Because this study analyzed deidentified, publically available data, the Stanford Institutional Review Board considered it exempt (May 22, 2012).
Cohort Optimization
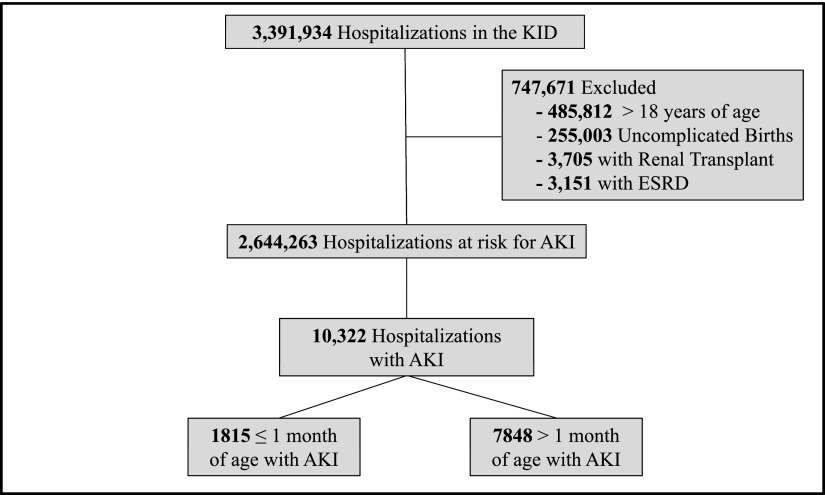
Cohort optimization is shown in Figure 1. To identify pediatric hospitalizations at risk for AKI, we excluded patients >18 years, uncomplicated births, patients with ESRD, and renal transplant recipients. Uncomplicated births were excluded using the HOSPBRTH=1 and UNCBRTH=1 variables, because by definition, they could not have been diagnosed with AKI; also, the KID undersamples this subset. Newborns experiencing complications were not excluded (HOSPBRTH=1 and UNCBRTH=0). Patients with ESRD were excluded using the ICD-9-CM codes 585.6 (ESRD), V56.31 (encounter for hemodialysis adequacy testing), V56.32 (encounter for peritoneal dialysis adequacy testing), V56.1 (fitting and adjustment of extracorporeal catheter), and V56.2 (fitting and adjustment of peritoneal dialysis catheter); these patients receive chronic dialysis and are not at risk for AKI. Renal transplant recipients were excluded using the ICD-9-CM codes V42.0 (kidney replaced by transplant) and 996.81 (complications of transplanted kidney), because these elevations in serum creatinine likely represent episodes of allograft dysfunction. Patients were excluded if either of these codes was present as a primary or secondary diagnosis.
Figure 1.
Cohort optimization. Patients >18 years old were excluded to create a cohort representative of pediatric disease. Uncomplicated births (by Kids’ Inpatient Database [KID] definition) and patients with ESRD (receiving chronic dialysis) are not at risk for AKI. Creatinine elevations in renal transplant recipients are likely to represent a diagnosis other than AKI (allograft dysfunction).
Identification of AKI Events
AKI was identified using ICD-9-CM codes 585.5–585.9: 584.5 (acute kidney failure with lesion of tubular necrosis), 584.6 (acute kidney failure with lesion of renal cortical necrosis), 584.7 (acute kidney failure with lesion of renal medullary [papillary] necrosis), 584.8 (acute kidney failure with other specified pathologic lesion in kidney), and 584.9 (acute kidney failure unspecified). Hospitalizations were considered to have been complicated by AKI if any of these codes appeared in any of 25 diagnosis categories.
AKI Subcategories
Neonatal AKI represents a unique spectrum of disease (26), and therefore, the AKI cohort was subdivided; hospitalizations among children ≤1 month of age were classified as neonatal AKI, and hospitalizations among children >1 month of age were classified as pediatric AKI. AKI hospitalizations were subclassified by need for renal replacement therapy. AKI requiring dialysis (AKI-D) was identified using ICD-9-CM codes for hemodialysis/hemofiltration (39.95) and peritoneal dialysis (54.98). Patients carrying both an AKI code and either dialysis code were considered AKI-D. AKI hospitalizations with concomitant critical care (AKI-ICU) were identified using ICD-9-CM critical care codes (27) for mechanical ventilation (96.70–96.72), arterial BP monitoring (89.61), and central venous pressure monitoring (89.62). The presence of one or more of these codes, which serve as surrogate markers of intensive care, resulted in categorization as AKI-ICU.
