Summary
Background and objectives
The role of peritoneal dialysis in the management of AKI is not well defined, although it remains frequently used, especially in low-resource settings. A systematic review was performed to describe outcomes in AKI treated with peritoneal dialysis and compare peritoneal dialysis with extracorporeal blood purification, such as continuous or intermittent hemodialysis.
Design, setting, participants, & measurements
MEDLINE, CINAHL, and Central Register of Controlled Trials were searched in July of 2012. Eligible studies selected were observational cohort or randomized adult population studies on peritoneal dialysis in the setting of AKI. The primary outcome of interest was all-cause mortality. Summary estimates of odds ratio were obtained using a random effects model.
Results
Of 982 citations, 24 studies (n=1556 patients) were identified. The overall methodological quality was low. Thirteen studies described patients (n=597) treated with peritoneal dialysis only; pooled mortality was 39.3%. In 11 studies (7 cohort studies and 4 randomized trials), patients received peritoneal dialysis (n=392, pooled mortality=58.0%) or extracorporeal blood purification (n=567, pooled mortality=56.1%). In the cohort studies, there was no difference in mortality between peritoneal dialysis and extracorporeal blood purification (odds ratio, 0.96; 95% confidence interval, 0.53 to 1.71). In four randomized trials, there was also no difference in mortality (odds ratio, 1.50; 95% confidence interval, 0.46 to 4.86); however, heterogeneity was significant (I2=73%, P=0.03).
Conclusions
There is currently no evidence to suggest significant differences in mortality between peritoneal dialysis and extracorporeal blood purification in AKI. There is a need for good-quality evidence in this important area.
Introduction
AKI is a common complication in critical illnesses (1). Extracorporeal blood purification (EBP) techniques such as continuous renal replacement therapy (CRRT) and intermittent hemodialysis (HD) are the most commonly used in the therapy of AKI in developed countries (2). There has, however, been renewed interest in the use of peritoneal dialysis (PD) in AKI (3,4). Indeed, there has been a recent flurry of publications reporting good results (5–8).
The use of PD for AKI remains widespread, especially in low-resource settings, because of its lower cost. Furthermore, in isolated areas or major disasters, PD is technically simple with fewer infrastructural requirements, such as electricity and municipal water supply (9). In young children for whom vascular access is problematic, PD is often used. The use of PD has also been shown to be associated with a shorter duration of dialysis dependence (10). There are, nevertheless, concerns that PD may not be adequate for acutely ill patients, particularly those patients who are hypercatabolic (11). It is, therefore, important to further evaluate the use of PD in AKI.
The aims of this systematic review were to describe the outcomes of AKI patients treated with PD and compare outcomes between PD and EBP.
Materials and Methods
Search Strategy
This study was conducted and reported according to preferred reporting items for systematic reviews and meta-analyses guidelines (12).
We searched the databases of MEDLINE, CINAHL, and Cochrane Central Register of Controlled Trials from 1966 to July of 2012 by a combination of Medical Subject Headings and text words using Boolean search strategies to identify studies assessing PD in the setting of AKI. Full search terms are listed in Supplemental Material.
Study Selection
All abstracts were reviewed independently by two authors (C.Y.C. and S.S.S.) to identify potentially relevant articles for full text review. Reference lists were also searched for relevant citations. Disagreements were resolved by a third reviewer (D.N.C.) or discussion and consensus.
Full text review was independently performed by two reviewers (as above) for the following specific eligibility criteria: (1) observational cohort and/or randomized/quasirandomized clinical trial (RCT) design; (2) adult population; (3) diagnosis of AKI; (4) at least two patients treated with PD (i.e., case reports were excluded); and (5) description of mortality and/or clinically relevant secondary outcomes (described below).
Data Abstraction and Synthesis
All data were extracted independently with standardized forms on study characteristics and quality, baseline characteristics (clinical/biochemical parameters at initiation of renal replacement therapy [RRT]), and details of PD and EBP (including dose). If data were not reported, attempts were made to contact the authors for additional details. Quality of RCTs was assessed using the Jadad score (13) ranging from zero (lowest quality) to five (highest quality). The primary outcome of interest was mortality. Secondary outcomes included length of stay, kidney recovery and/or dialysis dependence, and complications related to PD (e.g., peritonitis, hyperglycemia, and hypoalbuminemia) and EBP (e.g., intradialytic hypotension, line sepsis, and bleeding).
Statistical Analyses
Analyses were performed with Review Manager, version 5.0 (RevMan; The Nordic Cochrane Centre, The Cochrane Collaboration 2008, Copenhagen, Denmark). Data from eligible studies were combined by using a random effects model expressed as odds ratio with 95% confidence interval for outcomes in patients treated with PD compared with EBP. Level of statistical significance is set at P<0.05. Analysis was also done with the fixed effects model, which was also evaluated for robustness and susceptibility to outliers.
Statistical heterogeneity was quantified for pooled results using the Q (heterogeneity chi-squared) and the I2 statistic. Sensitivity analyses of the RCTs were planned a priori to evaluate potential sources of heterogeneity, including age, clinical setting (intensive care unit [ICU] or not), proportion of sepsis, and PD dose (standardized Kt/Vurea<2.1 or ≥2.1 per week) (11).
