Summary
Heart failure remains the leading cause of hospitalization in older patients and is considered a growing public health problem with a significant financial burden on the health care system. The suboptimal efficacy and safety profile of diuretic-based therapeutic regimens coupled with unsatisfactory results of the studies on novel pharmacologic agents have positioned ultrafiltration on the forefront as an appealing therapeutic option for patients with acute decompensated heart failure (ADHF). In recent years, substantial interest in the use of ultrafiltration has been generated due to the advent of dedicated portable devices and promising results of trials focusing both on mechanistic and clinical aspects of this therapeutic modality. This article briefly reviews the proposed benefits of ultrafiltration therapy in the setting of ADHF and summarizes the major findings of the currently available studies in this field. The results of more recent trials on cardiorenal syndrome that present a counterpoint to previous observations and highlight certain limitations of ultrafiltration therapy are then discussed, followed by identification of major challenges and unanswered questions that could potentially hinder its more widespread use. Future studies are warranted to shed light on less well characterized aspects of ultrafiltration therapy and to further define its role in ADHF and cardiorenal syndrome.
Introduction
Heart failure (HF) remains a major and growing public health problem worldwide largely due to its prevalence and the substantial cost for society. It is the leading cause of hospitalization among patients aged >65 years (1), with 1 million hospitalizations annually in the United States alone (2). Furthermore, with the disproportionate growth in the population aged >65 years that will occur over the next 20 years, the prevalence of HF is expected to increase from its current value of 2.8% to 3.5% by 2030 (3). Despite therapeutic advances in the overall care of chronic stable HF, the prognosis of the patients with acute decompensated heart failure (ADHF) remains unacceptably poor, with an in-hospital mortality rate of 4% (4) and a 30-day readmission rate of 27%, the highest among all medical conditions (5). The annual expenditure of the care for patients with HF is estimated at $60 billion in the United States (6), with the cost related to the acute in-hospital care accounting for almost 70% of the total expenses (7). Consequently, improvement in management of HF patients has gained priority in healthcare policies; rehospitalization rates are targeted by performance measures and government incentives (8).
Shortcomings of the Current Therapies
Several factors contribute to the steadily growing population of patients annually hospitalized for HF. The shortcomings of the currently available therapeutic regimens, which are heavily based on diuretics, are likely to play a key role in this regard.
Efficacy
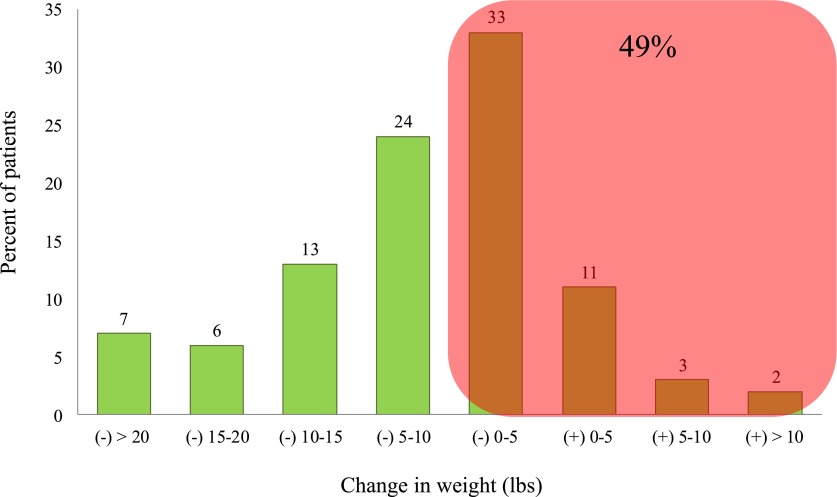
Congestion is the major cause for hospitalization in the great majority of patients with HF (4) and contributes to adverse outcomes (9). The efficacy of therapeutic options is then in part determined by how successfully they can affect patients’ congestion and weight after admission to the hospital. Recent studies suggest that lack of adequate decongestion is more common than previously appreciated (10,11). A report of >50,000 patients in the ADHF National Registry revealed that 33% of the patients lose as little as ≤2.3 kg (5 lbs), and another 16% even gain weight during the course of hospitalization (12); nearly half of the patients admitted for ADHF are discharged with unresolved congestion after receiving diuretic-based conventional therapies (Figure 1). Development of diuretic resistance is a well recognized challenge in the care of patients with ADHF and captures a subset of patients at high risk of morbidity and mortality. The adverse effect of persistent congestion on outcomes has been shown in several studies. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), elevated pulmonary capillary wedge pressure and reduced 6-minute walk were the strongest independent predictors in postdischarge mortality of patients who were hospitalized for worsening HF (13).