Data and Statistical Analyses
The KID contains deidentified data; thus, it is not possible to distinguish individual patients, and some admissions likely represent rehospitalizations. Therefore, for all results, the units of analysis are hospitalizations rather than patients. Because of the discharge sampling strategy of the KID, the data must be weighted to permit nationally representative estimates; all analyses were performed on data that had been weighted according to the standard Agency for Healthcare Research and Quality procedure (24). Weighted results were subjected to comparative testing between AKI and non-AKI cohorts as well as AKI subgroups using t tests and chi-squared/Fisher exact analyses. In large datasets, minor differences can seem to have high statistical significance. Thus, we calculated the standardized difference of the mean (SDM) for the demographic characteristics to provide an alternative method to assess intergroup differences; SDM>0.2 equates to small differences, SDM>0.5 equates to medium differences, and SDM>0.8 equates to large differences. When we compared patients with missing data values with patients with complete data, the statistical distributions between the two groups were similar and without bias; thus, listwise deletion was used to deal with missing values. Testing was two-sided testing, and data were reported with standard errors or 95% confidence intervals. Analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
The KID is multifeatured, and the AKI and non-AKI groups are highly imbalanced, making it challenging to describe them by simple linear statistics. Thus, to identify features effectively, our AKI association studies used statistical learning strategies; a predictive model was created to accurately determine the KID data elements that were highly associated with an AKI diagnosis. We used prediction analysis of microarrays (PAM), which is commonly applied to high-feature datasets such as DNA microarrays; PAM determines the data elements or features that best contribute to the predictive model or characterize individual classes/cohorts (28,29). Clinical Classification Software codes (286 diagnosis and 231 procedural codes) were used to bin ICD-9-CM codes (n=6722) and analyzed by PAM. PAM identified relevant AKI predictors and eliminated irrelevant data elements, which constitute noise. Subsequently, the data were subjected to machine learning/pattern recognition predictive modeling analyses using linear discriminant analysis (http://www.r-project.org/libraryMASS). Linear discriminant analysis maximizes the ratio of between-class variance to within-class variance, guaranteeing maximal separation between the AKI and non-AKI classes. At the outset, the data were randomly divided into a training dataset (two thirds of the records) and a testing/validation dataset (one third of the records); the training data were used to design the prediction model, and the testing/validation data were used to confirm its accuracy. The results of this analysis are presented as unadjusted odds ratios (ORs). Notably, the datasets are class-imbalanced, because one class (non-AKI) contains significantly more subjects than the other class (AKI). Thus, repeated random subsampling (n=100) was integrated with a voting mechanism to derive the final classification result. Receiver Operating Characteristic (ROC) analysis was performed (30,31) to evaluate the performance of the model. Area under the ROC curve was calculated using ROCR package (30). ORs of the PAM-selected features were computed using generalized linear modeling (http://www.r-project.org/ library) and EpiCalc (http://cran.r-project.org/doc/contrib/Epicalc_Book.pdf) methods.