Results
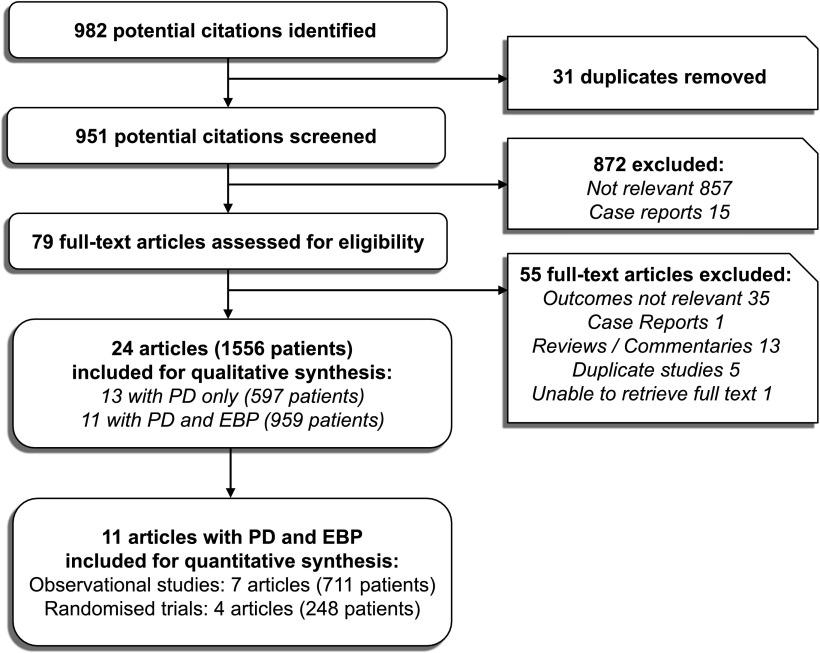
The initial search yielded 982 articles, and 903 articles were excluded after initial review (Figure 1). On full text review of 79 articles, 55 articles were excluded. One report was excluded from analysis (14), because it was a duplicate report of a prior study (10). In four studies, the same retrospective group for which PD was used was also used for comparison with different prospective EBP groups; of these four studies, only one study with the largest EBP group was included (15). We were not able to retrieve the full text of one article (16), despite attempts to contact the authors. A total of 24 articles with 1556 patients was included in this review.
Figure 1.
Flow chart of the article selection process based on preferred reporting items for systematic reviews and meta-analyses (PRISMA) (12). EBP, extracorporeal blood purification; PD, peritoneal dialysis.
Study Characteristics and Quality
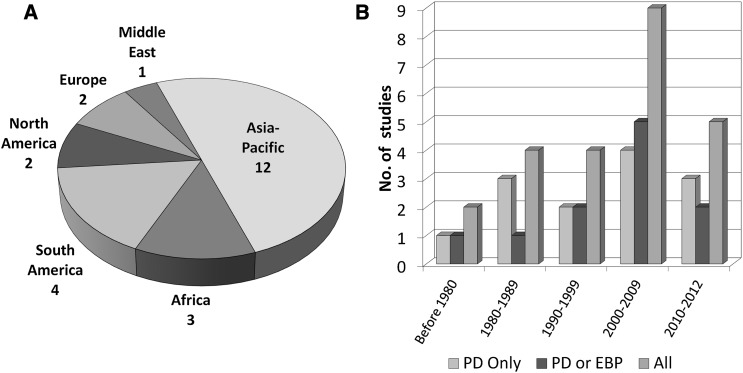
The characteristics of the relevant studies are summarized in Tables 1 and 2. The number of patients, results, and percentages represent only patients who received RRT. Thirteen studies (n=597 patients) were descriptive in nature, in which PD was the only mode of RRT (Table 1) (6,17–28). Three studies compared different subtypes of PD (18,25,26). Because this systematic review was not designed to compare the subtypes of PD, patients were regarded as having received PD treatment regardless of technique used. In 11 studies, there was a comparator group treated with EBP (Table 2) (10,15,29–37): 7 studies were cohort studies, whereas 4 studies were prospective RCTs. One observational study (36) described two distinct cohorts of patients in 1994 and 2004, and the data from each cohort were analyzed separately. In one RCT (34), only 8 of 40 patients had AKI; only these patients were included in the analysis. The number of studies by geographical region of origin of the studies as well as year of publication is shown in Figure 2. One half of the studies come from Asia–Pacific, four studies are from Brazil, and three studies are from Africa. Over one half of the studies were published in the year 2000 or later; six studies were published before 1990.
Table 1.
Characteristics of studies that included AKI patients treated with peritoneal dialysis only
| Reference | Study Type | Country | Study Period | Mean Age (yr) | ICU Patients (%) | Sepsis (%) | Mean APACHE II Score | Predominant Causes of AKI (Stated in the Studies) | N | Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ponce (17) | Pros. | Brazil | January 2004 to January 2011 | 63.8 | NA | 54.7 | NA | Sepsis (41.1%), acute tubular necrosis (34.1%) | 150 | 57.3 |
| Kilonzo (6) | Retr. | Tanzania | July 2009 to June 2011 | 34.9 | NA | 7.1 | NA | Acute tubular necrosis (40.0%), GN (20.0%) | 14 | 21.4 |
| Ponce (18) | Pros. | Brazil | January 2005 to January 2007 | 63.5 | NA | 49.2 | 25.6 | Sepsis (49.5%), heart failure (23.5%), postsurgery (12.5%) | 61 | 54.1 |
| Hayat (28) | Retr. | India | April 2004 to November 2005 | 35.0 | NA | 5.0 | NA | Gastroenteritis (75.0%) | 43 | 10.0 |
| Gabriel (27) | Pros. | Brazil | January 2004 to December 2004 | 59.0 | 76.0 | NA | 32.2 | Ischemic (67.0%), mixed (33.0%) | 30 | 57.0 |
| Chitalia (26) | Pros. | India | NA | 34.7 | NA | 0.0 | NA | Prerenal (30.0%), leptospirosis (17.2%), G6PD crisis (13.8%) | 87 | 1.1 |
| Thongboonkerd (25) | Pros. | Thailand | NA | 43.9 | 100.0 | NA | 25.2 | Shock (50%), nonshock (50%) | 20 | 15.0 |
| Trang (24) | Retr. | Vietnam | December 1987 to November 1988 | 29.1 | NA | 0.0 | NA | Malaria (100%) | 104 | 26.0 |
| Howdishell (23) | Retr. | United States | January 1989 to May 1990 | 45.2 | 100.0 | NA | NA | Trauma-related (100%) | 5 | 40.0 |
| Sonnenblick (22) | Retr. | Israel | 1975–1986 | 75.6 | 100.0 | 38.6 | NA | Sepsis (38.6%), prerenal (36.4%), other (25%) | 44 | 70.5 |
| Indraprasit (21) | Retr. | Thailand | NA | 30.7 | 100.0 | 0.0 | NA | Malaria (100%) | 10 | 10.0 |
| Ojogwu (20) | Pros. | Nigeria | NA | NA | NA | NA | NA | Hypertensive crisis (100%) | 20 | 100.0 |
| Cameron (19) | Retr. | United Kingdom | January 1965 to February 1967 | 57.3 | NA | NA | NA | Postcardiac surgery (33.3%), postaortic surgery (33.3%) | 9 | 66.7 |
ICU, intensive care unit; APACHE II, Acute Physiology, Age, Chronic Health Evaluation II; Pros., prospective; NA, results not available; Retr., retrospective; G6PD, glucose-6-phosphate dehydrogenase deficiency.