Figure 1.
Change in body weight at discharge based on Acute Decompensated Heart Failure National Registry database. Modified from reference 12, with permission.
Safety
The potential effect of diuretics on renal function remains of particular interest in the setting of ADHF. A significant subset of patients develop AKI or worsening renal function (WRF) only after admission to the hospital for ADHF (i.e., acute cardiorenal syndrome). In a study by Hata et al., 60% of the patients who were free of AKI upon admission developed AKI while being treated for ADHF (14). The mechanistic aspects of diuretic-associated WRF are beyond the scope of this article and are presumably related to a number of pathways, including exacerbation in renin-angiotensin-aldosterone system and activation of sympathetic nervous system. Not surprisingly, development of WRF during the treatment of ADHF portends poor outcomes independent of the baseline renal dysfunction (15).
An association between high doses of loop diuretics and adverse outcomes has been proposed by observational studies, although they could be confounded by the fact that patients with more severe disease are likely to receive higher doses of diuretics. Indeed, diuretic dose is possibly a marker of severity of the disease, rather than a cause for adverse outcomes (16). In a prospective study on 183 patients with HF in the outpatient setting, Mielniczuk et al. found that higher dose of diuretics (i.e., >80 mg furosemide equivalent) was a strong predictor of subsequent HF events in the univariate analysis (hazard ratio, 3.83; 95% confidence interval, 1.82 to 8.54), but it no longer remained significant after adjustment for clinical stability (hazard ratio, 1.53; 95% confidence interval, 0.58 to 4.03) (17). On the basis of these results, the authors concluded that in patients with HF, high-dose diuretics might be more of a marker than a cause for instability.
Finally, despite their ubiquitous use, there are still significant uncertainties about the very basic aspects of diuretic use, such as the optimal dosing or the mode of administration (i.e., intermittent versus bolus) in the setting of ADHF. National data indicate that conventional management of patients with ADHF is subject to substantial variability, possibly leading to significant variations in patients’ outcomes (12). The potential shortcomings of diuretics are summarized in Table 1.
Table 1.
Potential shortcomings of diuretic use in treatment of heart failure
| Direct activation of the renin-angiotensin-aldosterone system |
| Deterioration in renal function |
| Electrolyte abnormalities (e.g., hypokalemia and hypomagnesemia) |
| Suboptimal natriuresis (production of hypotonic urine) |
| Development of diuretic resistance |
| Unpredictability of the therapeutic response |
| Lack of clarity on the practical aspects of use (e.g., optimal dosing strategy) |
| Nonrenal adverse effects (e.g., ototoxicity and hypersensitivity) |
Pharmacologic Alternatives
A number of pharmacologic agents (e.g., endothelin receptor antagonists, vasopressin receptor antagonists, and adenosine-A1 receptor antagonists) have been used in trials in the hope of replacing or complementing conventional therapies but so far large-scale studies have unfortunately proven them to be suboptimal, ineffective, or unsafe (18).
Proposed Advantages of UF Therapy
Over the past few years, mechanical extraction of fluid (i.e., ultrafiltration [UF]) has been progressively positioned on the forefront as an alternative for pharmacologic approaches of ADHF. Earlier investigations were primarily focused on the effect of UF on pathophysiologic mechanisms (e.g., neurohormonal mediators), whereas recent trials have evaluated practical aspects of its use such as the effect on the length of hospital stay. The proposed benefits of UF therapy are summarized in Table 2.