Results
AKI Demographics
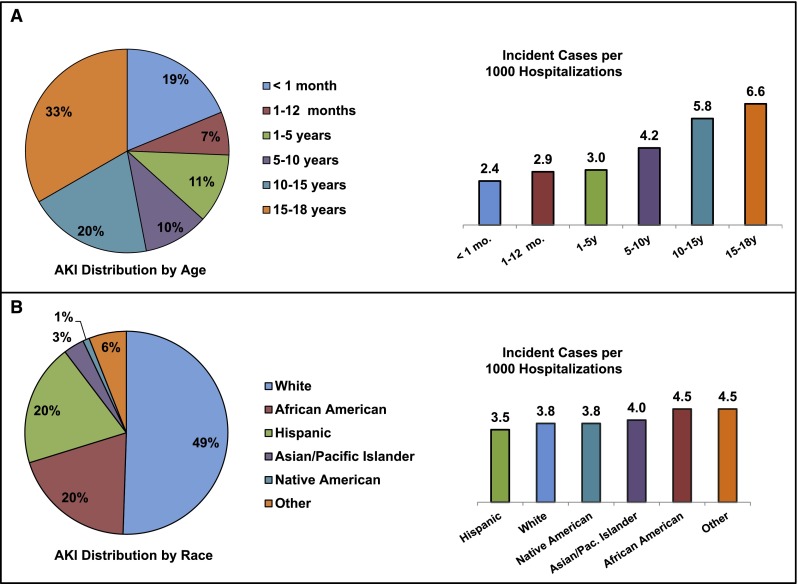
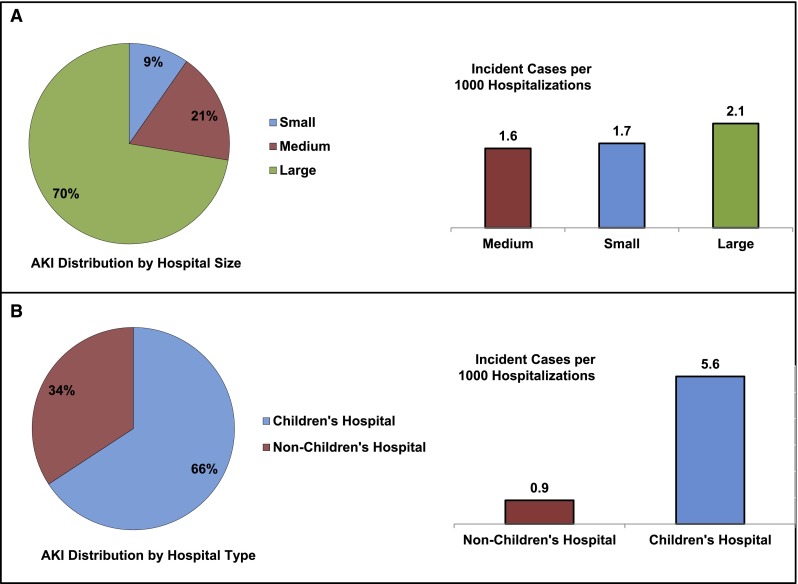
The hospitalized population at risk for AKI included 2,644,263 pediatric admissions (Figure 1); 10,322 patients were diagnosed with AKI, yielding an incidence of 0.39% (95% confidence interval [95% CI], 0.38% to 0.40%) or 3.9 cases per 1000 admissions. Demographic data are shown in Table 1. The median age of the AKI cohort was 10.8 years (interquartile range [IQR]=1.0–16.8). This age was higher than patient age for hospitalizations not complicated by AKI (median=2.0 years, IQR=0.1–12.8, P<0.001). One third of AKI events occurred in children 15–18 years of age (Figure 2A). Children ≤1 month of age represented a large proportion of the cohort (19%); however, the incidence of AKI was lowest among this age group. AKI incidence increased in parallel with age (Figure 2A) and was greatest in 15- to 18-year-old patients (6.6 events per 1000 hospitalizations). AKI was more common in boys (56.4% versus 43.6%), whereas non-AKI hospitalizations showed equal sex distribution (50.5% versus 49.5%, P<0.001). African Americans had a greater representation among AKI admissions (20.1% versus 17.4%, P<0.001) whereas Hispanic children were less represented among AKI hospitalizations (20.1% versus 22.4%, P<0.001). Nearly one half of the AKI population was white; however, AKI incidence was highest among African Americans at 4.5 events per 1000 admissions (Figure 2B). Most AKI admissions occurred at large hospitals (69.8%, 2.1 events per 1000 hospitalizations) (Figure 3A) and children’s hospitals (66.2%, 5.6 events per 1000 hospitalizations) (Figure 3B). Of these demographic characteristics, only age and hospital type had an SDM greater than 0.2 (Table 1).
Table 1.
Demographics of AKI and non-AKI hospitalizations
| AKI Cohort | Non-AKI Cohort | P Value | Standardized Difference of the Mean | |
|---|---|---|---|---|
| Age (yr) | ||||
| Median (interquartile range) | 10.8 (1.0–16.8) | 2.0 (0–12.8) | <0.001 | 0.50 |
| Sex | ||||
| Girls (%) | 43.6 | 49.5 | <0.001 | 0.12 |
| Boys (%) | 56.4 | 50.5 | 0.12 | |
| Race | <0.001 | |||
| White (%) | 49.2 | 50.6 | 0.03 | |
| African American (%) | 20.1 | 17.4 | <0.001 | 0.07 |
| Hispanic (%) | 20.1 | 22.4 | 0.06 | |
| Asian/Pacific Islander (%) | 3.2 | 3.1 | 0.01 | |
| Native American (%) | 1.0 | 1.0 | 0.00 | |
| Other (%) | 6.4 | 5.5 | 0.04 | |
| Hospital type | ||||
| Children’s hospital (%) | 66.2 | 34.6 | <0.001 | 0.67 |
| Nonchildren’s hospital (%) | 33.8 | 65.4 | 0.67 | |
| Hospital size | ||||
| Large (%) | 69.8 | 64.4 | 0.12 | |
| Medium (%) | 20.8 | 25.0 | <0.001 | 0.10 |
| Small (%) | 9.4 | 10.6 | 0.04 |
When given, the percentage represents the percentage of hospitalizations with available data. The number of patients available for analysis in the AKI and non-AKI cohorts, respectively, are as follows: age (9663 and 2,359,501), gender (10,315 and 2,618,318), race (8515 and 2,233,926), hospital type (9027 and 2,375,714), hospital size (9225 and 2,455,973).
