Summary
Background and objectives
Among patients receiving maintenance dialysis, weight loss at any body mass index is associated with mortality. However, it is not known whether weight changes before dialysis initiation are associated with mortality and if so, what risks are associated with weight gain or loss.
Design, setting, participants, and measurements
Linking data from the US Renal Data System to a national registry of nursing home residents, this study identified 11,090 patients who started dialysis between January of 2000 and December of 2006. Patients were categorized according to weight measured between 3 and 6 months before dialysis initiation and the percentage change in body weight before dialysis initiation (divided into quintiles). The outcome was mortality within 1 year of starting dialysis.
Results
There were 361 patients (3.3%) who were underweight (Quételet’s [body mass] index<18.5 kg/m2) and 4046 patients (36.5%) who were obese (body mass index≥30 kg/m2) before dialysis initiation. The median percentage change in body weight before dialysis initiation was −6% (interquartile range=−13% to 1%). There were 6063 deaths (54.7%) over 1 year of follow-up. Compared with patients with minimal weight changes (−3% to 3%, quintile 4), patients with weight loss ≥15% (quintile 1) had 35% higher risk for mortality (95% confidence interval, 1.25 to 1.47), whereas those patients with weight gain≥4% (quintile 5) had a 24% higher risk for mortality (95% confidence interval, 1.14 to 1.35) adjusted for baseline body mass index and other confounders.
Conclusions
Among nursing home residents, changes in body weight in advance of dialysis initiation are associated with significantly higher 1-year mortality.
Introduction
In 2009, more than 110,000 patients started maintenance dialysis for ESRD. Despite considerable effort on the part of physicians, nurses, social workers, dietitians, and other care providers, mortality rates remain persistently high, averaging 18%–20% annually and 26% in the first year after initiation of dialysis. Moreover, among certain subgroups, such as patients over the age of 80 years, patients with multiple chronic conditions, and patients with frailty or disability, mortality rates are considerably higher (1–3). Given these grim statistics, there is an urgent need to identify modifiable risk factors to improve survival.
Among patients with ESRD, a higher body mass index (BMI) is associated with lower mortality, including when BMI is in the range of obesity (≥30 kg/m2), an observation that has been termed the obesity paradox (4–7). Higher BMI seems to be protective, even when accompanied by additional cardiovascular risk factors, including hypertension and hypercholesterolemia (8–10). Moreover, weight loss at any BMI is associated with mortality among patients receiving maintenance dialysis (11).
The significance of changes in body weight among patients approaching ESRD has not been carefully studied. As patients progress from CKD to ESRD, changes in body weight may reflect changes in fat mass, muscle mass, or extracellular fluid. Correctly interpreting changes in body weight is important for determining the appropriate timing of dialysis initiation but may be especially difficult among frail older adults; they may be more likely to lose muscle mass but also more likely to retain fluid. Linking data from national registries of nursing home residents and patients receiving dialysis, we hypothesized that weight loss before dialysis initiation would be associated with mortality and that these associations would be consistent across the range of body composition.
Materials and Methods
We used data from the US Renal Data System (USRDS) that had been linked with data from the Minimum Data Set (MDS) using name, date of birth, social security number, health insurance claim number, and beneficiary identity code. USRDS contains data on >99% of persons starting dialysis in the United States. The MDS is a national registry of residents of nursing homes in the United States. In this manner, we identified 30,747 persons who were residents of a nursing home before the start of dialysis and started dialysis between January 1, 2000 and December 31, 2006. We excluded 15,355 patients with missing body weight information at the start of dialysis and 437 patients with a prior kidney transplant. We also excluded 3615 individuals missing pre-ESRD body weight, 54 individuals with absolute changes in body weight of more than 45 kg or more than 60% of body weight, 81 individuals with body weight>150 kg, and 115 individuals without Medicare as a primary payer, resulting in a final cohort of 11,090 patients. We restricted inclusion to patients with Medicare as a primary payer so that we could capture hospitalizations before the initiation of dialysis. Excluded patients had similar demographic characteristics and 1-year mortality (55.1% versus 54.7%, P value=0.40). The study received a determination of nonhuman subjects research from the Institutional Review Boards at Stanford University and the University of California at San Francisco.