Table 2.
Proposed advantages and disadvantages of ultrafiltration
| Advantages | Disadvantages |
| Reduction in renal venous congestion and improvement in renal hemodynamics | Lack of protective effect on renal functionLack of effect on markers of mortality (i.e., serum sodium level and BUN)Possible need for placement of midline or central venous catheterNeed for additional training for staff and physiciansNeed for anticoagulationComplications related to extracorporeal circuit (e.g., allergic reaction, air embolism, hemolysis, infection, and bio-incompatibility)Lack of widely accepted guidelines for its use (e.g., patient population, indications, timing of initiation and termination, and ultrafiltration rate/volume)Lack of knowledge on the long-term outcomesHigh cost (device and disposables) |
| Rapid and adjustable removal of fluid and improvement in symptoms of congestion | |
| Higher mass clearance of sodiumDecreased risk of electrolyte abnormalities (e.g., hypokalemia)Lack of neurohormonal activation (SNS, RAAS, and AVP)Sustainability of the beneficial effects (e.g., effect on neurohormonal axis)Improvement in diuretic resistance, natriuresis, and urine outputDecreased rate of heart failure–related rehospitalizationsDecreased hospital length of stayAvailability of dedicated ultrafiltration devices that are portable, user-friendly, with minimal extracorporeal volume (33 ml), and have the ability of functioning with low blood flow rates (10–40 ml/min) |
SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system; AVP, arginine vasopressin.
Neurohormonal Axis
An intriguing aspect of UF therapy is its beneficial effect on neurohormonal mediators and hemodynamic status. Guazzi et al. reported that a 20% reduction in plasma volume through UF paradoxically led to significant decrease in plasma renin activity and the serum levels of NE and aldosterone (19). They also observed a substantial increase in the urine volume and sodium excretion after UF. Subsequently, Marenzi et al. invasively measured cardiopulmonary pressures in patients with refractory HF who underwent UF. Fluid removal was followed by a progressive decrease in right atrial and pulmonary capillary wedge pressures while stroke volume and cardiac output increased (20). They observed no change in the heart rate or systemic vascular resistance. The intravascular volume reduction through use of diuretics does not often portend these benefits and even tends to produce opposite effects such as activation of renin-angiotensin-aldosterone system and sympathetic nervous system (21,22). The beneficial effects of UF on contractile cardiac efficiency and performance without an adverse effect on heart rate or BP were also reported by other investigators (23). In a recent randomized controlled study on patients with ADHF using a minimally invasive cardiocirculatory monitoring system, UF resulted in substantial increase in several parameters such as cardiac index and cardiac power output while simultaneously reducing systemic vascular resistance; these effects were not observed in the diuretic group (22). In that study, UF had no adverse effect on BP or heart rate and was associated with a significant reduction in serum aldosterone levels, whereas patients who received diuretics showed a decrease in BP, an increase in heart rate, and no change in serum aldosterone levels (22). It is noteworthy that the UF volume and rate could play an important role in the potential effect of UF on the neurohormonal axis and hemodynamic status. During UF therapy, fluid that is removed from the intravascular sector is replenished from the extravascular compartment and this helps maintain hemodynamic stability. The rate of this shift is variable and depends on a number of factors such as serum albumin level. The UF volume and rate should be carefully matched to the degree of fluid overload and anticipated plasma refill rate, respectively. For example, in malnourished patients, the resultant low serum oncotic pressure could contribute to an impaired plasma refill rate during UF, leading to intravascular volume contraction, hemodynamic instability, and exacerbated neurohormonal activation.
