Summary
Background and objectives
This study evaluated predictors for patient and renal survival in patients with ANCA-associated vasculitis (AAV) with and without renal involvement.
Design, setting, participants, & measurements
There were 273 consecutive AAV patients from January 1990 until December 2007 who were followed until death, loss to follow-up, or December 2010. Based on organ involvement, patients were divided into renal (n=212) and nonrenal groups (n=61). The primary end point was ESRD requiring renal replacement therapy (RRT) or renal transplantation or death.
Results
Patient survival was significantly better in the nonrenal group compared with the renal group (hazard ratio, 0.55; 95% confidence interval, 0.33 to 0.92; P=0.02). In the renal group, renal survival was significantly worse in MPO-ANCA–positive patients (n=65) compared with PR3-ANCA–positive patients (n=138) (hazard ratio, 2.1; 95% confidence interval, 1.11 to 3.8; P=0.01). Of 48 patients who needed RRT at diagnosis, 11 patients (23%) died within 6 months and 14 patients (29%) did not regain renal function. Of all 23 patients who regained renal function after RRT, 7 patients (30%) were temporarily dialysis independent and needed dialysis later (range, 13–63 months). Five patients had a renal relapse in the 6 months before restart of RRT. Of all 203 PR3-ANCA–positive and MPO-ANCA–positive patients with renal involvement, 12 patients (6%) developed ESRD during follow-up. These patients were classified as CKD stage 4 or 5 after initial treatment and eight patients had a renal relapse before becoming dialysis dependent.
Conclusions
AAV patients with renal involvement who needed RRT had the worst survival probability. In multivariate analysis, the only major determinants for long-term renal survival were renal function at 6 months and renal relapses.
Introduction
Systemic small vessel vasculitis and necrotizing GN with little or no immune deposits (pauci-immune) is frequently associated with the presence of ANCA against proteinase 3 (PR3-ANCA) and myeloperoxidase (MPO-ANCA) (1–3). It is estimated that PR3-ANCA or MPO-ANCA are found in >90% of patients with ANCA-associated vasculitis (AAV), with a specificity as high as 98% for PR3-ANCA and 91% for MPO-ANCA (2,4,5).
Both types of antibodies are associated with granulomatosis with polyangiitis, microscopic polyangiitis, and renal limited vasculitis (1–3). It is recognized that there are meaningful clinical differences between patients with PR3-AAV and MPO-AAV and it has been suggested that PR3-AAV and MPO-AAV are distinct diseases because PR3-ANCA–positive and MPO-ANCA–positive patients demonstrate a different disease spectrum (2,6,7). In general, patients with PR3-ANCA have more widespread extrarenal organ involvement and more active renal lesions at the time of diagnosis compared with MPO-ANCA–positive patients who have more chronic lesions (2,8,9). Another important difference is the higher relapse rate found in PR3-ANCA–positive AAV patients, which may be a major prognostic factor for renal survival during long-term follow-up (3).
In clinical practice, the differences between PR3-AAV and MPO-AAV could possibly be used for therapeutic diversity in future treatment, such as intensity of treatment or duration of maintenance therapy. Questions also remain about differences in prognosis between AAV without and with renal involvement, especially in those patients who need renal replacement therapy (RRT).
To answer these questions, we retrospectively studied our cohort of patients with systemic AAV to determine the differences between PR3-ANCA–positive and MPO-ANCA–positive patients and to evaluate other determinants for renal outcome (renal function at 6 months, relapse, hypertension, and proteinuria) during long-term follow-up.
Materials and Methods
We included all 273 consecutive patients diagnosed and treated with systemic AAV at our center between January 1990 and December 2007. Patients were followed until death, loss to follow-up, or December 2010. Median duration of follow-up for all patients was 88 months (interquartile range [IQR], 47.5–144.5 months). Thirty-two patients (12%) were lost to follow-up, mainly because of transfer of care to other hospitals. In our cohort, 155 patients (64%) were alive in December 2010.
First, we divided all 273 AAV patients into groups with (n=212) and without (n=61) renal involvement at diagnosis and compared clinical characteristics and outcome. Then, we divided all patients with renal involvement according to ANCA specificity, i.e., PR3-ANCA (n=138), MPO-ANCA (n=65), and PR3-ANCA–negative and MPO-ANCA–negative patients (n=9). In these groups, we compared clinical characteristics and patient and renal outcome according to ANCA specificity as well as the influence of relapses on renal function.
Finally, we looked for differences in all patients with renal involvement who were in need of RRT at diagnosis (n=48) and compared outcomes with the renal involvement group who did not need RRT (n=164).