Body Weight Measurements
MDS assessments are completed by nursing staff at admission and then, every 3 months as well as at the time of acute changes in status and readmission from hospital. From these assessments, we recorded the first measurement of body weight and height between 3 and 6 months before the start of dialysis (hereafter referred to as baseline). Weight measurements in the MDS are strongly correlated (r=0.95) with measurements performed by trained research staff (12). From the MDS measurements, we calculated the baseline Quételet’s BMI and categorized nursing home residents as underweight (<18.5 kg/m2), normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), grade 1 obesity (30 to <35 kg/m2), and grades 2 and 3 obesity (≥35 kg/m2) per the National Institutes of Health consensus statement (13). We also categorized nursing home residents according to quintiles of baseline body weight as follows: <61.9, 61.9–71.4, 71.5–81.8, 81.9–95.5, and ≥95.5 kg. We calculated the percentage change in pre-ESRD body weight for each patient as the difference between the first recorded (baseline) and dialysis initiation body weights (from the Medical Evidence Form) indexed to the baseline body weight. We categorized patients according to quintiles of weight change as follows: −59% to −15%, −14% to −9%, −8% to −4%, −3% to 3%, and 4% to 58%.
Nutritional Support
We ascertained pre-ESRD nutritional patterns of care from MDS nursing assessments. From these assessments, we identified patients with low oral intake (defined as leaving 25% or more food uneaten at meals), oral problems (difficulty chewing or swallowing), tube feeding in the last 7 days, and parenteral nutrition in the last 7 days. Among patients who received enteral or parenteral nutrition, we also determined the percentage of patients who received >50% of their calories through enteral or parenteral feedings and the percentage of patients who had an average daily fluid intake by tube or intravenous administration >1500 ml/d.
Mortality
Death was ascertained from the USRDS patient files. Patients were censored at 1 year after initiation of dialysis (no patients received a transplant). The primary cause of death and withdrawal from dialysis before death was determined from the Death Notification Form. Causes of death were categorized as heart failure, arrhythmia/sudden death, atherosclerotic heart disease, infectious, or other.
Covariates
Patients were considered to have the following comorbid conditions if a yes was coded on either the USRDS Medical Evidence Report or the MDS assessments before dialysis initiation: diabetes, congestive heart failure, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, cancer, and chronic lung disease. Edema was recorded as present or absent at each assessment. We used Medicare claims to identify patients who were hospitalized between the baseline assessment of body weight and dialysis initiation.
Statistical Analyses
We assessed differences in patient characteristics at the start of dialysis and patterns of pre-ESRD nutritional support according to quintiles of pre-ESRD body weight change using chi-squared tests and one-way ANOVA as appropriate. We used logistic regression to determine the association between pre-ESRD nutritional patterns and weight loss, which was defined as loss of body weight≥15% (the highest quintile). Models were adjusted for baseline body weight, age, sex, race, comorbid conditions, edema, and hospitalization pre-ESRD.
We used Kaplan–Meier curves and Cox proportional hazards models to determine the association, expressed as a hazard ratio and 95% confidence interval, of baseline weight and pre-ESRD weight change with survival during the first 1 year after dialysis initiation. Models were adjusted for age, sex, race, comorbid conditions, edema, and hospitalization pre-ESRD. We tested for effect modification of weight change by baseline weight by conducting stratified analyses and including an interaction term of weight by weight change in the proportional hazards models. The proportional hazards assumption was checked using −log(log) plots and Schoenfeld residuals.
We conducted several sensitivity analyses to determine if the findings were robust. First, we substituted the annualized percentage change in body weight for the absolute percentage change in body weight to account for differences in the timing of the pre-ESRD body weight assessment. Second, we repeated the analyses after stratifying patients according to their baseline BMI or baseline height rather than baseline weight. Finally, we repeated the analyses after excluding individuals with documented pre-ESRD hospitalizations to assess whether intercurrent illness may explain the findings. We also repeated the analyses after excluding individuals with edema to assess whether clinically evident fluid retention influenced the findings. Analyses were conducted with SAS v9.1 (Cary, NC).