Figure 2.
AKI patient demographics and incident rates. AKI analysis by (A) age and (B) race. Each percent figure represents the percentage of the entire AKI cohort. Additionally, AKI incidence (AKI events per 1000 hospitalizations) is shown from lowest to highest.
Figure 3.
AKI hospital demographics and incident rates. AKI analysis by (A) hospital size and (B) hospital type. Each percent figure represents the percentage of the entire AKI cohort. Additionally, AKI incidence (AKI events per 1000 hospitalizations) is shown from lowest to highest.
Case Complexity and Severity of Illness
Data regarding case complexity and disease severity are shown in Table 2. Compared with non-AKI hospitalizations, at discharge, AKI hospitalizations had more diagnoses listed (median=11, IQR=7–15 versus median=3, IQR=2–5; P<0.001), were considered to suffer from more chronic conditions (median=3, IQR=1–4 versus median=0, IQR=0–1; P<0.001), and had undergone significantly more procedures (median=3, IQR=0–8, versus median=1, IQR=0–2; P<0.001). Based on all patient-refined, diagnosis-related group data, hospitalized children with AKI experienced significantly greater morbidity and mortality risk (Table 2). Among AKI hospitalizations, 96.4% experienced at least a major loss of function, and 59.4% experienced an extreme loss of function; these results are compared with 15.1% and 2.7%, respectively, among non-AKI hospitalizations (P<0.001). Moreover, although only 1.0% of non-AKI hospitalizations qualified as having an extreme likelihood of dying, 46.4% of AKI hospitalizations were classified as having an extreme likelihood of dying (P<0.001). All of these variables, with the exception of moderate likelihood of dying and moderate loss of function, had an SDM>0.8.
Table 2.
Disease severity and case complexity of AKI and non-AKI hospitalizations
| AKI Cohort | Non-AKI Cohort | P Value | Standardized Difference of the Mean | |
|---|---|---|---|---|
| Number of diagnoses | <0.001 | |||
| Median (interquartile range) | 11 (7–15) | 3 (2–5) | 1.60 | |
| Number of chronic conditions | <0.001 | |||
| Median (interquartile range) | 3 (1–4) | 0 (0–1) | 1.15 | |
| Number of procedures | <0.001 | |||
| Median (interquartile range) | 3 (0–8) | 1 (0–2) | 0.94 | |
| Major operating room procedure performed (%) | 29.4 | 22.2 | <0.001 | 0.17 |
| APR-DRG severity score | ||||
| Minor loss of function/no complication or comorbidity (%) | 0.6 | 48.2 | 1.33 | |
| Moderate loss of function (%) | 3.0 | 36.7 | <0.001 | 0.93 |
| Major loss of function (%) | 37.0 | 12.4 | 0.60 | |
| Extreme loss of function (%) | 59.4 | 2.7 | 1.55 | |
| APR-DRG risk mortality score | ||||
| Minor likelihood of dying (%) | 1.8 | 89.1 | 3.64 | |
| Moderate likelihood of dying (%) | 17.4 | 7.5 | <0.001 | 0.30 |
| Major likelihood of dying (%) | 34.4 | 2.4 | 0.91 | |
| Extreme likelihood of dying (%) | 46.4 | 1.0 | 1.26 |
When given, the percentage represents the percentage of hospitalizations with available data. The number of patients available for analysis in the AKI and non-AKI cohorts, respectively, are as follows: number of diagnoses, chronic conditions, procedures and operating room procedures (10,322 and 2,633,941), severity score and mortality risk (10,290 and 2,629,456). APR-DRG, all patient-refined, diagnosis related group.