Clinical Diagnosis and ANCA Analyses
Based on clinical characteristics, patients were suspected of having AAV and tested for ANCA. When a positive ANCA was found in indirect immunofluorescence, its specificity was confirmed by antigen-specific ELISA, as previously described (10).
When clinically indicated, biopsies were performed. Disease severity at diagnosis and relapse was scored using the Birmingham Vasculitis Activity Score (BVAS) (11).
Definitions
Renal involvement was based on clinical data (active urinary sediment, proteinuria, impaired renal function) or by biopsy. Need for dialysis at baseline was defined as RRT within 6 weeks after diagnosis.
Relapse was defined as recurrence or new appearance of organ involvement attributable to active vasculitis and requiring increase in or reintroduction of immunosuppression. Renal relapse was defined as a rise in or appearance of new proteinuria and hematuria, a rise in serum creatinine (mg/dl) >10% with active urinary sediment, or renal biopsy results showing disease activity. During follow-up, renal function was assessed as the estimated GFR (eGFR) (ml/min per 1.73 m2) using the Modification of Diet in Renal Disease formula.
Treatment
Induction treatment consisted of daily oral cyclophosphamide (2 mg/kg, and adjusted for age >65 years to 1.5 mg/kg) and prednisolone (1 mg/kg; maximal dosage of 60 mg/d). The patients with severe renal involvement (serum creatinine >5,66, dialysis dependency at diagnosis or progressive disease during the first weeks) received additional therapy with plasma exchange (three times per week for three weeks). Doses of cyclophosphamide were adjusted to maintain the white blood cell count above 4×10*9/L. After 4–6 weeks, the daily prednisolone dose was tapered by 10 mg every 2 weeks until the dose reached 30 mg and thereafter by 5 mg every 2–4 weeks.
During the period from 1990 to 1996, once remission was achieved, maintenance therapy consisted of oral cyclophosphamide, with a daily dose tapered by 25 mg every 3 months (n=117). From 1996 onward, patients were switched to azathioprine maintenance therapy (1.5–2 mg/kg body weight daily) after 3 months of stable remission (n=156). From 1 year after diagnosis, azathioprine was tapered by 25 mg every 3 months.
Statistical Analyses
Data were analyzed using SPSS16 and GraphPad Prism (version 5.01) software. Values are given as mean ± SD and median (range). Groups were compared using the unpaired t test or chi-squared test. For paired data, a paired t test was used. Relapse-free survival was assessed with actuarial survival curves, calculated using Kaplan–Meier estimates for survival distribution. Differences between groups in survival were analyzed with the log-rank test. Multivariate backward stepwise Cox proportional hazard analysis with time to death, time to start or restart of RRT, and relapse as the time-dependent variables was performed to determine the significance of different risk factors in patient and renal outcomes. Other covariates in our multivariable model were age, sex, ANCA specificity, dialysis at diagnosis, nonrenal relapse, hypertension and proteinuria at 6 months, and eGFR at 6 months. A two-sided P value <0.05 was considered statistically significant. Decline in renal function was estimated as the slope (ml/min per 1.73 m2) per year of the individual linear regression line of eGFR over time until relapse or end of follow-up. Frequency of relapse was expressed as number of relapses per patient-year.
Results
Patient Baseline Clinical Characteristics
Patients were divided according to presence (n=212) or absence (n=61) of renal involvement at diagnosis (Table 1). Compared with patients without renal involvement at diagnosis, patients in the renal involvement group were older (P=0.01), and had higher levels of baseline C-reactive protein (P=0.001) and BVAS (P<0.001). A higher creatinine level at baseline was also found in the renal group.
Table 1.
Clinical characteristics at baseline of 273 patients with ANCA-associated vasculitis: Differences between patients without and with renal involvement
| Characteristic | Patients without Renal Involvement | Patients with Renal Involvement | P Value |
|---|---|---|---|
| Number | 61 | 212 | |
| Age (yr) | 52±14 | 58±16 | 0.01a |
| Sex | 32 | 126 | 0.94 |
| Diagnosis | |||
| Granulomatosis with polyangiitis | 55 | 132 | <0.001a |
| Microscopic polyangiitis | 6 | 52 | 0.01a |
| Renal limited vasculitis | 0 | 28 | |
| Comorbidity | |||
| Hypertension | 1 | 4 | 0.90 |
| Diabetes mellitus | 1 | 15 | 0.11 |
| Cardiovascular disease | 6 | 20 | 0.34 |
| Autoimmune diseasesb | 1 | 9 | 0.34 |
| Malignancy | 4 | 15 | 0.89 |
| Ear, nose, and throat | 52 | 124 | <0.001a |
| Pulmonary | 26 | 40 | <0.001a |
| Birmingham Vasculitis Activity Score | 13 (7–27) | 23 (7–48) | <0.001a |
| Creatinine (mg/dl) | 0.94±0.20 | 3.63±3.42 | <0.001a |
| C-reactive protein | 7.6±8.3 | 10.8±9.0 | 0.001a |
| Proteinuria (g/24 h) | 0.09 | 1.7 | <0.001a |
| Renal replacement therapy | 0 | 49c | |
| Plasmapheresis | 0 | 48 |
Data are presented as the number, mean ± SD, or median (range).