Table 2.
Characteristics of studies that included AKI patients treated with either peritoneal dialysis or extracorporeal blood purification
| Reference | Study Type(Country) | Study Period | Mean Age (yr) | ICU Patients (%) | Sepsis (%) | EBP Used | Mean APACHE II Score | Predominant Causes of AKI (Stated in the Studies) | PD | EBP | Total on RRT | Overall Mortality (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mortality | N | Mortality | |||||||||||
| Watcharotone (29) | Retr.(Thailand) | January 2005 to December 2009 | 61.6 | 69.7 | 84.8 | Int. HD | 23.1 | Not stated | 62 | 47 (75.8%) | 83 | 52 (62.7%) | 145 | 68.3 |
| George (37) | RCT(India) | June 2005 to June 2008 | 46.9 | 100.0 | 38.0 | CVVHDF | 18.1 | Sepsis (38.0%), prerenal (34.0%), leptospirosis (10.0%), snake bite (6.0%) | 25 | 18 (72.0%) | 25 | 21 (84.0%) | 50 | 78.0 |
| Gabriel (10) | RCT(Brazil) | January 2004 to December 2006 | 63.4 | 77.4 | 44.5 | Daily HD | 25.1 | Sepsis (44.5%), prerenal (39.2%), postsurgery (22.5%) | 60 | 35 (58.0%) | 60 | 32 (53.0%) | 120 | 55.5 |
| Chow A (36) | Pros.(Malaysia) | March 1994 to June 1994 | 57.7 | 29.5 | 41.0 | Int. HD, CVVHDF | NA | Prerenal (43.6%), sepsis (41.0%), toxins (10.3%) | 9 | 6 (66.7%) | 3 | 2 (66.7%) | 12 | 66.7 |
| Chow B (36) | Pros.(Malaysia) | November 2004 to February 2005 | 55.6 | 13.3 | 37.9 | Int. HD, CVVHDF | NA | Prerenal (53.5%), sepsis (37.9%), toxins (6.2%) | 26 | 12 (46.2%) | 4 | 3 (75.0%) | 30 | 46.7 |
| Mahajan (35) | Retr.(India) | April 2000 to March 2004 | 66.4 | NA | 21.6 | Int. HD | NA | Prerenal (33.0%), sepsis (21.6%), toxins (16.1%) | 95 | 46 (48.4%) | 37 | 25 (67.6%) | 132 | 53.8 |
| Arogundade (34) | RCT(Nigeria) | December 1998 to February 2001 | 44.0 | NA | 17.5 | Int. HD | NA | Sepsis (87.5%), obstruction (12.5%) | 4 | 0 (0.0%) | 4 | 0 (0.0%) | 8 | 0.0 |
| Phu (33) | RCT(Vietnam) | 1993–1998 | 35.5 | 100.0 | 31.4 | CVVHDF | NA | Malaria (68.6%), sepsis (31.4%) | 36 | 17 (47.0%) | 34 | 5 (15.0%) | 70 | 31.5 |
| Bellomo (15) | Retr.(Australia) | 1983–1993 | 58.3 | 100.0 | 66.6 | HDF, Int. HD | 27.3 | Sepsis (66.0%) | 16 | 12 (75.0%) | 218 | 139 (63.8%) | 234 | 64.5 |
| Kumar (32) | Retr.(India) | July 1987 to March 1988 | 46.3 | NA | NA | Int. HD | NA | Diarrheal illness (100%) | 42 | 25 (59.5%) | 3 | 2 (66.7%) | 45 | 60.0 |
| Hadidy (31) | Retr.(Serbia) | 1980–1986 | 38.0 | NA | NA | Int. HD | NA | Obstruction, surgery, trauma (64.0% of men); pregnancy, GN (56.0% of women) | 4 | 0 (0.0%) | 77 | 26 (33.8%) | 81 | 30.9 |
| Werb (30) | Retr.(Canada) | July 1974 to June 1976 | NA | 100.0 | 28.0 | Int. HD | NA | Sepsis (28.0%), prerenal (17.0%) | 13 | 9 (69.2%) | 19 | 12 (65.0%) | 32 | 65.5 |
ICU, intensive care unit; EBP, extracorporeal blood purification; APACHE II, Acute Physiology, Age, Chronic Health Evaluation II; PD, peritoneal dialysis; RRT, renal replacement therapy; Retr., retrospective; Int., intermittent; HD, hemodialysis; RCT, randomized control trial; CVVHDF, continuous venovenous hemodiafiltration; Pros., prospective; NA, results not available; HDF, hemodiafiltration.
Figure 2.
Distribution of studies included for review. The number of studies is illustrated according to (A) geographical region of origin and (B) year of study publication. Australia is included under Asia–Pacific. EBP, extracorporeal blood purification; PD, peritoneal dialysis.
The mean age of patients ranged from 29.1 to 75.6 years. The causes of AKI were variable, with the most common causes being reported as sepsis (10,15,17,18,22,25,27–30,33–37), prerenal causes or hypovolemia (6,17,22,26,28,30,32,35–37), cardiac failure (10,18,22,30,35,36), postsurgery (10,18,19,26,31,35), and nephrotoxic agents (22,26,35,36). Mortality was reported at hospital discharge (6,15,23,35), at 30 days (10,17,18,27), or not specified. Mortality at the last reported follow-up was used in the analysis. In many studies, data on other parameters of interest (dialysis dose, length of stay, and duration of dialysis dependence) and complications (hyperglycemia, hypoalbuminemia, peritonitis, and hypotension) were unavailable. Four studies (19,20,31,32) did not report parameters that indicate the risk profile of the cohort, such as the proportion of septic and ICU patients. Only seven studies reported an illness severity score (10,15,18,25,27,29,37).