Results
Patient Characteristics
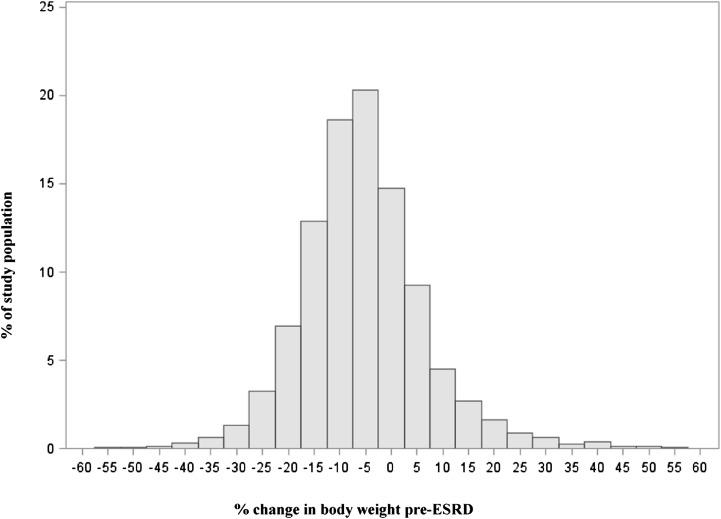
Pre-ESRD weight and height were measured a median of 4.7 months (interquartile range=3.8–5.5 months) before dialysis initiation. At baseline, the prevalence of underweight was 3.3%, whereas the prevalence of grade 1 obesity was 17.5%; the prevalence of grades 2 and 3 obesity was 19.0%. The median change in body weight from baseline to the initiation of dialysis was −6.2% (interquartile range=−12.8%–0.7%) (Figure 1). Table 1 shows patient characteristics according to quintiles of weight change. Several patient characteristics had a U-shaped distribution across weight change quintiles. For example, patients with extremes of weight loss or weight gain were younger, had a lower serum albumin concentration, and were more likely to be nonwhite and have heart failure. Patients with weight loss were more likely to have a lower pre-ESRD BMI, ischemic heart disease or cerebrovascular disease, and higher serum creatinine concentration at dialysis initiation.
Figure 1.
Histogram of pre-ESRD percentage change in body weight among 11,090 nursing home residents starting maintenance dialysis. Q, quintile.
Table 1.
Characteristics of nursing home residents at the initiation of dialysis according to pre-ESRD weight change quintiles
| Characteristic | Percentage Change in Body Weight before Dialysis Initiation | P Value | ||||
|---|---|---|---|---|---|---|
| Quintile 1 (−59 to −15) n=2205 | Quintile 2 (−14 to −9) n=2226 | Quintile 3 (−8 to −4) n=2222 | Quintile 4 (−3 to 3) n=2218 | Quintile 5 (4 to 58) n=2219 | ||
| Age (yr) | 72.4±10.9 | 73.7±10.8 | 73.6±11.3 | 72.5±11.5 | 72.3±11.9 | <0.001 |
| Women | 61.2% | 59.2% | 58.1% | 58.6% | 60.4% | 0.18 |
| Nonwhite | 38.0% | 34.8% | 32.7% | 33.1% | 38.7% | <0.001 |
| Diabetes | 59.3% | 57.9% | 59.6% | 61.1% | 59.7% | 0.30 |
| Heart failure | 50.7% | 49.8% | 48.1% | 46.6% | 50.5% | 0.03 |
| Ischemic heart disease | 32.9% | 33.2% | 31.1% | 32.2% | 29.3% | 0.04 |
| Peripheral vascular disease | 25.9% | 24.1% | 23.2% | 25.0% | 22.7% | 0.08 |
| Cerebrovascular disease | 24.9% | 27.5% | 23.8% | 26.3% | 22.9% | 0.003 |
| Chronic lung disease | 14.4% | 15.1% | 13.1% | 13.3% | 12.9% | 0.16 |
| Cancer | 5.3% | 5.6% | 6.3% | 5.6% | 4.7% | 0.24 |
| Hospitalized within 6 mo of dialysis initiation | 61.1% | 57.6% | 53.9% | 50.8% | 56.1% | <0.001 |
| Body mass index (kg/m2) | 24.9±6.6 | 26.5±6.9 | 27.6±7.4 | 28.4±7.4 | 29.7±8.2 | <0.001 |
| Weight (kg) | 67.2±16.4 | 71.9±17.2 | 75.3±19.1 | 77.4±19.3 | 80.8±21.4 | <0.001 |
| Serum creatinine (mg/dl) | 5.5±2.5 | 5.6±2.5 | 5.5±2.5 | 5.4±2.3 | 5.2±2.3 | <0.001 |
| BUN (mg/dl) | 87.3±38.1 | 87.5±36.6 | 88.2±36.1 | 84.9±32.7 | 87.9±34.8 | 0.10 |