Sodium Extraction
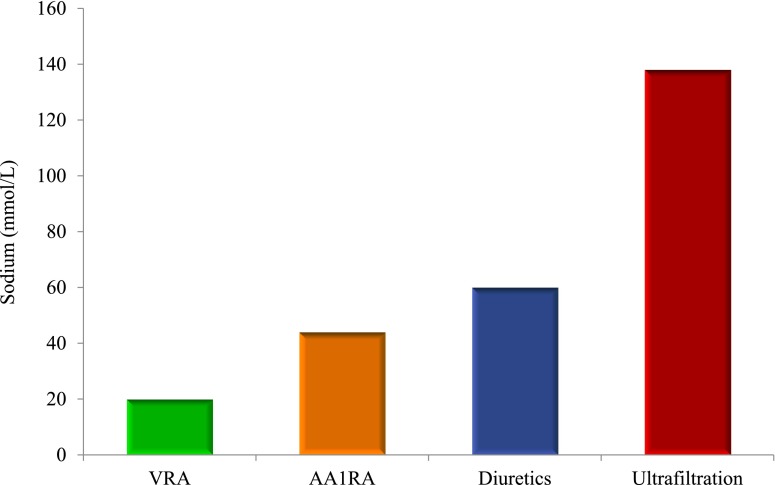
The common pathway for several mechanisms involved in the development of ADHF is sodium retention with resultant extracellular fluid expansion; the great majority of ADHF admissions are due to volume overload and congestion (4). Thus, any therapeutic modality with greater sodium extraction will be advantageous in this setting. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, addition of a vasopressin receptor antagonist to standard therapy (with resultant enhanced free water excretion) did not reduce all-cause mortality, cardiovascular death, or rehospitalization for HF despite adequate decongestion, suggesting the paramount role of sodium removal (24,25). Because ultrafiltrate extracted through convective forces is isotonic, it removes a significantly higher amount of sodium compared with hypotonic urine produced by diuretics and other available pharmacologic agents (Figure 2). Given that UF also extracts fluid more efficiently and rapidly compared with diuretics (26,27), and that it is reported to enhance natriuresis (19,20), this effect can be further potentiated possibly leading to longer-lasting results (see below). A secondary analysis of the Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) trial found that despite comparable fluid volume removal with UF and diuretic infusion, fewer HF rehospitalization equivalents occurred with UF (28).
Figure 2.
Comparison of sodium removal with various treatment options. Whereas ultrafiltration extracts isotonic fluid from plasma, pharmacologic agents produce hypotonic urine containing lower concentrations of sodium. VRA, vasopressin receptor antagonists; AA1RA, adenosine-A1 receptor antagonists.
Sustainability of the Effects
A less well characterized advantage of UF therapy could be persistence of its beneficial effects. In a study on 36 patients with HF, Agostoni et al. reported an improvement in several respiratory parameters (e.g., tidal volume and peak exercise ventilation) that lasted over the next 6 months after one single session of UF (29). In a similar study, those HF patients who underwent one UF session showed improvement in cardio-respiratory parameters (e.g., exercise tolerance time) that persisted at 1- and 3-month follow-up assessments; these effects were not observed in the control group (30). In line with these studies, patients in a more recent trial tended to be less often rehospitalized for ADHF within 3 months after treatment with UF therapy compared with those who received diuretics (27). It is still unclear whether durability of beneficial effects is related to lack of neurohormonal activation, enhanced sodium removal, or other less well understood mechanisms.
Venous Decongestion
The precise pathways underlying ADHF and cardiorenal syndrome remain elusive. Earlier animal studies had suggested that isolated elevation in renal venous pressure could adversely affect renal hemodynamics and increase sodium retention (31), but only recently has renal venous congestion been implicated as an important factor in the complex interactions between the heart and the kidney. Damman et al. reported that high central venous pressure (CVP) correlated with low estimated GFR (eGFR) and could predict mortality independent of the cardiac index in HF patients (32). Similarly, Guglin et al. found that low eGFR was associated with elevated CVP but not with cardiac index or left ventricular ejection fraction in these patients (33). A primary role of venous congestion in ADHF and cardiorenal syndrome is especially appealing because it could potentially be amenable to fluid removal by UF. In a study by Mullens et al., mechanical fluid removal (whether via UF or paracentesis) in patients with HF was associated with reduction in intra-abdominal pressure and improvement in renal function (34). The complex pathophysiologic pathways and maladaptive mechanisms underlying ADHF and renal dysfunction, as well as the proposed role of UF on reversal of these parameters, are depicted in Figure 3.