Statistically significant.
Concomitant autoimmune diseases included Crohn’s disease (n=1) in patients without renal involvement, and Sjögren’s syndrome (n=1), sarcoidosis (n=1), psoriasis (n=1), hypothyroidism (n=4), and hyperthyroidism (n=2) in patients with renal involvement.
There were 48 PR3-ANCA–positive and MPO-ANCA–positive patients, and 1 PR3-ANCA–negative and MPO-ANCA–negative patient.
Within the renal involvement group, we divided the cohorts to differences in ANCA specificity (Table 2). Patients in the PR3 group were more often men (66%) compared with the MPO group (48%) (P=0.01). MPO-ANCA–positive patients were older (P=0.02) and had higher levels of creatinine (P<0.001) at baseline. Of patients with MPO-AAV, 31% needed dialysis at diagnosis versus 20% in the PR3 group (P=0.10). PR3-ANCA–positive patients more often had ear, nose, and throat involvement (P<0.001) and had higher C-reactive protein levels and BVAS at baseline (P<0.001).
Table 2.
Clinical characteristics at baseline of 212 AAV patients with renal involvement: differences between PR3-ANCA–positive patients, MPO-ANCA–positive patients, and PR3-ANCA–negative and MPO-ANCA–negative patients (statistical analysis for PR3 versus MPO)
| Characteristic | PR3 | MPO | P Value | ANCA Negative |
|---|---|---|---|---|
| Number | 138 | 65 | 9 | |
| Age (mean) | 56±16 | 61±15 | 0.02a | 60±15 |
| Sex | 91 | 31 | 0.01a | 4 |
| Comorbidity | ||||
| Hypertension | 3 | 0 | 0.23 | 1 |
| Diabetes mellitus | 8 | 7 | 0.21 | 0 |
| Cardiovascular disease | 10 | 8 | 0.24 | 2 |
| Autoimmune diseasesb | 3 | 5 | 0.06 | 1 |
| Malignancy | 12 | 2 | 0.14 | 1 |
| Diagnosis | ||||
| Granulomatosis with polyangiitis | 122 | 7 | 3 | |
| Microscopic polyangiitis | 13 | 38 | 1 | |
| Renal limited vasculitis | 3 | 20 | 5 | |
| Ear, nose, and throat | 106 | 15 | <0.001a | 3 |
| Pulmonary | 31 | 8 | 0.08 | 1 |
| Birmingham Vasculitis Activity Score | 26 (7–48) | 18 (10–32) | <0.001a | 18 (12–25) |
| Creatinine (mg/dl) | (3.04±2.84) | 4.82±4.22 | <0.001a | 3.54±2.75 |
| C-reactive protein | 13±9.5 | 7.2±6.5 | <0.001a | 4.3± 4.2 |
| Proteinuria (g/24 h) | 1.6 | 2.4 | 0.004a | 1.4 |
| Renal replacement therapy | 28 | 20 | 0.10 | 1 |
| Plasmapheresis | 33 | 15 | 0.90 | 0 |
Data are presented as the number, mean ± SD, or median (range). PR3, proteinase 3; MPO, myeloperoxidase; AAV, ANCA-associated vasculitis.
Statistically significant.
Concomitant autoimmune diseases included hypothyroidism (n=2) and psoriasis (n=1) in PR3-ANCA–positive patients; hypothyroidism (n=2), hyperthyroidism (n=1), sarcoidosis (n=1), and Sjögren’s syndrome (n=1) in MPO-ANCA–positive patients; and hyperthyroidism (n=1) in ANCA–negative patients.
Patient Survival
During follow-up, 87 patients (32%) died; cumulative estimated patient survival rates at 1, 5, and 10 years were 90%, 83%, and 74% respectively.