| Serum albumin (g/dl) | 2.9±0.6 | 2.9±0.6 | 3.0±0.6 | 3.0±0.6 | 2.9±0.6 | <0.001 |
ESRD, end-stage renal disease; BUN, blood urea nitrogen.
Pre-ESRD Nutritional Patterns
Patients with lower pre-ESRD weight were more likely to have low oral intake and oral problems and more likely to receive tube feeding and parenteral nutrition. Among those patients receiving tube feeding or parenteral nutrition, patients with lower pre-ESRD weight were more likely to receive a majority of their calories from enteral or parenteral sources and more likely to have high fluid intake through tube or intravenous administration (Figure 2A). Patients who experienced weight loss pre-ESRD were less likely to have an oral problem and receive tube feeding or parenteral nutrition; among those patients receiving enteral or parenteral nutrition, they were less likely to receive a majority of their calories from these sources and less likely to have high fluid intake through tube or intravenous administration (Figure 2B). After adjustment for baseline body weight, age, sex, race, comorbid conditions, and hospitalization, low oral intake was significantly associated with weight loss≥15% of body weight (odds ratio, 1.27; 95% confidence interval, 1.13 to 1.42). There was no significant association between oral problems, tube feeding, or parenteral nutrition and weight loss after adjusting for confounders. Because of the small number of individuals receiving enteral or parenteral nutrition, we were unable to determine the adjusted association between high caloric or fluid intake from supplemental sources and weight loss.
Figure 2.
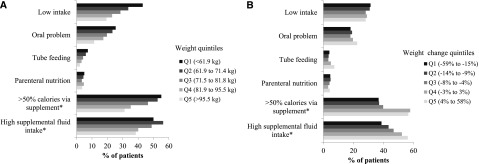
Pre-ESRD nutritional patterns among 11,090 nursing home residents starting maintenance dialysis (A) by body weight and (B) change in body weight. (A) N=8930 for not eating and N=930 for calorie and fluid intake variables. P value <0.05 across weight quintiles for all measures. (B) N=8930 for not eating and N=930 for calorie and fluid intake variables. P value<0.05 across weight change quintiles for oral problem, tube feeding, supplemental caloric intake, and supplemental fluid intake. Q, quintile. *These measures only pertain to individuals receiving enteral or parenteral nutrition.
Mortality
There were 6063 deaths (54.7%) over 1 year of follow-up after dialysis initiation. The association of pre-ESRD weight with mortality after dialysis initiation is shown in Table 2. In unadjusted analysis, mortality was lower with higher levels of pre-ESRD weight. This relationship persisted after adjustment for age, sex, race, chronic disease, edema, pre-ESRD hospitalization, and pre-ESRD change in weight. A similar relationship was present between pre-ESRD BMI and mortality (Table 2).
Table 2.