Outcomes
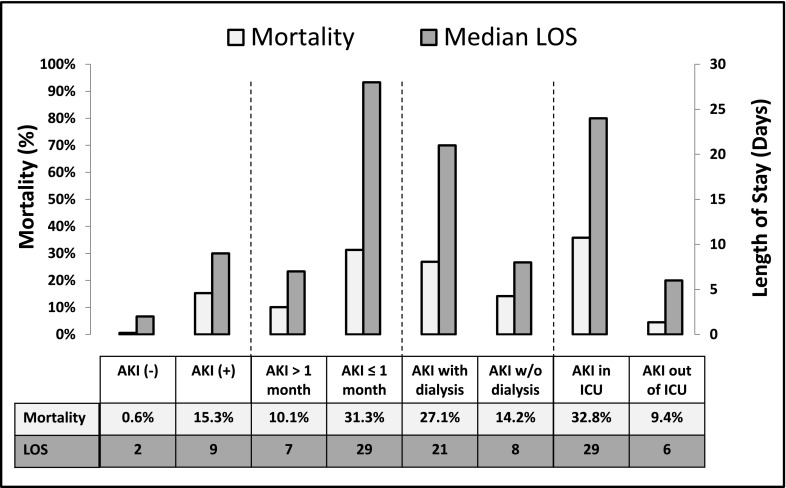
Outcomes are shown in Figure 4. Hospitalizations complicated by AKI experienced a mortality rate of 15.3% compared with a mortality rate of 0.6% among non-AKI hospitalizations (P<0.001). Mortality within the AKI cohort was significantly higher in neonates than children >1 month of age (31.3% versus 10.1%, P<0.001); 34.5% of AKI hospitalizations required critical care (AKI-ICU) during their stay (71.6% patients ≤1 month versus 23.4% patients >1 month, P<0.001), and mortality was higher in AKI-ICU hospitalizations (32.8% versus 9.4%, P<0.001). Dialysis was required in 8.8% of AKI cases (AKI-D) and used more commonly in children >1 month of age (9.8% versus 4.3%, P<0.001). AKI-D hospitalizations experienced higher mortality (27.1% versus 14.2%, P<0.001).
Figure 4.
Outcomes. Mortality rates (%) and length of stay (median days) are shown for patients with and without AKI, AKI patients >1 and ≤1 month of age, AKI patients who required and did not require dialysis, and AKI patients who received and did not receive critical care. ICU, intensive care unit; LOS, length of stay.
Length of stay (LOS) data showed a trend similar to the trend seen for mortality (Figure 4). AKI was associated with a prolonged median LOS (9 versus 2 days, P<0.001). Median LOS was longer among neonates (29 versus 7 days, P<0.001), AKI requiring dialysis (21 versus 8 days, P<0.001), and AKI-ICU (29 versus 6 days, P<0.001).
Factors Associated with the Diagnosis of AKI
AKI-associated clinical factors are shown in Table 3. In hospitalizations among children >1 month of age (n=7848), liver disease (OR, 1.24; 95% CI, 1.18 to 1.28), respiratory failure (OR, 1.21; 95% CI, 1.17 to 1.25), and pulmonary collapse/pulmonary inflammation (OR, 1.15; 95% CI, 1.11 to 1.19) were associated with AKI. Likewise, shock (OR, 2.15; 95% CI, 1.95 to 2.36), septicemia (OR, 1.37; 95% CI, 1.32 to 1.47), and coagulation disorders (OR, 1.23; 95% CI, 1.18 to 1.28) were associated with AKI. Procedural associations included intubation and mechanical ventilation (OR, 1.2; 95% CI, 1.16 to 1.25), vascular catheterization (OR, 1.18; 95% CI, 1.14 to 1.22), delivery of enteral/parental nutrition (OR, 1.14; 95% CI, 1.09 to 1.19), and blood transfusions (OR, 1.11; 95% CI, 1.08 to 1.15. The analysis also identified several factors likely to be a result of AKI rather than a potential cause; these factors included hypertension, anemia, and electrolyte abnormalities (P values<0.001). In hospitalizations among children ≤1 month of age (n=1815), a similar pattern emerged (Table 3). However, the analysis also identified circulatory diseases (OR, 1.47; 95% CI, 1.32 to 1.65), congenital cardiac disease (OR, 1.18; 95% CI, 1.13 to 1.23), and postoperative complications (OR, 1.42; 95% CI, 1.24 to 1.63) as being associated with neonatal AKI. The procedural factors in this cohort also highlight extracorporeal membrane oxygenation and intraoperative bypass (OR, 2.58; 95% CI, 2.04 to 3.26) and operating room vascular procedures (OR, 2.07; 95% CI, 1.78 to 2.41) as associated with AKI. The overall predictive model for AKI in hospitalizations among children ≤1 and >1 month of age resulted in ROC areas under the curve of 0.94 and 0.98, respectively.
Table 3.