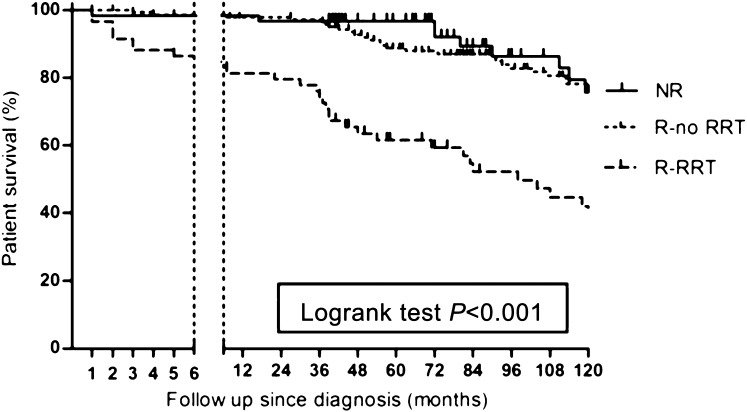
Patients without renal involvement showed higher survival rates compared with all patients with renal involvement (hazard ratio [HR], 0.55; 95% confidence interval [95% CI], 0.33 to 0.92; P=0.02). The need for RRT at diagnosis accounted for this difference (HR, 0.27; 95% CI, 0.15 to 0.47; P<0.001) (Figure 1), because mortality in patients with renal involvement who did not need RRT was not different compared with patients in the nonrenal involvement group (HR, 0.72; 95% CI, 0.38 to 1.46; P=0.32). In addition, patients who needed RRT were more prone to death due to infectious disease compared with patients who did not need RRT (P=0.04).
Figure 1.
Patient survival in ANCA-associated vasculitis without renal involvement compared with patients with renal involvement and renal replacement therapy and patients with renal involvement without renal replacement therapy. NR, nonrenal; R-RRT, renal involvement and renal replacement therapy; R-no RRT, renal involvement without renal replacement therapy.
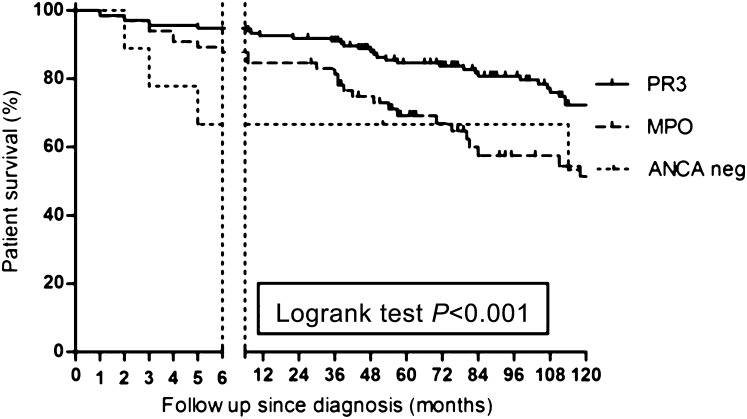
We compared patient survival according to ANCA specificity in all patients with renal involvement (Figure 2). During the first 6 months after diagnosis, a survival disadvantage was found for patients with MPO-ANCA compared with PR3-ANCA (HR, 0.38; 95% CI, 0.22 to 0.65; P=0.003). However, in multivariate analysis with inclusion of age at diagnosis, this survival difference was no longer significant (95% CI, 0.35 to 2.14; P=0.76). As shown, during follow-up after 6 months, the survival curves are parallel in course.
Figure 2.
Differences in patient survival in PR3-ANCA–positive patients, MPO-ANCA–positive patients, and ANCA-negative patients (statistical analysis for PR3 versus MPO). PR3, proteinase 3; MPO, myeloperoxidase; neg, negative.
Renal Survival
In the nonrenal group, no patient developed ESRD during follow-up, despite occurrence of 17 renal relapses in 13 patients in this group (1 MPO-ANCA–positive patient and 12 PR3-ANCA–positive patients).
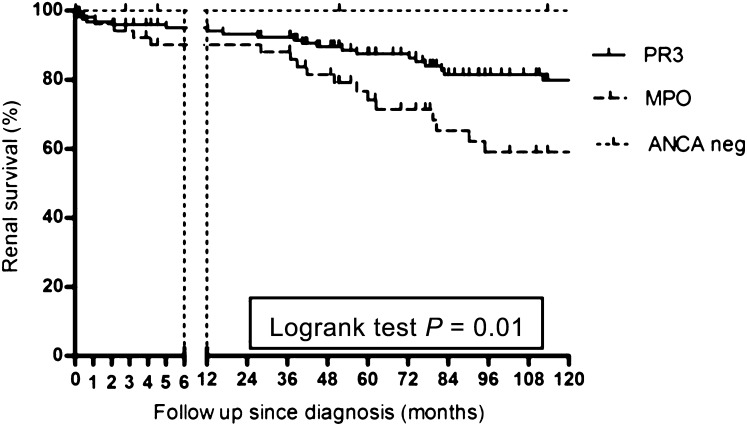
In the renal involvement group, renal survival was worse in MPO-ANCA–positive patients, compared with PR3-ANCA–positive patients and ANCA-negative patients (HR, 2.1; 95% CI, 1.11 to 3.8; P=0.01) (Figure 3).