Association of pre-ESRD weight and body mass index with mortality within 1 year after dialysis initiation
| Characteristic | Crude 1-Yr Mortality Rate N (%) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) |
|---|---|---|---|
| Pre-ESRD weight (kg) | |||
| <61.9 | 1395 (63.5) | 1.15 (1.07 to 1.24) | 1.11 (1.02 to 1.19) |
| 61.9–71.4 | 1288 (57.4) | 1.00 (reference) | 1.00 (reference) |
| 71.5–81.8 | 1248 (54.2) | 0.92 (0.85 to 0.99) | 0.92 (0.85 to 0.99) |
| 81.9–95.5 | 1111 (51.1) | 0.84 (0.77 to 0.91) | 0.88 (0.81 to 0.95) |
| ≥95.5 | 1021 (47.0) | 0.74 (0.68 to 0.81) | 0.85 (0.78 to 0.93) |
| Pre-ESRD BMI (kg/m2) | |||
| <18.5 | 230 (65.2) | 1.14 (1.00 to 1.31) | 1.17 (1.02 to 1.34) |
| 18.5 to <25 | 2026 (60.2) | 1.00 (reference) | 1.00 (reference) |
| 25 to <30 | 1790 (54.5) | 0.86 (0.81 to 0.92) | 0.87 (0.81 to 0.93) |
| 30 to <35 | 1046 (53.6) | 0.84 (0.78 to 0.90) | 0.86 (0.80 to 0.93) |
| ≥35 | 971 (45.5) | 0.66 (0.61 to 0.72) | 0.77 (0.71 to 0.84) |
Pre-ESRD weight and body mass index (BMI) were measured at a median of 4.7 months before dialysis initiation. HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, race, diabetes, heart failure, ischemic heart disease, peripheral vascular disease, chronic lung disease, stroke, cancer, presence of edema, pre-ESRD hospitalization, and change in weight.
The association of pre-ESRD weight change with mortality after dialysis initiation is shown in Table 3. In unadjusted analyses, there was a U-shaped association between weight change and mortality, with both weight loss and weight gain associated with higher risk. Compared with those patients with minimal weight change, among those patients with weight gain≥4%, the risk for mortality was 24% higher. Among those patients with the weight loss≥15%, the risk for mortality was 28% higher, whereas lesser degrees of weight loss were associated with nominal differences in mortality. After adjustment for age, sex, race, comorbid conditions, edema, pre-ESRD hospitalization, and pre-ESRD weight, the magnitude of the associations was similar. In sensitivity analyses substituting the annualized change in body weight for the percentage change in body weight, a similar U-shaped relationship was observed. Exclusion of individuals with pre-ESRD hospitalization or edema also did not substantially change the results.
Table 3.
Association of pre-ESRD weight change with mortality within 1 year after dialysis initiation
| Change in Weight before Dialysis Initiation | Crude 1-Yr Mortality Rate N (%) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) |
|---|---|---|---|
| Quintile 1 (−59% to −15%) | 1286 (58.3) | 1.28 (1.18 to 1.38) | 1.35 (1.25 to 1.47) |
| Quintile 2 (−14% to −9%) | 1189 (53.4) | 1.10 (1.02 to 1.20) | 1.07 (1.00 to 1.17) |
| Quintile 3 (−8% to −4%) | 1198 (53.9) | 1.10 (1.01 to 1.19) | 1.07 (0.99 to 1.16) |
| Quintile 4 (−3 to –3%) | 1112 (50.1) | 1.00 (reference) | 1.00 (reference) |
| Quintile 5 (4% to 58%) | 1278 (57.6) | 1.24 (1.14 to 1.34) | 1.24 (1.14 to 1.35) |
Pre-ESRD weight was measured a median of 4.7 months before dialysis initiation. HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, race, diabetes, heart failure, ischemic heart disease, peripheral vascular disease, chronic lung disease, stroke, cancer, edema, pre-ESRD weight, and pre-ESRD hospitalizations.