Unadjusted AKI associations
| Patients >1 Mo of Age | Patients ≤1 Mo of Age | ||
|---|---|---|---|
| Associated Factor | Odds Ratio (95% Confidence Interval) | Associated Factor | Odds Ratio (95% Confidence Interval) |
| Diagnosis category associations | Diagnosis category associations | ||
| Shock | 2.15 (1.95 to 2.36) | Condition due to external cause | 1.61 (1.39 to 1.87) |
| Septicemia | 1.37 (1.32 to 1.43) | Severe sepsis | |
| Liver diseases | 1.24 (1.18 to 1.28) | Sepsis | |
| Coagulation/bleeding disorders | 1.23 (1.18 to 1.28) | ||
| Thrombocytopenia | Liver diseases | 1.58 (1.32 to 1.89) | |
| Disseminated intravascular coagulation | Circulatory disease | 1.47 (1.32 to 1.65) | |
| Coagulation defect not otherwise specified | Complication of surgical care | 1.42 (1.24 to 1.63) | |
| Respiratory failure | 1.21 (1.17 to 1.25) | Bleeding complicating procedure | |
| Hypertension | 1.2 (1.14 to 1.27) | Cardiac surgical complication | |
| Pulmonary collapse/pleurisy | 1.15 (1.11 to 1.19) | Postoperative infection | |
| Anemia | 1.1 (1.07 to 1.12) | ||
| Fluid/electrolyte disorders | 1.09 (1.07 to 1.1) | Fluid/electrolyte disorders | 1.33 (1.25 to 1.42) |
| Nutritional/endocrine/metabolic disorders | 1.05 (1.03 to 1.07) | Perinatal conditions not otherwise specified | 1.2 (1.16 to 1.25) |
| Disorder phosphorous metabolism | Neonatal arrhythmia | ||
| Hypocalcemia | Neonatal dehydration | ||
| Disorder of magnesium metabolism | |||
| Condition caused by external cause | 1.05 (1.02 to 1.07) | Cardiac congenital anomalies | 1.18 (1.13 to 1.23) |
| Severe sepsis | Respiratory distress syndrome | 1.06 (1.01 to 1.1) | |
| Sepsis | |||
| Hypoxemia | |||
| Procedural category associations | Procedural category associations | ||
| Intubation/mechanical ventilation | 1.2 (1.16 to 1.25) | Extracorporeal circulatory support | 2.58 (2.04 to 3.26) |
| Vascular catherization | 1.18 (1.14 to 1.22) | Extracorporeal membrane oxygenation | |
| Parenteral/enteral nutrition | 1.14 (1.09 to 1.19) | Extracorporeal membrane oxygenation for cardiac surgery | |
| Blood transfusion | 1.11 (1.08 to 1.15) | Operating room procedure on vessel | 2.07 (1.78 to 2.41) |
| Occlusion of thoracic vessel | |||
| Arterial suture | |||
| Resection of thoracic vessel | |||
| Blood transfusion | 1.42 (1.32 to 1.53) | ||
| Vascular catherization | 1.16 (1.11 to 1.21) | ||
| Intubation/mechanical ventilation | 1.14 (1.1 to 1.18) | ||
| Parenteral/enteral nutrition | 1.1 (1.05 to 1.14) | ||
Discussion
This study represents the most extensive epidemiologic description of AKI among hospitalized children in the United States. Across 2,644,263 hospitalizations encompassing all hospital sizes, types, and acuities, a wide range of disease severities, and both critical and noncritical care populations, we found a national AKI incidence of 3.9 cases per 1000 admissions. Studies using highly sensitive creatinine-based definitions, such as the Acute Kidney Injury Network (AKIN) and Pediatric Risk, Injury, Failure, Loss, and End Stage Renal Disease criteria (pRIFLE), in selective, high-risk populations have found higher incidences; rates of 17.9%–52% have been seen in the ICU or after corrective cardiac surgery (5,9,32). However, our findings are comparable with those findings of a single-center study, which used similar diagnostic criteria (4.6–9.9 per 1000 hospitalizations) (2).
The large scale of our study allowed identification of novel AKI demographics in children. The incidence of AKI was highest among African Americans; although AKI has been shown to be more common among African-American adults (1,33), this study is the first study to show this pattern in children. Additionally, AKI was associated with boys. This finding has also been seen among adults (1) but never established in pediatrics. Finally, increasing age was associated with rising AKI incidence; the greatest incidence was among 15–18 year olds. This finding is in contrast with several small studies, which found younger age to be associated with greater AKI risk (9,34,35). Although the overall incidence of AKI among infants ≤1 month of age was lower than in other age groups, they comprised approximately 20% of the entire AKI cohort, despite the fact that they represent less than 0.5% of the age span studied. Although our study suggests that AKI is not necessarily common across the massive neonatal population, neonatal AKI clearly represents a substantial proportion of the total pediatric AKI burden. Of note, many of the studies referenced above used creatinine-based AKI definitions rather than ICD-9-CM coding. Because younger children tend to have lower serum creatinine values, smaller absolute changes can result in an AKI diagnosis when using the creatinine-based definitions. Additionally, given the lower initial creatinine values, it is possible that clinicians, many of whom use less objective AKI definitions in practice, still interpret creatinines that have technically increased to normal in smaller children.