Figure 3.
Renal survival according to ANCA specificity in ANCA-associated vasculitis patients with renal involvement. PR3, proteinase 3; MPO, myeloperoxidase; neg, negative.
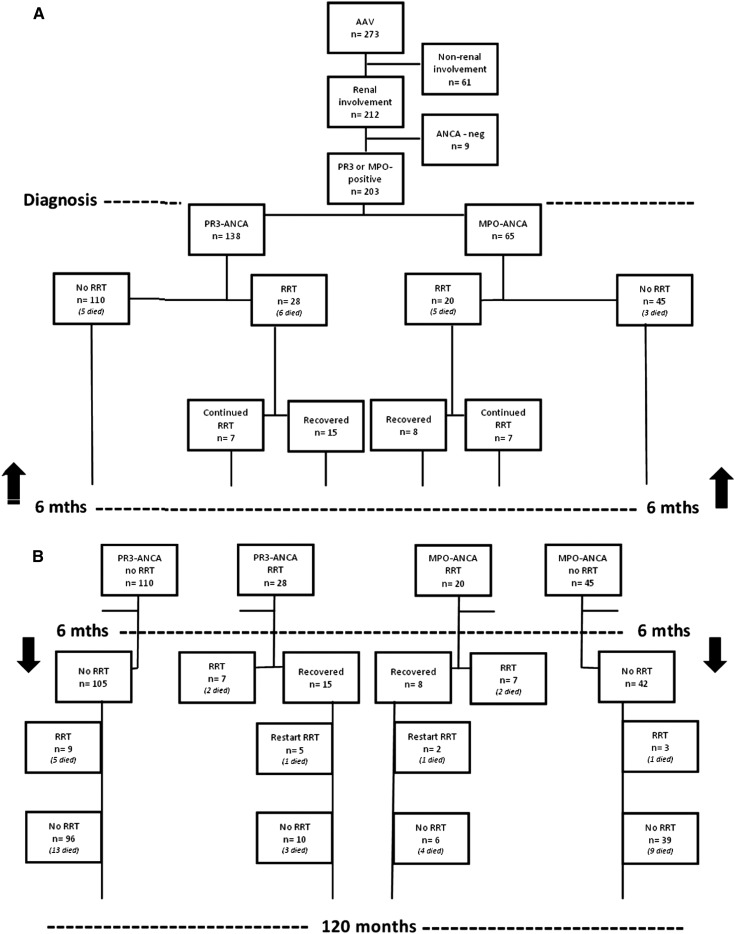
Forty-eight PR3-ANCA–positive and MPO-ANCA–positive patients needed RRT at diagnosis. Eleven patients (23%) died within 6 months (2 patients were independent of RRT at time of death), and 14 patients (29%) did not regain renal function at all. Seven patients (15%) regained renal function only temporarily and needed RRT later (range, 13–63 months after diagnosis). Sixteen patients (33%) regained and maintained renal function. Of all 164 patients with renal involvement who were dialysis independent at diagnosis, 12 patients (7%) developed ESRD during follow-up and then needed RRT. Further details are given in Figure 4.
Figure 4.
Flowchart of renal survival in PR3-AAV and MPO-AAV (A) <6 months after diagnosis (B) >6 months after diagnosis. AAV, ANCA-associated vasculitis; RRT, renal replacement therapy; PR3, proteinase 3; MPO, myeloperoxidase.
Of all PR3-ANCA–positive patients who became dialysis dependent during follow-up (n=14), the mean eGFR at 6 months was 27 ml/min per 1.73 m2. Thirteen patients experienced relapse in the 6 months before becoming dialysis dependent; only one patient, whose eGFR was 20 ml/min per 1.73 m2 at 6 months showed a slow decline in renal function during follow-up and reached ESRD 178 months after diagnosis. All five MPO-ANCA–positive patients who became dialysis dependent during follow-up were classified as CKD stage 4 or 5 after initial treatment. They did not experience relapses, but showed a more rapid decline in renal function (range in slope, −1.3 to −4.75 ml/min per year) compared with the mean slope of 0.06 ml/min per year in the other MPO-ANCA–positive patients.