Figure 3.
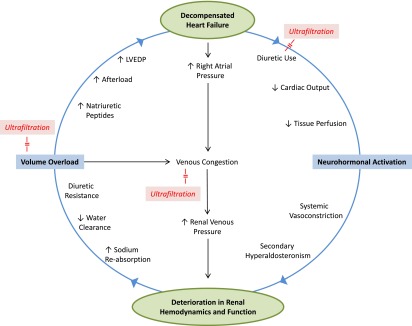
Proposed pathophysiologic pathways underlying decompensated heart failure and renal dysfunction. Ultrafiltration could potentially counter certain interactions and break this vicious cycle via correction of fluid overload (while sparing the kidneys), venous decongestion, and increase in cardiac output (left shift of Frank-Starling curve). In addition, diuretics can be held during ultrafiltration therapy potentially avoiding their downstream adverse effects (i.e., biologic holiday for cardiorenal interactions). LVEDP, left ventricular end-diastolic pressure.
Renal Replacement Therapy for Heart Failure: Earlier Clinical Studies
Renal replacement therapy for management of volume overload in HF was proposed for the first time in the 1970s (35). Over the next 3 decades, several investigators reported favorable results with regard to the efficacy and safety of this therapy (36). In one of the largest of these studies, Canaud et al. used slow UF (continuous or daily) in 52 patients with severe congestive HF (class IV of the New York Heart Association classification) (37). The UF sessions were tolerated well with an average weight loss of 9.2 kg. Interestingly, there were 39 responders or partial responders: 26 patients became class III and 13 became class II. Although an improvement in natriuresis and renal function was also observed in a subset of patients, the findings were variable and not as prominent as those related to cardiac status. It is noteworthy that not all earlier studies, which were either case reports or had small patient populations without a control group, performed isolated UF; some used other modalities such as continuous venovenous hemofiltration or hemodiafiltration (36). Because these therapies provide significant clearance in addition to extraction of fluid, their results should be cautiously interpreted to avoid unjustified extrapolation.
Due to the overall positive tone of these early feasibility studies, UF was recognized in many centers as a mechanistically relevant therapeutic alternative for HF and refractory fluid overload. However, due to a lack of large-scale controlled multicenter trials as well as the relative complexity of this therapeutic modality, support for its use especially in the United States remained anecdotal until the early 2000s.
UF Therapy for Heart Failure: The New Era
The advent of portable devices with newer technology rendered UF more appealing and led to a second generation of clinical trials. These simplified and user-friendly machines have the advantages of small size, portability, blood flow rates of as low as 40 ml/min, and an extracorporeal blood volume of <50 ml. They can provide UF rate within a large spectrum (0–500 ml/h), do not mandate admission to intensive care unit, and have been marketed with the ability of even using peripheral veins.
There is a limited number of clinical studies on the use of UF in management of HF, of which <10 involve randomization, and only 6 are randomized controlled trials comparing UF with diuretics. In a first study using peripherally-inserted catheters, Jaski et al. performed 25 sessions of UF on 21 fluid-overloaded HF patients with an average fluid removal of 2.6 L per treatment (38). The primary endpoint of this feasibility study (i.e., assessing the safety of rapid fluid removal of ≥1 L in ≤8 hours) was achieved in 93% of treatment sessions without a significant change in serum creatinine levels. In order to further explore the indications of UF therapy, Costanzo et al. used it in 20 patients with ADHF who also presented with diuretic resistance or renal dysfunction (serum creatinine ≥1.5 mg/dl) (39). They reported favorable results with successful decongestion, significant shorter length of stay, and lower readmission rates. The largest trial in this field (i.e., the UNLOAD trial), compared UF with intravenous diuretics in patients with ADHF (27). Shortly after admission (i.e., within 24 hours), 200 patients with ADHF were randomized to either receive intravenous diuretics or UF therapy. Patients with severe renal dysfunction and those with hemodynamic instability were excluded. The UF group showed a greater fluid loss at 48 hours without a significant change in serum creatinine or BP. Interestingly, the UF group also demonstrated a greater freedom from rehospitalization during the 90-day follow-up period. Moreover, rehospitalization days and HF-related unscheduled visits were fewer for these patients, and UF was associated with a 53% reduction in the risk of rehospitalization for ADHF. The positive results of the UNLOAD trial further