The four RCTs scored 1 (India) (37), 1 (Nigeria) (34), 2 (Vietnam) (33), and 3 (Brazil) (10) points on the Jadad scale. In the Indian study comparing CRRT and PD (37), 5 of 55 randomized patients were excluded because of death within 6 hours of enrolment, of whom 1 patient was from the PD group. In the Brazilian RCT comparing high-volume PD with daily HD (10), 34 of 154 randomized patients were withdrawn and not included in the final analysis; of these patients, 9 of 79 patients in the PD group were withdrawn because of mechanical complications with the peritoneal catheter within the first 24 hours of dialysis. The other two RCTs did not report any dropout from study.
PD Techniques
The PD techniques used are summarized in Table 3. Details of the PD technique were often not reported. Where data were available, the studies used either rigid catheters (19,26,28,33,35,37) or flexible Tenckhoff catheters (10,15,17,18,21,23,27,34). The automated cycler was used in four studies (10,17,18,23,26,27), and closed drainage systems were commonly used (10,15,17,18,22,23,26,27,37). As buffer, lactate (n=10 studies) (10,15,17–19,22,23,25–27), acetate (n=3) (24,33,37), and bicarbonate (n=1) were used (25). PD dose is discussed separately below.
Table 3.
Characteristics of peritoneal dialysis techniques and peritonitis rates
| Reference | Access Type | Cycler Based/Manual | Open/Closed Drainage | Buffer | Peritonitis Incidence (%) |
|---|---|---|---|---|---|
| Observational studies (PD only) | |||||
| Ponce (17) | Tenckhoff | Cycler | Closed | Lactate | 12.0 |
| Kilonzo (6) | NA | Manual | NA | NA | 11.1 |
| Ponce (18) | Tenckhoff | Cycler | Closed | Lactate | 13.1 |
| Hayat (28) | Rigid | NA | NA | NA | NA |
| Gabriel (27) | Tenckhoff | Cycler | Closed | Lactate | 16.7 |
| Chitalia (26) | Rigid | Cycler/manuala | Closed | Lactate | 3.4 |
| Thongboonkerd (25) | NA | NA | NA | Lactate/bicarbonateb | 0.0 |
| Trang (24) | NA | NA | Open | Acetate | 8.7 |
| Howdieshell (23) | Tenckhoff | Cycler | Closed | Lactate | 40.0 |
| Sonnenblick (22) | NA | NA | Closed | Lactate | 27.0 |
| Indraprasit (21) | Tenckhoff | NA | NA | NA | 20.0 |
| Ojogwu (20) | NA | NA | NA | NA | NA |
| Cameron (19) | Rigid | Manual | NA | Lactate | NA |
| Studies with PD and EBP | |||||
| Watcharotone (29) | NA | NA | NA | NA | 3.2 |
| George (37) | Rigid | Manual | Closed | Acetate | 4.0 |
| Gabriel (10) | Tenckhoff | Cycler | Closed | Lactate | 18.0 |
| Chow (36) | NA | NA | NA | NA | NA |
| Mahajan (35) | Rigid | NA | NA | NA | NA |
| Arogundade (34) | Tenckhoff | Manual | NA | NA | 65.0 |
| Phu (33) | Rigid | Manual | Open | Acetate | 2.8 (41.6 cloudy dialysate) |
| Bellomo (15) | Tenckhoff | Manual | Closed | Lactate | 25.0 |
| Kumar (32) | NA | NA | NA | NA | NA |
| Hadidy (31) | NA | NA | NA | NA | NA |
| Werb (30) | NA | NA | NA | NA | NA |
The peritoneal dialysis techniques are tabulated according to the type of catheters, use of automated cyclers or manual exchanges, drainage system, and dialysate buffer. The peritonitis incidence listed is based on the rates reported in the studies. PD, peritoneal dialysis; NA, not available; EBP, extracorporeal blood purification.
The study compared cycler-based tidal PD with conventional PD using manual exchanges.
The study assessed differences in outcomes between bicarbonate and lactate-based PD fluids in AKI.
EBP Techniques
EBP modalities included intermittent HD (n=7 studies) (29–32,34–36), daily HD (n=1 study) (10), and CRRT (n=4 studies) (15,33,36,37) (Table 4). Details of the vascular access, prescription, and dose were often not reported. Three studies reported the use of high-efficiency dialyzers (10,33,36), whereas flux was variable. Parallel plate filters were used in earlier studies (15,30).
Table 4.
Characteristics and dose of extracorporeal blood purification techniques
| Reference | EBP Mode | Machine | Buffer | Intensity | Dose | Efficiency | Flux | Dialyzer/Membrane |
|---|---|---|---|---|---|---|---|---|
| Watcharotone (29) | IHD | NA | NA | NA | NA | NA | NA | NA |
| George (37) | CVVHDF | 2008B; Fresenius, Germany | Acetate | Continuous | Kurea=21.72±10.41; KCr=22.13±9.61 | NA | NA | Polysulfone |
| Gabriel (10) | Daily HD | 4008F; Fresenius, Germany | Bicarb | 6 times/wk | Kt/V=1.2/session | H | L | Polysulfone (HF 6 or 8) |
| Chow (36) | HD and/or CVVHDF | NA | NA | NA | NA | Ha | HD: L; CVVHDF: Ha | NA |
| Mahajan (35) | IHD | NA | Bicarb | NA | NA | NA | NA | NA |
| Arogundade (34) | IHD | COBE Centry 2× | Acetate | Intermittent | KCr=13.7±39.4 | NA | NA | Cuprophane |
| Phu (33) | CVVHF | BS1 Balancing System; Gambro, Sweden | Lactate | Continuous | 25 L/d | H | H | FH-66 |
| Bellomo (15) | HDF | CVVHDF: AK 10; Gambro, Sweden | NA | NA | NA | NA | NA | HD: Cuprophane; CAHDF: AN69S parallel plate filters |
| Kumar (32) | IHD | NA | NA | NA | NA | NA | NA | NA |
| Hadidy (31) | IHD | NA | NA | NA | NA | NA | NA | NA |
| Werb (30) | IHD | Recirculating Single Pass Dialysis Machine; Travenol Laboratories Inc. | NA | NA | NA | NA | NA | Parallel plate filters |
The reported dose of dialysis and the characteristics of the EBP modes and techniques are summarized in this table. EBP, extracorporeal blood purification; IHD, intermittent hemodialysis; NA, not available; CVVHDF, continuous venovenous hemodiafiltration; HD, hemodialysis; H, high; L, low; CVVHF, continuous venovenous hemofiltration; HDF, hemodiafiltration.