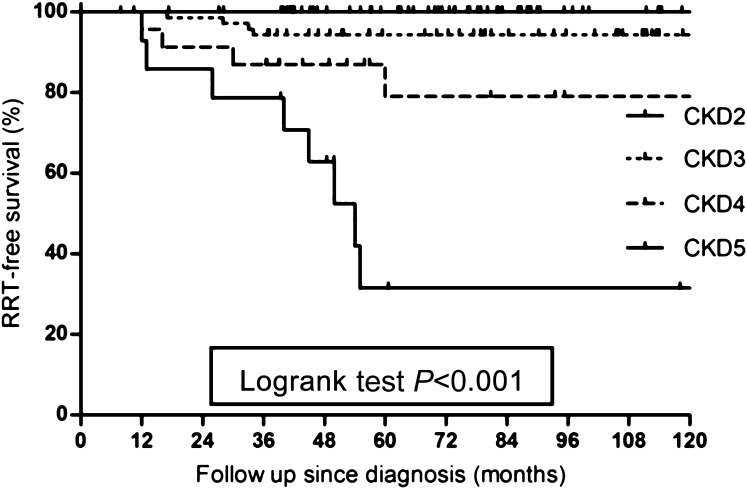
These findings indicate that renal function after initial treatment is a major prognostic factor for reaching ESRD (P<0.001), as also shown in Figure 5. In multivariate analysis, renal relapses were also significantly associated with the development of ESRD (P=0.02), whereas other covariates were not (Table 3).
Figure 5.
Renal replacement therapy-free survival according to CKD stage for all patients with renal involvement alive >6 months after diagnosis. RRT, renal replacement therapy.
Table 3.
Relative risk for renal failure for CKD stage and renal relapse in multivariate analysis
| CKD Stage | Relative Risk (95% Confidence Interval) | P Value |
|---|---|---|
| 3 | Reference | |
| 4 | 16.4 (4.5 to 59.7) | <0.001a |
| 5 | 43.1 (11.7 to 158.6) | <0.001a |
| Renal relapse | 3.27 (1.24 to 8.63) | 0.01a |
| Mean arterial pressure (per mmHg) | 0.99 (0.96 to 1.01) | 0.29 |
| Proteinuria (per g/24 h) | 1.14 (0.62 to 2.1) | 0.62 |
Variables in multivariate analysis include time to death, time to start or restart of renal replacement therapy, and relapse as time-dependent variables. Other covariates are age, sex, ANCA specificity, dialysis at diagnosis, nonrenal relapse, hypertension, and proteinuria and eGFR at 6 months.
Statistically significant.
Of all 212 AAV patients with renal involvement, 138 patients were PR3-ANCA positive and 65 patients were MPO-ANCA positive at diagnosis.
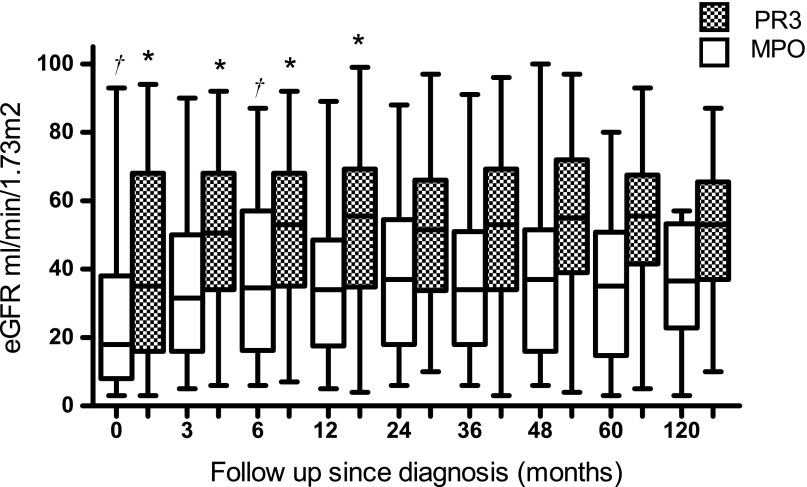
No statistical difference was found in the number of patients who needed RRT at diagnosis between MPO-ANCA–positive patients (31%) versus PR3-ANCA–positive patients (20%) (P=0.10), or in the number of patients who recovered renal function (P=0.29). However, for patients who presented with CKD stage 4 or 5 at diagnosis, recovery of renal function in the first 6 months was less for MPO-ANCA–positive patients (median 8.5; IQR, 1.75–15) compared with PR3-ANCA–positive patients (median 12.5; IQR, 5–21) (data not shown, Supplemental Figures 1–3). For patients with eGFR >30 ml/min per 1.73 m2, recovery was not different. Overall, we found a difference in renal function at baseline and during follow-up (P<0.001) between MPO-ANCA–positive patients and PR3-ANCA–positive patients with renal involvement (Figure 6). Within both groups, no differences were found comparing eGFR at 6 months and at 5 years. During follow-up, 11% of MPO-positive patients had a decline in renal function of 1 CKD stage compared with 3% of PR3-positive patients. In those patients who did not experience a relapse, the slope of decline in renal function was −0.06 ml/min per year (SD 3.13) for MPO-ANCA–positive patients compared with a slope of −0.26 ml/min per year (SD 3.18) for PR3-ANCA–positive patients. Therefore, we concluded that although MPO-ANCA–positive patients present with more advanced renal damage and show less recovery of eGFR after induction therapy, long-term outcome shows little tendency to deterioration to ESRD. Inclusion of patients without renal involvement did not substantially change this finding.