promoted UF and, although controversial, some authors even suggested that it should replace diuretics as the first-line therapy for ADHF (24). Nevertheless, this trial had a number of important shortcomings. For example, only patients with hemodynamic stability were studied; those with a systolic BP of ≤90 mmHg or requiring intravenous pressors/vasoactive agents were excluded. Because patients in the UF group are theoretically at greater risk of hemodynamic instability due to the nature of the extracorporeal therapy, elimination of the unstable patients might potentially have acted in favor of UF. Moreover, study groups were not controlled for the total amount of volume loss at the end of therapy. In fact, weight loss at 48 hours was significantly greater for UF than for the diuretic group (5 kg versus 3.1 kg, respectively; P=0.001). This was, in part, due to suboptimal use of diuretics because the treatment goal was not achieved in this group; per the study protocol, the minimum dose of intravenous diuretics was planned to be at least twice the before-hospitalization daily oral dose (i.e., 119 ±116 mg), but it showed only a 50% increase (i.e., 181±121 mg). Finally, although the investigators did not observe a statistically significant difference in the serum creatinine between the two groups, there was a trend toward higher serum creatinine levels for the UF arm. It is conceivable that the adverse effect on renal function could have been significant had the patient population been hemodynamically less stable.
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial, the second largest and the most recent study in this field, started recruitment of the patients about 1 year after the results of the UNLOAD trial were released (40). In this multicenter study, those patients with ADHF who also presented with cardiorenal syndrome and persistent congestion despite conventional treatment were randomized to receive either an algorithm-based pharmacologic regimen or UF as a rescue therapy. The primary bivariate endpoint was the change in serum creatinine level and body weight at 96 hours after enrollment. Interestingly, not only patients in the UF group failed to have greater weight loss (5.7 kg for UF versus 5.5 kg for medical therapy; P=0.58), but they also presented with a significant increase in serum creatinine level (+0.23 mg/dl for UF versus −0.04 mg/dl for medical therapy; P=0.003) as well as a higher rate of serious adverse events such as sepsis. Due to a lack of evidence of benefit for UF as well as an excess number of adverse events, the enrollment had to be ended after the first 188 patients were included. There are a number of concerns with regard to the design of the CARRESS-HF trial. Although careful attention was paid in trying to optimize both study arms, they were not totally comparable. Use of inotropes and vasodilators was prohibited in the UF group, whereas they were permitted in the pharmacologic care arm after 48 hours if urine output was inadequate. Moreover, although dosing of the diuretics was adjusted in the pharmacologic therapy group based on their therapeutic response, the UF rate was uniformly delivered at 200 ml/h. The importance of customizing UF rate to plasma refill rate is discussed above. In this trial, the early weight loss tended to be faster in the UF arm, potentially contributing to the increased incidence of AKI. Finally, the use of UF is often reserved for those patients that are not fully responsive to diuretics. Patients in the CARRESS-HF trial had a urine output of 2.8–3.4 L/d during medical therapy, which raises questions regarding the applicability of these results to routine clinical practice.
Although disappointing, the results of the CARRESS-HF trial were not totally unexpected. Recent uncontrolled retrospective studies on ADHF patients who also presented refractoriness to medical therapy previously reported similar findings. In a retrospective study by Dev et al., 72 ADHF patients underwent UF therapy for diuretic resistance and failure to respond to conventional therapies (41). The investigators observed significant deterioration in renal function: 43% of the patients experienced a ≥20% reduction in eGFR and 10% required dialysis. In another study on 63 patients with refractory ADHF, UF was used as a rescue therapy (42). Although improvement in several hemodynamic variables and significant weight loss was observed after UF, it was also associated with subsequent requirement of renal replacement therapy in a significant number of patients (59%) and overall poor outcomes (30% in-hospital mortality and 6% discharge to hospice care).