As seen in Figure 3, the association between weight change and mortality did not differ significantly by baseline weight (P value for interaction=0.87). When we stratified by baseline BMI rather than weight, the findings were generally similar, although attenuated at extremes of BMI (Supplemental Table 1). There was a statistically significant interaction between height and weight change (P value for interaction=0.01) (Supplemental Table 2). Specifically, weight loss≥15% was a stronger predictor of mortality among tall individuals compared with shorter individuals, whereas the association between weight gain and mortality was consistent across a range of height.
Figure 3.
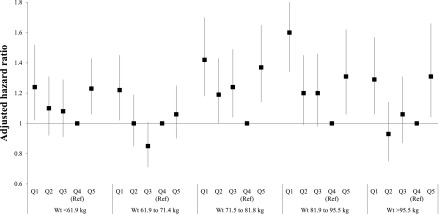
Association of quintile of pre-ESRD percentage weight change with mortality stratified by pre-ESRD weight. Model is adjusted for age, sex, race, diabetes, heart failure, ischemic heart disease, peripheral vascular disease, chronic lung disease, stroke, cancer, edema, and pre-ESRD hospitalizations. Q, quintile.
There were no significant differences in the major causes of death across weight change quintiles. However, withdrawal from dialysis occurred less frequently among those patients with large weight loss or weight gain compared with those patients with moderate or no weight loss (for quintiles 1–5: 29.8%, 36.2%, 31.8%, 32.2%, and 29.0%; P<0.01).
Discussion
In this study of nursing home residents starting dialysis, loss or gain of weight during the months before the start of dialysis was associated with increased mortality after dialysis initiation. These relationships were independent of acute and chronic comorbid conditions and consistent across baseline weight or BMI categories. Nursing home residents who were underweight, who were surprisingly uncommon in this cohort, experienced significantly higher mortality regardless of weight gain or weight loss.
Several studies have sought to elucidate an explanation for the obesity paradox. The study by Johansen et al. (4) was among the first studies to show an obesity paradox among patients beginning dialysis. The study by Johansen et al. (4) showed an 8% increase in survival for every 1 kg/m2 higher BMI. The paradoxical association persisted when estimates of fat mass were taken into consideration. Beddhu et al. (14) reported that the protective effect of a high BMI was limited to those patients with normal or high muscle mass. Subsequent studies examined the association between changes in BMI or muscle mass after starting dialysis and mortality. In these studies, changes in serum creatinine concentration (a presumed marker for changes in muscle mass) were more predictive of mortality than weight loss alone (15,16). In fact, the mortality risk associated with weight loss was offset by gaining muscle mass, whereas the benefit of weight gain was diminished by losing muscle mass.
Two prior studies have also described the obesity paradox in patients with advanced CKD (17,18). Our study extends these observations by showing that the obesity paradox is also present among adults residing in nursing homes and highlights the association of weight loss before dialysis initiation with mortality. We also show that weight gain in the months leading to dialysis may be an underappreciated risk factor. We suspect that the risk associated with weight gain might be attributable to fluid retention rather than gain of muscle mass or even body fat, although we lack the tools to distinguish among intracellular (muscle) and extracellular (edema) water and adipose tissue. Our results would suggest that, although higher weight or BMI in pre-ESRD patients confers a mortality benefit, those patients who are normal weight to overweight do best without weight change.
Several theories explaining the obesity paradox have been proposed. For example, in wasting diseases such as ESRD, the harmful effects of obesity may be outweighed in the short term by the harmful effects of malnutrition (19,20). Adipose tissue may produce beneficial adipokines and/or sequester harmful cytokines (19,20). Individuals who survive long enough to reach ESRD may be genetically selected (a hearty survivor effect) and therefore, different from the general CKD population.
Not surprisingly, reduced oral intake was common and independently associated with weight loss pre-ESRD. There are multiple potential reasons for reduced intake among patients approaching ESRD, including anorexia, altered taste sensation, mechanical impediments to chewing or swallowing, and inability to access or prepare food (21,22). The Kidney Disease Outcomes Quality Initiative nutrition guidelines for patients approaching ESRD suggest that supplemental enteral nutrition may be considered when intake is not adequate to maintain nutritional status (22). We found that supplemental enteral and/or parenteral feeding was infrequent in this cohort, perhaps reflecting the lack of evidence supporting its effectiveness for patients with ESRD, therapeutic nihilism, and/or patient or surrogate preference. Among patients receiving supplemental feeding, there was a trend to weight gain among those patients receiving a larger fraction of their caloric and fluid intake through supplemental routes. However, we were not able to determine whether these associations were independent of comorbid conditions due to the small sample. Thus, it remains unclear whether a supplemental feeding approach effectively mitigates weight loss and other signs of malnutrition.