Information obtained from correspondence with authors.
Study Outcomes
Mortality.
Studies That Used PD Only.
Thirteen studies (total n=597, median sample size=30) were included (Table 1), with five studies conducted predominantly in the ICU setting (21–23,25,27). The pooled mortality was 39.3%, whereas reported mortality in the individual studies ranged from 1.1% (26) to 100% (20). Although 5 of 13 studies did not report the number of septic patients, studies that included only 0%–10% septic patients (21,24,26,28) had lower mortality rates ranging from 1.1% (26) to 26% (24). However, 100% mortality was observed for 20 consecutive patients with hypertensive emergency, oliguria, and uremia (20), likely reflecting the severity of the underlying condition.
Studies That Used PD or EBP.
Eleven studies (total n=959, median sample size=60) were included, of which four studies were conducted only in the ICU (15,30,33,37) and four studies were RCTs (10,33,34,37) (Table 2). In total, 392 patients underwent PD, whereas 567 patients underwent EBP. For PD patients, reported mortality rates ranged from 25.0% to 75.8%, except for two studies with 0% mortality on PD (31,34). In comparison, mortality for EBP patients ranged from 15.0% to 84.0% in individual studies.
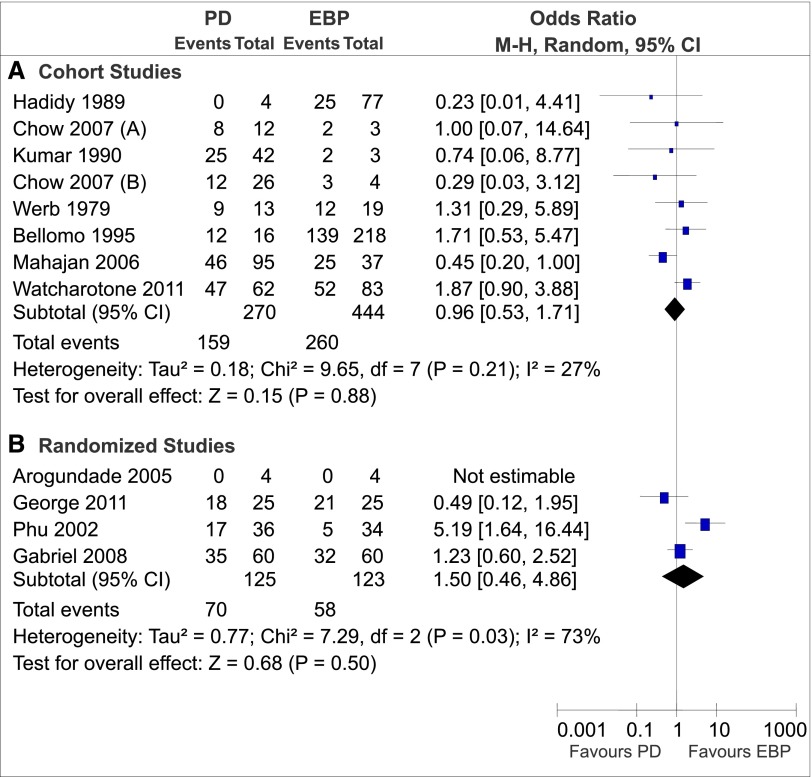
Pooled mortality was 58.0% for PD and 56.1% for EBP. Quantitative analysis was performed separately for observational studies and RCTs. Among the observational studies, there was no significant difference in mortality between PD and EBP (odds ratio, 0.96; 95% confidence interval, 0.53 to 1.71) (Figure 3).
Figure 3.
Effect of renal replacement therapy modality on mortality in patients with AKI grouped by study design. The pooled odds ratio with 95% confidence interval (95% CI) was calculated using the Mantel–Haenszel (M-H) random effects model. (A) Cohort studies. (B) Randomized studies. EBP, extracorporeal blood purification; PD, peritoneal dialysis.
Among the RCTs, there was significant intertrial heterogeneity (I2=73%, P=0.03) (Figure 3). PD was inferior to continuous venovenous hemofiltration in one study (33), whereas mortality rates were comparable for the other three studies (10,34,37). Of note, the first study enrolled patients with severe falciparum malaria (68.6% of cases) (33) in contrast to the other studies with AKI, which were mainly caused by sepsis or hemodynamic disturbances. These factors likely contribute to the heterogeneity among these studies. Because of the small number of RCTs, there was insufficient data to conduct a sensitivity analysis to determine the cause of heterogeneity.
Complications Related to PD.
Sixteen studies reported on peritonitis, which was diagnosed based on signs and symptoms (6,25), positive bacterial culture (18,21,22,26,33), presence of white cells in the dialysate (23,24), or unspecified diagnostic criteria. Only one study had clearly specified criteria for peritonitis (cloudy effluent, >100 white cells/mm3) (23). The method of collecting samples for analysis was not stated in any of the studies. Overall incidence of peritonitis was 12.4%, and it ranged from 0% to 40% in individual studies (Table 3). One study reported that 41.6% of patients on PD developed a cloudy dialysate during the study, but it reported the peritonitis rate as 2.8% as defined by a positive culture (33).
No data were available on other complications, such as hyperglycemia and hypoalbuminemia.
Dose of Dialysis.
Variable measures were used to represent dose in PD. The total volume of peritoneal dialysate used was reported in eight studies as ranging from 13 to 70 L/d (Table 5). Six studies reported the urea clearance (Kurea), which ranged from 9.4 (37) to 29.6 (25) ml/min. However, it is not possible to use Kurea for comparisons between studies, because this measurement was not normalized for patient weight or the volume of distribution of urea. Five studies presented data from which the standardized Kt/Vurea could be calculated. Of these studies, four studies from the same center (10,17,18,27) used a higher dose (mean Kt/Vurea≥3.5), whereas one study (26) used a Kt/V of 1.8–2.4. This last study was conducted outside an ICU setting in a young cohort without sepsis, and mortality in this study was 1.1%. Additional analysis of the relationship between PD dose and mortality was not possible.