Figure 6.
Course in eGFR (ml/min per 1.73 m2) during long-term follow-up of patients with PR3- and MPO-AAV with renal involvement at diagnosis. eGFR, estimated GFR; PR3, proteinase 3; MPO, myeloperoxidase.
Relapses
During a follow-up period from 6 months after diagnosis to 120 months, 223 relapses occurred, 105 of which were renal relapses (47%). Of 249 patients alive after the first 6 months of treatment, 124 patients (50%) did not experience a relapse and 60 patients (24%) experienced at least two relapses. Overall frequency of relapses per patient-years was 0.10; this frequency was 0.051 during the first year after diagnosis, 0.17 from 1 year to 5 years, and 0.056 after 5 years.
PR3-ANCA–positive positive patients experienced more relapses than MPO-ANCA–positive positive patients (P<0.001, data not shown) (Supplemental Figures 4 and 5). We analyzed the influence of these renal relapses on renal function by comparing renal function 1 year before and 1 year after relapse. We found that renal relapse caused a significant loss of eGFR of 8±4 ml/min (P<0.001, data not shown) (Supplemental Figure 6). In addition, a difference in eGFR was found comparing those patients with renal relapses and those without at 120 months after diagnosis (P=0.01) (Supplemental Figure 7).
PR3-ANCA–Negative and MPO-ANCA–Negative Patients
Nine PR3-ANCA–negative and MPO-ANCA–negative patients were diagnosed with vasculitis with renal involvement. Four patients died during follow-up, three of them within 6 months after diagnosis. One patient needed RRT at diagnosis. His kidney function recovered during the first months of treatment but he died due to a severe infection. Of all six patients who were alive >6 months after diagnosis, no other patient developed ESRD despite five relapses (three of which were renal relapses) in three patients. During long-term follow-up, the slope of decline in kidney function was comparable with the ANCA-positive patients with renal involvement (data not shown).
Discussion
Although induction therapy for AAV has become standardized currently, questions remain regarding possible individualized treatment strategies for AAV with and without renal involvement and potential differences between PR3-ANCA–positive and MPO-ANCA–positive patients (6). In this retrospective study, we evaluated both patient and renal survival in patients with AAV and aimed to report differences between the aforementioned groups.
Overall cumulative survival rates in our cohort were similar to previous studies, with 1-year patient survival of 90% and 10-year survival of 74% (1,2,4). Patients with renal involvement at diagnosis had worse survival outcomes. In addition, severity of renal involvement at diagnosis affected both patient and renal survival. In multivariate analysis, a low eGFR at 6 months and renal relapses were associated with reaching ESRD. However, traditional risk factors like hypertension and proteinuria at diagnosis and 6 months had no significant prognostic value.
Patients in need of RRT had worse survival rates, were prone for remaining dependent of RRT, and showed a 30% risk for loss of renal function and becoming RRT dependent if renal function improved after initial treatment. Renal survival was 100% in patients without renal involvement at diagnosis.
In our cohort, renal relapse was associated with a median decline of 8 ml/min in renal function comparable with previously reported data of Slot et al. (3). This decline in renal function involves patients with PR3-AAV in particular, because these patients are especially at risk for relapse of disease (2,4,5). However, during long-term follow-up, only a small percentage of patients (3%) moved downward in CKD stage. The fact that almost all PR3-ANCA–positive patients (13 of 14) who became dialysis dependent during follow-up had a relapse before reaching ESRD highlights our finding that relapse is a significant predictor for renal function during follow-up.