There are several limitations of our study. We were unable to ascertain if weight changes were unintentional. The MDS does not include laboratory measurements, such as serum or urine creatinine or markers of inflammation. Our cohort included only patients who survived long enough to initiate dialysis. There may be residual confounding caused by the severity of comorbid conditions that we were not able to capture using administrative claims. Finally, because this study is an observational study, we cannot determine whether the associations between weight change and mortality are causal.
In summary, we confirm the ESRD obesity paradox among a national cohort of older nursing home residents. We also highlight a statistically significant and sizeable increase in risk among patients who lose 15% or more or gain more than 4% of their body weight in the months preceding initiation of dialysis. Additional studies are needed to determine the optimal nutritional management of overweight and obese patients with advanced CKD approaching dialysis.
Disclosures
G.M.C. serves on the Board of Directors of Satellite Healthcare and the Scientific Advisory Committee of DaVita Clinical Research. K.L.J. serves on the National Nephrology Advisory Board for Amgen and is Deputy Editor of CJASN. No other disclosures were reported.
Supplementary Material
Acknowledgments
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Contract N01-DK-7-0005.
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01410213/-/DCSupplemental.
See related editorial, “Changes in Body Weight and Subsequent Mortality: Are We Any Closer to Knowing How to Deal with Obesity in ESRD?,” on pages 1640–1642.
References
- 1.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kurella M, Covinsky KE, Collins AJ, Chertow GM: Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 146: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Kutner NG, Young B, Chertow GM: Association of body size with health status in patients beginning dialysis. Am J Clin Nutr 83: 543–549, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Friedman AN: Adiposity in dialysis: Good or bad? Semin Dial 19: 136–140, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Yen TH, Lin JL, Lin-Tan DT, Hsu CW: Association between body mass and mortality in maintenance hemodialysis patients. Ther Apher Dial 14: 400–408, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bansal N, Vittinghoff E, Plantinga L, Hsu CY: Does chronic kidney disease modify the association between body mass index and cardiovascular disease risk factors. J Nephrol 25: 317–324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowden RG, La Bounty P, Shelmadine B, Beaujean AA, Wilson RL, Hebert S: Reverse epidemiology of lipid-death associations in a cohort of end-stage renal disease patients. Nephron Clin Pract 119: c214–c219, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Chang TI: Systolic blood pressure and mortality in patients on hemodialysis. Curr Hypertens Rep 13: 362–369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD: The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clinic Proc 85: 991–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons SF, Peterson EN, You C: The accuracy of monthly weight assessments in nursing homes: Implications for the identification of weight loss. J Nutr Health Aging 13: 284–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health : Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 6[Suppl 2]: 51S–209S, 1998 [PubMed] [Google Scholar]
- 14.Beddhu S, Pappas LM, Ramkumar N, Samore M: Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14: 2366–2372, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, Greenland S: Mortality prediction by surrogates of body composition: An examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol 175: 793–803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, Krishnan M, Nissenson AR, Danovitch GM, Kalantar-Zadeh K: Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant 11: 725–736, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans M, Fryzek JP, Elinder CG, Cohen SS, McLaughlin JK, Nyrén O, Fored CM: The natural history of chronic renal failure: Results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 46: 863–870, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49: 581–591, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Anderson JE: Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial 20: 566–569, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE: Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 10: 433–442, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lim VS, Kopple JD: Protein metabolism in patients with chronic renal failure: Role of uremia and dialysis. Kidney Int 58: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI. National Kidney Foundation : Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 35[6 Suppl 2]: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.