Table 5.
Reported indicators of dose of peritoneal dialysis
| Reference | Std-Kt/Vurea (per wk) | Kurea (ml/min) | KCr (ml/min) | PD Volume (L/d) |
|---|---|---|---|---|
| Ponce (17) | 3.5±0.68 | NA | NA | 32.0–44.0 |
| Kilonzo (6) | NA | NA | NA | 7.5 |
| Ponce D (18) | 3.6 | NA | NA | NA |
| George (28) | NA | 9.4±4.9 | 10.5±6.1 | NA |
| Gabriel (10) | 3.6±0.6 | 16.1±4.0a | NA | 42.8±5.72a |
| Gabriel (27) | 3.9±0.6 | 17.3±5.0 | 15.8±4.2 | 43.2±5.1a |
| Arogundade (34) | NA | NA | 8.1±2.8 | 8.0±0.6 |
| Phu (33) | NA | NA | NA | 70 |
| Chitalia (26) | 1.8–2.4 | 10.6–19.8 | 5.8–6.8 | 13.0–26.3 |
| Thongboonkerd (25) | NA | 29.6 | 23.9 | 26.7 |
| Trang (24) | NA | NA | NA | 48.0 |
| Sonnenblick (22) | NA | NA | NA | 48.0 |
| Indraprasit (21) | NA | 12.1 | NA | 24.0–36.0 |
| Cameron (19) | NA | NA | NA | 40.8 |
Dose is represented by the standardized weekly Kt/Vurea (std-Kt/Vurea), urea clearance (Kurea), creatinine clearance (KCr), and volume of PD effluent per day (PD volume). The dose is listed according to how the original article had presented the data: mean, mean ± SD, or range. PD, peritoneal dialysis; NA, results not available.
Information obtained from correspondence with authors.
Other Outcomes.
One RCT (10) observed a significantly shorter duration of dialysis dependence with PD than daily HD (5.5±2.7 versus 7.5±3.1 days; P=0.02). However, one RCT reported that patients treated with PD were more likely to require more than one session of dialysis (PD=70% versus continuous venovenous hemofiltration=37%; P=0.04) (33), whereas the third RCT reported that PD patients required more time on dialysis (PD=20 hours, interquartile range=19 hours versus continuous venovenous hemodiafiltration=48 hours, interquartile range=74.5 hours; P=0.01) (37).
Discussion
We performed a systematic review of PD in the management of AKI in adults and identified 24 studies from 13 countries, of which 11 studies compared PD with EBP. Together, this review represents a total of 1556 patients (PD, n=1005; EBP, n=583), and to our knowledge, it is the most comprehensive review of PD for renal replacement in AKI to date. The majority (19/24) of these studies come from low-resource regions, such as Asia, Africa, and South America (Figure 2). From the developed economies (Canada, United States, United Kingdom, and Australia), there were only one study each. We had three main findings. First, there is a paucity of good-quality data, with only four relatively small RCTs. Second, pooled analysis of 11 studies showed no difference in mortality between PD and EBP. Third, reporting was poor regarding PD dose and other important outcomes, such as renal recovery and PD-related complications.
Although there has recently been an increased interest in using PD to treat patients with AKI in low-resource settings (5,6,8,28,37), the use of PD for such patients in developed countries has been extremely limited. This finding may be considered surprising, because it has been postulated that PD may be more physiologic and less inflammatory than EBP, which involves the exposure of blood to synthetic surfaces. The local renal hemodynamics may be better preserved in PD, because no extracorporeal circulation is required, which could theoretically result in better outcomes. Indeed, in a physician survey, 36%–51% of respondents considered PD to be a suitable therapeutic option in most cases of AKI. However, only 16%–22% actually used PD for AKI in their own practice (38). The gap between opinion and practice was highest in North America and Europe and lowest in Asia–Pacific. This finding is in concordance with the ample representation of Asia among the studies in this review. Interestingly, this divide between opinion and actual practice of PD in AKI is similar to the situation seen in CKD stage 5 (39). Furthermore, PD use to treat AKI is limited to small children in many countries, especially in Western countries. The reason for this limited use of PD is not clear but likely multifactorial. Contributing factors include the development of efficient and easy-to-place central venous catheters, the expanded use of CRRTs and slow extended daily dialysis in affluent countries, and the perception that PD offers inferior care. Also, the etiology of AKI varies in developed and emerging economies (40). AKI treated in Western countries is often in the setting of hypercatabolic multiorgan failure in the ICU in elderly patients with multiple comorbidities. In these cases, efficient solute removal by EBP may be considered more desirable. In contrast, in developing countries, AKI occurs more often among young, previously healthy individuals and/or in the context of a single disease or condition, often infection and toxins (Tables 1 and 2). Moreover, the exposure or lack thereof to a particular dialysis modality during fellowship training may influence future practice patterns (38,39). In the United States, 30% of new nephrologists felt competent with acute PD compared with 90% of new nephrologists felt competent with acute HD (41). The recent reports suggesting acceptable outcomes with PD may stimulate nephrologists from Western countries to re-examine, and potentially broaden, their approach to treating patients with AKI and make appropriate changes in the curriculum of training programs.
It has been suggested that there is more rapid recovery of renal function in AKI patients treated with PD. However, published studies have conflicting results. Although one Brazilian study (10) showed shorter time to renal recovery with PD, studies from Vietnam and India noted that patients on PD required more or longer dialysis sessions (33,37). The latter finding could be attributed to lower solute clearances with PD compared with EBP (42). Unfortunately, the rate of renal recovery is not reported in other studies. Whether PD could potentially be detrimental in specific clinical situations remains unclear. In the RCT on patients with falciparum malaria, mortality with PD was threefold higher than mortality with hemofiltration (33). It has been postulated that the erythrocytic phase of malaria parasites was accelerated because of high splanchnic blood glucose levels resulting from glucose-based peritoneal dialysate (42). Of note, the mortality rate of 47% in this study is higher than the 10%–26% mortality reported in two observational studies on malaria-related AKI also treated with PD (21,24). However, baseline characteristics and illness severity among these studies were different.