Our study also offers a broad description of AKI-associated outcomes. Children whose hospitalizations were complicated by AKI experienced a mortality rate of 15.3%. Subgroup analysis suggests that this mortality is driven by neonatal AKI (31.2%), AKI-ICU (32.8%), and AKI-D (27.1%). The substantial mortality seen among neonates with AKI is consistent with previously published data (36–38). Likewise, ICU-associated AKI has been associated with higher mortality rates (5,11). Perhaps more importantly, our study suggests that children with non-ICU AKI experience a reduced but nontrivial mortality rate of 9.4%. Notably, LOS followed an identical pattern to mortality, with longer stays seen in children ≤1 month of age, patients needing dialysis, and children with concomitant critical care.
Our study also shows the heightened case complexity and health risks associated with AKI. Hospitalizations complicated by AKI had significantly more diagnoses, chronic conditions, and procedures documented at discharge. Furthermore, patients with AKI were over 20 times more likely to experience an extreme loss of function and over 40 times more likely to experience an extreme likelihood of dying. Additionally, our ensemble learning analysis revealed a striking pattern; all of the diagnostic and procedural AKI associations identified by the predictive model emphasized the multiorgan system nature of pediatric AKI and highlighted the frequent need for critical care. The study provides strong evidence for the prevailing notion that, in the current hospital setting, children are developing AKI because of systemic disease, injury to other organ systems, and treatment required to manage these disease states rather than primary renal disease (20,23,34). Among children >1 month of age, shock and sepsis were highly associated with AKI. The same is true of liver disease, respiratory failure, and mechanical ventilation. Not surprisingly, nearly one quarter of AKI hospitalizations received concomitant intensive care. This figure reached 70% among children ≤1 month of age, establishing the ICU as the primary setting for neonatal AKI. Although sepsis, liver disease, and mechanical ventilation were also associated with neonatal AKI, the analysis highlighted the link between cardiac disease/surgery and AKI in this population (9,26).
It is important to interpret these findings in the context of their limitations. One limitation is our reliance on ICD-9-CM codes for AKI diagnosis. The use of standard, staged definitions, such as in RIFLE (39), pRIFLE (40), AKIN (41), and Kidney Disease Improving Global Outcomes (KDIGO) (42) studies, will likely improve future analyses. However, we feel that the limitations associated with ICD-9-CM codes, the use of which is necessary for analysis of the KID, are outweighed by the ability to generate such a large, comprehensive AKI cohort. Notably, although the use of ICD-9-CM codes has yet to be validated in children, this approach has been successfully used in adults (43–45). Validating these codes in children is an important subsequent step in pediatric AKI research. Also, unique patient identifiers are not present in the KID. Thus, it was not possible to identify and eliminate readmissions. Another limitation is the lack of a temporal relationship between discharge data elements. It is not possible to determine if a diagnosis of sepsis occurred before or after a diagnosis of AKI; thus, it is challenging to define AKI causality in this study, and it was not possible to control for additional risk factors in patients with AKI. However, the innovative design of the analysis did allow us to identify significant AKI associations, which we reported in lieu of AKI cause. This same issue also limited our ability to determine the location where AKI occurred. Although we feel that the inclusion of intensive care procedure codes was successful in differentiating ICU-associated AKI from general care AKI, it is possible that we did not capture all such patients.
In summary, this study represents the largest epidemiologic description of pediatric AKI to date. Use of a large discharge database and an innovative ensemble learning approach allowed us to describe the incidence, demographics, outcomes, and associations for pediatric AKI across the United States. Future studies using the same analytic strategy with the granular, temporally related data available within the electronic medical record and standard AKIN/pRIFLE/KDIGO definitions will allow more accurate assessment of AKI causality and development of AKI predictive strategies, and they may lead to improved pediatric AKI-related care.
Disclosures
None.
Acknowledgments
We would like to thank Harvey Cohen and Atul Butte for their guidance and mentorship as well as Qiaojun Wen for his assistance with algorithm interpretation.
J.J. was supported by China Scholarship Council Grant 2011632151. X.B.L. was supported by an internal Stanford University Children's Health Research Initiative (CHRI) Grant.