The sobering findings of the CARRESS-HF, coupled with previous similar studies, imply that although early UF could be advantageous for management of patients with ADHF, it might not be the ideal option for salvage therapy after development of diuretic resistance, progressive renal dysfunction, and cardiorenal syndrome. These results also suggest that implementation of a well designed algorithm to guide management of ADHF using currently available pharmacologic options could be associated with improvement in the outcomes. Indeed, the patients in the CARRESS-HF trial receiving stepped medical therapy had a mean weight loss of 5.5 kg, whereas based on the national data, 73% of the patients lose <4.5 kg during their hospital admission (12). This concept is supported by the results of a recent observational study on 596 patients by Barsuk et al., who found that implementation of a diuretic-based protocol for patients with ADHF could be associated with significant improvement in weight loss and the 30-day readmission rate compared with usual care (43). In that study, there was no difference between the two groups in terms of the effect on renal function but the diuretic dosing protocol group showed an increased length of stay. The design and findings of the most recent UF studies (randomized and nonrandomized) are summarized in Tables 3 and 4.
Table 3.
Recent randomized controlled trials on the use of ultrafiltration in heart failure
| Reference | Study Design and Protocol | Patients (n) | Ultrafiltration Therapy | Effect on Renal Function | Main Findings |
| Bart (2005), RAPID-CHF (26) | Early single 8-h UF plus usual care versus usual care alone (additional courses of UF were allowed only after 24-h goals were assessed) | 40 | Maximum rate was 500 ml/h. UF group had a volume removal of 4650 ml at 24 h. UF was used as an adjunct rather than an alternative treatment | No significant difference in renal function between UF and diuretic groups | UF was well tolerated. Greater fluid removal with UF but weight loss was similar in both groups |
| Costanzo (2007), UNLOAD (27) | Single session early UF therapy (within first 24 h of admission). Duration and rate of removal at discretion of physician | 200 | Maximum rate was 500 ml/h. The average rate of removal 241 ml/h for 12.3±12 h | No significant difference in renal function between UF and diuretic groups. Percentage of patients with >0.3 mg/dl rise in creatinine consistently higher in UF group at 24 h, 48 h, and at discharge (not statistically significant) | Greater net fluid loss with UF. Fewer patients in the UF group rehospitalized at 90 d with fewer rate of hospitalization, hospitalization days, and unscheduled visits |
| Rogers (2008) (47) |
Single session UF therapy (exclusive UF therapy during the first 24 h of admission). Substudy of UNLOAD trial |
20 | Maximum rate was 500 ml/h. The target weight and rate of fluid removal were at the discretion of the physician. Fluid removal by UF was 3.7 L | No significant difference in GFR, RBF, and FF between UF and diuretic groups. Iothalamate was used to measure GFR; it decreased by 3.4 and 3.6 ml/min in UF and diuretic groups, respectively | No significant difference in net fluid removal between the two groups. The changes in renal hemodynamics were comparable for UF and diuretics |
| Giglioli (2011), ULTRADISCO (22) | Slow continuous UF. Duration and rate of removal at discretion of physician. Hemodynamic changes were monitored by the Pressure recording analytical method | 30 | Median UF time was 46 h. Maximum UF rate 300 ml/h. After 36 h, the cumulative fluid removal was 9.7 L for the UF group | No significant difference was observed in serum creatinine levels between baseline and post-therapy and between diuretic and UF groups | Weight loss significantly greater in the UF group, UF resulted in better hemodynamic status, reduction in serum aldosterone levels and systemic vascular resistance, and improvement in cardiac index and stroke volume |
| Hanna (2012) (53) | Patients with NYHA class III/IV heart failure and ejection fraction <40%, UF rate 400 ml/h for 6 h and 200 ml/h thereafter | 36 | UF group had a fluid removal rate of 3.4 ml/kg per hour with a total volume removal of 5.2 L. The mean time to achieve a PCWP of ≤18 mmHg was 22 h in the UF group | No significant difference in serum creatinine and cystatin C levels before and after therapy between the two groups | Fluid removal was faster and more efficient in the UF group with shorter hospital length of stay, no change in inflammatory markers, NT-pro-BNP, hospital readmissions (trend toward worsening), and emergency department visits |
| Bart (2012) [CARRESS-HF] (40) | UF was used as rescue therapy after patients had worsening renal function (UF rate 200 ml/h) | 188 | Median duration of UF was 40 h. Post-UF weight loss was 5.7 kg | Serum creatinine level increased significantly after UF. No change in serum creatinine level with medical therapy | Although weight loss was similar with UF and pharmacologic therapy, patients in the UF group had higher rate of serious adverse events. Enrollment ended prematurely due to a lack of benefit and an excess of adverse events with UF |
UF, ultrafiltration; RBF, renal blood flow; FF, filtration fraction; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Table 4.