Details of PD dose were either absent or inconsistently reported among various studies, making it difficult to examine the relationship between PD dose and mortality. In a recent review on PD dose in AKI (11), it was recommended that continuous forms of PD should be prescribed, with a minimum standardized Kt/Vurea of at least 2.1 per week. Of note, intermittent PD is the more commonly used modality in clinical practice, and over 50% of practitioners professed uncertainty regarding the appropriate PD dose in AKI (38). This uncertainty is likely because of the paucity of strong evidence or consensus on this aspect.
The most commonly described complication related to acute PD was peritonitis, and the incidence was as high as 40%. PD can theoretically disrupt local host–defense mechanisms with dilution of Igs (43) and reduce phagocytic function (44). Although established guidelines (45) for chronic PD recommend that 1 L dialysate be allowed to dwell for 1–2 hours before being collected for examination, this guideline may be less feasible in the acute setting, especially in automated PD targeting a higher dose. Another important but underreported complication is technique failure or catheter-related problems. Only one RCT specifically reported this complication, which resulted in discontinuation of PD in over 10% of the patients randomized to that arm (10). Other complications (e.g., hyperglycemia) were simply not reported.
To our knowledge, this review is the first systematic review of the literature on the use of PD in AKI specifically focusing on its effect on survival compared with EBP. This analysis is limited by the low quality of evidence, with most of the data drawn from retrospective observational studies. The RCTs showed marked intertrial heterogeneity and were limited by small sample sizes and methodological considerations. Furthermore, relevant clinical parameters and outcomes were not reported, precluding more comprehensive analysis to determine the importance of other factors, such as the dose of RRT and metabolic derangements.
Possible confounders are identified in this review. The studies span over four decades; both PD and EBP techniques evolved significantly during this timeframe. In the earlier studies, HD was undertaken with machines that lacked precise flow and volumetric control and membranes that were bioincompatible with low efficiency and flux. More recent studies used dialyzers with high efficiency (10,36) and high flux (33) for EBP. Similarly, PD is evolving from manual exchanges at low doses to automated PD and higher doses. Aside from technology, the epidemiology of AKI has also evolved over this period. We now tend to see older patients with multiple comorbid conditions who have undergone interventions, such as radiocontrast procedures, high-risk surgery, and invasive ICU care (46). Also, selection bias is likely among the nonrandomized studies. One modality could have been preferentially chosen for more unstable patients on the belief that EBP results in better clearance or PD results in less hemodynamic disturbances. In centers with limited resources, only patients expected to have the most benefit from RRT may have been selectively offered therapy.
On systematic review of the published literature, there were limited good-quality data on PD for AKI management in adults. Current available literature does not support a significant difference in outcomes between PD and EBP, suggesting that PD may be a viable option. In the absence of precise data, the clinician needs to exercise judgment in selecting a dialysis modality. The choice should depend on the clinical status of the patient as well as the expertise and resources of the center. Given the paucity of good-quality evidence in this important area, additional well designed and powered randomized trials are needed to evaluate clinically important outcomes as well as cost. Standardized reporting of technique, dose, complications, and cost should be encouraged for observational studies.
Disclosures
None.
Acknowledgments
The authors would like to thank the investigators of the included studies who had provided copies of their papers and additional information on their studies.
The authors wish to acknowledge that this systematic review was initiated during the international fellowship of C.Y.C. at the Department of Nephrology, Dialysis and Transplantation, San Bortolo Hospital, Vicenza, Italy, and C.R. is the department director.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ricci Z, Ronco C, D’Amico G, De Felice R, Rossi S, Bolgan I, Bonello M, Zamperetti N, Petras D, Salvatori G, Dan M, Piccinni P: Practice patterns in the management of acute renal failure in the critically ill patient: An international survey. Nephrol Dial Transplant 21: 690–696, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Blake PG: A renaissance for PD in acute kidney injury. Perit Dial Int 32: 237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeates K, Cruz DN, Finkelstein FO: Re-examination of the role of peritoneal dialysis to treat patients with acute kidney injury. Perit Dial Int 32: 238–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ademola AD, Asinobi AO, Ogunkunle OO, Yusuf BN, Ojo OE: Peritoneal dialysis in childhood acute kidney injury: Experience in southwest Nigeria. Perit Dial Int 32: 267–272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilonzo KG, Ghosh S, Temu SA, Maro V, Callegari J, Carter M, Handelman G, Finkelstein FO, Levin N, Yeates K: Outcome of acute peritoneal dialysis in northern Tanzania. Perit Dial Int 32: 261–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponce D, Caramori JT, Barretti P, Balbi AL: Peritoneal dialysis in acute kidney injury: Brazilian experience. Perit Dial Int 32: 242–246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos CR, Branco PQ, Gaspar A, Bruges M, Anjos R, Gonçalves MS, Abecasis M, Meneses C, Barata JD: Use of peritoneal dialysis after surgery for congenital heart disease in children. Perit Dial Int 32: 273–279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sever MS, Vanholder R, Lameire N: Management of crush-related injuries after disasters. N Engl J Med 354: 1052–1063, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL: High volume peritoneal dialysis vs daily hemodialysis: A randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl 108: S87–S93, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Chionh CY, Ronco C, Finkelstein FO, Soni SS, Cruz DN: Acute peritoneal dialysis: What is the ‘adequate’ dose for acute kidney injury? Nephrol Dial Transplant 25: 3155–3160, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 151: W65–W94, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 17: 1–12, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Gabriel DP, Caramori JT, Martin LC, Barretti P, Balbi AL: Continuous peritoneal dialysis compared with daily hemodialysis in patients with acute kidney injury. Perit Dial Int 29[Suppl 2]: S62–S71, 2009 [PubMed] [Google Scholar]