Footnotes
S.M.S. and J.J. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E: Childhood acute renal failure: 22-year experience in a university hospital in southern Thailand. Pediatrics 118: e786–e791, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SL: Acute kidney injury biomarkers: Renal angina and the need for a renal troponin I. BMC Med 9: 135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: A two-center retrospective cohort study. Crit Care 15: R146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider J, Khemani R, Grushkin C, Bart R: Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38: 933–939, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hayes LW, Oster RA, Tofil NM, Tolwani AJ: Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24: 394–400, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Symons JM, Chua AN, Somers MJG, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL: Demographic characteristics of pediatric continuous renal replacement therapy: A report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2: 732–738, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zappitelli M, Bernier P-L, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O: A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76: 885–892, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Sinha R, Nandi M, Tullus K, Marks SD, Taraphder A: Ten-year follow-up of children after acute renal failure from a developing country. Nephrol Dial Transplant 24: 829–833, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, Bagga A, Levin A: Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 3: 881–886, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Ball EF, Kara T: Epidemiology and outcome of acute kidney injury in New Zealand children. J Paediatr Child Health 44: 642–646, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD: Pediatric acute renal failure: Outcome by modality and disease. Pediatr Nephrol 16: 1067–1071, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Moghal NE, Brocklebank JT, Meadow SR: A review of acute renal failure in children: Incidence, etiology and outcome. Clin Nephrol 49: 91–95, 1998 [PubMed] [Google Scholar]
- 17.Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, Gauvin F: Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med 8: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Counahan R, Cameron JS, Ogg CS, Spurgeon P, Williams DG, Winder E, Chantler C: Presentation, management, complications, and outcome of acute renal failure in childhood: Five years’ experience. BMJ 1: 599–602, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallego N, Gallego A, Pascual J, Liaño F, Estepa R, Ortuño J: Prognosis of children with acute renal failure: A study of 138 cases. Nephron 64: 399–404, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Hui-Stickle S, Brewer ED, Goldstein SL: Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Williams DM, Sreedhar SS, Mickell JJ, Chan JCM: Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med 156: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Mehta P, Sinha A, Sami A, Hari P, Kalaivani M, Gulati A, Kabra M, Kabra SK, Lodha R, Bagga A: Incidence of acute kidney injury in hospitalized children. Indian Pediatr 49: 537–542, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SL: Acute kidney injury in children: Prevention, treatment and rehabilitation. Contrib Nephrol 174: 163–172, 2011 [DOI] [PubMed] [Google Scholar]
- 24.HCUP : Overview of the Kids' Inpatient Database, Kids’ Inpatient Database, 2009. Available at: http://www.hcup-us.ahrq.gov/kidoverview.jsp [Google Scholar]
- 25.3M Health Information Systems: All Patient Refined Diagnosis Related Groups (APR-DRGs), Version 20.0 Methodology Overview, Wallingford, CT, 3M Health Information Systems, 2003
- 26.Jetton JG, Askenazi DJ: Update on acute kidney injury in the neonate. Curr Opin Pediatr 24: 191–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statler KD, Dong L, Nielsen DM, Bratton SL: Pediatric stroke: Clinical characteristics, acute care utilization patterns, and mortality. Childs Nerv Syst 27: 565–573, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Tibshirani R, Hastie T, Narasimhan B, Chu G: Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A 99: 6567–6572, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T: Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med 13: 1359–1362, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Sing T, Sander O, Beerenwinkel N, Lengauer T: ROCR: Visualizing classifier performance in R. Bioinformatics 21: 3940–3941, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Zweig MH, Campbell G: Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 39: 561–577, 1993 [PubMed] [Google Scholar]
- 32.Blinder JJ, Goldstein SL, Lee V-V, Baycroft A, Fraser CD, Nelson D, Jefferies JL: Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 143: 368–374, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Waikar SS, Curhan GC, Ayanian JZ, Chertow GM: Race and mortality after acute renal failure. J Am Soc Nephrol 18: 2740–2748, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffett BS, Goldstein SL: Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6: 856–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiravuri SD, Riegger LQ, Christensen R, Butler RR, Malviya S, Tait AR, Voepel-Lewis T: Factors associated with acute kidney injury or failure in children undergoing cardiopulmonary bypass: A case-controlled study. Paediatr Anaesth 21: 880–886, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP: Renal failure in asphyxiated neonates. Indian Pediatr 42: 928–934, 2005 [PubMed] [Google Scholar]
- 37.Mathur NB, Agarwal HS, Maria A: Acute renal failure in neonatal sepsis. Indian J Pediatr 73: 499–502, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Agras PI, Tarcan A, Baskin E, Cengiz N, Gürakan B, Saatci U: Acute renal failure in the neonatal period. Ren Fail 26: 305–309, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 43.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa M-A, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 45.James M, Pannu N: Methodological considerations for observational studies of acute kidney injury using existing data sources. J Nephrol 22: 295–305, 2009 [PubMed] [Google Scholar]