MPO-ANCA–positive patients presented with worse renal function and higher levels of proteinuria, probably due to advanced chronic damage at presentation. Those patients who presented with CKD stage 4 or 5 also showed less recovery of renal function. Renal survival was thus significantly worse (HR, 2.1; 95% CI, 1.11 to 3.8; P=0.01) compared with PR3-ANCA–positive patients. The majority of MPO-positive patients had a marginal decline in renal function during follow-up (slope −0.06 ml/min; SD 3,13); however, patients who became dialysis dependent had somewhat steeper slopes (especially a mean eGFR of only 14.5 ml/min per 1.73 m2 at 6 months after diagnosis). Because MPO-ANCA–positive patients seldom experience relapses, this underscores our finding that renal survival is also predicted by a marginal or low eGFR at 6 months after diagnosis.
Our study has several limitations. First, this is a retrospective analysis of a single-center cohort, encompassing >2 decades of clinical observation. Renal biopsies were not undertaken in all patients and information on comorbidities before presentation is scarce. Although induction therapy was standardized, maintenance regimes and treatment of traditional risk factors like proteinuria and hypertension at baseline and at 6 months were not. Our population consists mainly of Caucasians and geographically it includes more PR3-positive patients than MPO-positive patients. However, to our knowledge, this is one of the largest series of long-term follow-up in AAV especially focused on differences between PR3-ANCA–positive and MPO-ANCA–positive patients.
PR3-AAV and MPO-AAV present as different entities at diagnosis because MPO-ANCA–positive patients are older and have higher serum creatinine at baseline. Both factors are of prognostic value for patient survival as well as renal survival in AAV (12). During follow-up, PR3-ANCA–positive patients show a good recovery in renal function during the first 6 months and although relapses occur frequently, they seldom reach ESRD. In contrast, MPO-ANCA–positive patients present with worse renal function and have less recovery of renal function, independent of hypertension or proteinuria. Therefore, although relapses are scarce, they still result in ESRD.
Thus, although presentation and disease course are different, renal function at diagnosis and especially the regained renal function after initial treatment at 6 months are major predictors for renal survival in both groups, next to renal relapses. In particular, traditional risk factors such as hypertension or proteinuria at 6 months seemed not to be associated with reaching ESRD and becoming dialysis dependent.
On the basis of our observations, it seems to be important to regain as much renal function as possible after diagnosis has been made. Therefore, one could argue to expand treatment options when response to standard initial treatment is limited, for instance by adding plasmapheresis.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01020113/-/DCSupplemental.
References
- 1.Falk RJF, Jennette JC: ANCA small-vessel vasculitis. J Am Soc Nephrol 8: 314–322, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Franssen CFM, Stegeman CA, Kallenberg CGM, Gans ROB, De Jong PE, Hoorntje SJ, Tervaert JW: Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int 57: 2195–2206, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Slot MC, Tervaert JW, Franssen CFM, Stegeman CA: Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int 63: 670–677, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Westman KWA, Bygren PG, Olsson H, Ranstam J, Wieslander J: Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 9: 842–852, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Booth AD, Almond MKA, Burns A, Ellis P, Gaskin G, Neild GH, Plaisance M, Pusey CD, Jayne DR, Pan-Thames Renal Research Group : Outcome of ANCA-associated renal vasculitis: A 5-year retrospective study. Am J Kidney Dis 41: 776–784, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, Cohen Tervaert JW, Deloukas P, Feighery C, Gross WL, Guillevin L, Gunnarsson I, Harper L, Hrušková Z, Little MA, Martorana D, Neumann T, Ohlsson S, Padmanabhan S, Pusey CD, Salama AD, Sanders JS, Savage CO, Segelmark M, Stegeman CA, Tesař V, Vaglio A, Wieczorek S, Wilde B, Zwerina J, Rees AJ, Clayton DG, Smith KG: Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 367: 214–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CGM, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman CA, Ytterberg SR, Specks U, RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Determinants of outcome in ANCA-associated glomerulonephritis: A prospective clinico-histopathological analysis of 96 patients. Kidney Int 62: 1732–1742, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Rihova Z, Jancova E, Merta M, Rysava R, Reiterova J, Zabka J, Tesar V: Long-term outcome of patients with antineutrophil cytoplasmic autoantibody-associated vasculitis with renal involvement. Kidney Blood Press Res 28: 144–152, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D: Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 87: 671–678, 1994 [PubMed] [Google Scholar]
- 11.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Carruthers DM, Moots RJ: Examination of disease severity in systemic vasculitis from the novel perspective of damage using the vasculitis damage index (VDI). Br J Rheumatol 37: 57–63, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Franssen CFM, Stegeman CA, Oost-Kort WW, Kallenberg CGM, Limburg PC, Tiebosch A, De Jong PE, Tervaert JW: Determinants of renal outcome in anti-myeloperoxidase-associated necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 9: 1915–1923, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.