- 15.Bellomo R, Farmer M, Parkin G, Wright C, Boyce N: Severe acute renal failure: A comparison of acute continuous hemodiafiltration and conventional dialytic therapy. Nephron 71: 59–64, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Arogundade FA, Sanusi AA, Okunola OO, Soyinka FO, Ojo OE, Akinsola A: Acute renal failure (ARF) in developing countries: Which factors actually influence survival. Cent Afr J Med 53: 34–39, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ponce D, Berbel MN, Regina de Goes C, Almeida CT, Balbi AL: High-volume peritoneal dialysis in acute kidney injury: Indications and limitations. Clin J Am Soc Nephrol 7: 887–894, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Ponce D, Brito GA, Abrão JG, Balb AL: Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. Adv Perit Dial 27: 118–124, 2011 [PubMed] [Google Scholar]
- 19.Cameron JS, Ogg C, Trounce JR: Peritoneal dialysis in hypercatabolic acute renal failure. Lancet 1: 1188–1191, 1967 [DOI] [PubMed] [Google Scholar]
- 20.Ojogwu LI: Peritoneal dialysis in the management of hypertensive acute oliguric renal failure. Trop Geogr Med 35: 385–388, 1983 [PubMed] [Google Scholar]
- 21.Indraprasit S, Charoenpan P, Suvachittanont O, Mavichak V, Kiatboonsri S, Tanomsup S: Continuous peritoneal dialysis in acute renal failure from severe falciparum malaria. Clin Nephrol 29: 137–143, 1988 [PubMed] [Google Scholar]
- 22.Sonnenblick M, Slotki IN, Friedlander Y, Kramer MR: Acute renal failure in the elderly treated by one-time peritoneal dialysis. J Am Geriatr Soc 36: 1039–1044, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Howdieshell TR, Blalock WE, Bowen PA, Hawkins ML, Hess C: Management of post-traumatic acute renal failure with peritoneal dialysis. Am Surg 58: 378–382, 1992 [PubMed] [Google Scholar]
- 24.Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TT, Mai NT, Waller DJ, White NJ: Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 15: 874–880, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Thongboonkerd V, Lumlertgul D, Supajatura V: Better correction of metabolic acidosis, blood pressure control, and phagocytosis with bicarbonate compared to lactate solution in acute peritoneal dialysis. Artif Organs 25: 99–108, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Chitalia VC, Almeida AF, Rai H, Bapat M, Chitalia KV, Acharya VN, Khanna R: Is peritoneal dialysis adequate for hypercatabolic acute renal failure in developing countries? Kidney Int 61: 747–757, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Gabriel DP, Nascimento GV, Caramori JT, Martim LC, Barretti P, Balbi AL: High volume peritoneal dialysis for acute renal failure. Perit Dial Int 27: 277–282, 2007 [PubMed] [Google Scholar]
- 28.Hayat A, Kamili MA, Samia R, Yaseen M, Shakeel R, Qureshi W, Malik GM: Peritoneal dialysis for adults with acute renal failure: An underutilized modality. Saudi J Kidney Dis Transpl 18: 195–199, 2007 [PubMed] [Google Scholar]
- 29.Watcharotone N, Sayumpoorujinant W, Udompon U, Leeaphorn N, Kanjanabuch T: Intermittent peritoneal dialysis in acute kidney injury. J Med Assoc Thai 94[Suppl 4]: S126–S130, 2011 [PubMed] [Google Scholar]
- 30.Werb R, Linton AL: Aetiology, diagnosis, treatment and prognosis of acute renal failure in an intensive care unit. Resuscitation 7: 95–100, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Hadidy S, Asfari R, Shammaa MZ, Hanifi MI: Acute renal failure among a Syrian population. Incidence, aetiology, treatment and outcome. Int Urol Nephrol 21: 455–461, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Kumar SS, Paramananthan R, Muthusethupathi MA: Acute renal failure due to acute diarrhoeal diseases. J Assoc Physicians India 38: 164–166, 1990 [PubMed] [Google Scholar]
- 33.Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, Winearls C, Farrar J, White N, Day N: Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 347: 895–902, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Arogundade FA, Ishola DA, Jr, Sanusi AA, Akinsola A: An analysis of the effectiveness and benefits of peritoneal dialysis and haemodialysis using Nigerian made PD fluids. Afr J Med Med Sci 34: 227–233, 2005 [PubMed] [Google Scholar]
- 35.Mahajan S, Tiwari S, Bhowmik D, Agarwal SK, Tiwari SC, Dash SC: Factors affecting the outcome of acute renal failure among the elderly population in India: A hospital based study. Int Urol Nephrol 38: 391–396, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Chow YW, Lim BB, Hooi LS: Acute renal failure in the same hospital ten years apart. Med J Malaysia 62: 27–32, 2007 [PubMed] [Google Scholar]
- 37.George J, Varma S, Kumar S, Thomas J, Gopi S, Pisharody R: Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: A pilot study. Perit Dial Int 31: 422–429, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Gaião S, Finkelstein FO, de Cal M, Ronco C, Cruz DN: Acute kidney injury: Are we biased against peritoneal dialysis? Perit Dial Int 32: 351–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blake PG, Finkelstein FO: Why is the proportion of patients doing peritoneal dialysis declining in North America? Perit Dial Int 21: 107–114, 2001 [PubMed] [Google Scholar]
- 40.Cerdá J, Bagga A, Kher V, Chakravarthi RM: The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol 4: 138–153, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Berns JS: A survey-based evaluation of self-perceived competency after nephrology fellowship training. Clin J Am Soc Nephrol 5: 490–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daugirdas JT: Peritoneal dialysis in acute renal failure—why the bad outcome? N Engl J Med 347: 933–935, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Lamperi S, Carozzi S, Icardi A, Nasini MG: Peritoneal membrane defense mechanism in CAPD. Trans Am Soc Artif Intern Organs 31: 33–37, 1985 [PubMed] [Google Scholar]
- 44.Alobaidi HM, Coles GA, Davies M, Lloyd D: Host defence in continuous ambulatory peritoneal dialysis: The effect of the dialysate on phagocyte function. Nephrol Dial Transplant 1: 16–21, 1986 [PubMed] [Google Scholar]
- 45.Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, Kuijper EJ, Li PK, Lye WC, Mujais S, Paterson DL, Fontan MP, Ramos A, Schaefer F, Uttley L, ISPD Ad Hoc Advisory Committee : Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 25: 107–131, 2005 [PubMed] [Google Scholar]
- 46.Lameire N, Van Biesen W, Vanholder R: The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2: 364–377, 2006 [DOI] [PubMed] [Google Scholar]