Recent nonrandomized studies on the use of ultrafiltration in heart failure
| Reference | Study Design and Protocol | Patients (n) | Ultrafiltration | Effect on Renal Function | Main Findings |
| Jaski (2003) [SAFE] (38) | No control group. A total of 25 treatment sessions; upon removal of 1 L, UF could be stopped or continued for a maximum of 8 h | 21 | Maximum rate was 500 ml/h. UF removed 2.6 L of fluid per treatment. Treatments were 6.8 h on average | No significant change in renal function. The patients did not need to be diuretic-resistant to be included | The goal of removing >1 L in ≤8 h could be achieved in 92% of all treatments without adverse effects |
| Costanzo (2005) [EUPHORIA] (39) | No control group, one session of UF for each patient | 20 | Maximum rate 500 ml/h, UF stopped when symptoms resolved. Fluid removal by UF 8.6 L, UF initiated within 4.7±3.5 h of admission | No significant change in serum creatinine or clearance of creatinine | Aggressive fluid removal with early UF was not associated with worsening renal function; 60% of patients were discharged in ≤3 d |
| Liang (2006) (45) | No control group, number of UF sessions at the discretion of physician (1–5 sessions) | 11 | A total of 32 UF sessions, each 8 h. In 41% of the sessions, >3.5 L was removed | No significant change in mean creatinine levels although 45% of the patients developed worsening renal function. Three patients were dialyzed on the same admission and one on a subsequent admission | UF can efficiently remove fluid. Fluid removal with UF is safe but variable |
| Dahle (2006) (55) | No control group (case series). UF sessions stopped at the discretion of physician | 9 | UF rate 400 ml/h for 4 h, 200 ml/hr thereafter. Mean length of UF time: 33.3±20 h | No significant change in renal function | Standard peripheral intravenous catheters can be used for UF therapy |
| Jaski (2008) (56) | No control group. Retrospective cohort, each patient with ≥1 sessions of UF therapy | 100 | 2–6 L of fluid removed over 8–12 h in each session. (total: 7 L during 2.1 sessions per hospitalization) | No significant change in renal function | Despite severe volume overload, the inpatient outcomes of the cohort after UF therapy were similar to national standards |
| Bartone (2009) (46) | Retrospective chart review of 25 patients with UF therapy compared with 25 patients with usual care and 25 patients with usual care plus nesiritide | 75 | Mean UF rate was 325 ml/h with a mean duration of 37.5 h. The target weight and rate of fluid removal were at the discretion of the physician | Significant increase in serum creatinine level in UF group. No significant change in renal function for the other two groups | More efficient fluid removal with UF compared with usual care with or without nesiritide |
| Dev (2012) (41) | Retrospective chart review. Choice of daily or continuous UF sessions at the discretion of physician. UF was typically used after failure of medical therapy | 72 | Median UF rate 200 ml/h (maximum 500 ml/h) for a median of 3 d. Median fluid removal 11.3 L | Median BUN and serum creatinine increased by 10 and 0.3 mg/dl respectively, 43% of patients experienced a ≥20% decrease in eGFR, 10% required dialysis | Overall poor outcomes with 6% mortality and 4% discharge to hospice care, and LVAD/transplant in 3% |
| Patarroyo (2012) (42) | Retrospective chart review. Slow continuous UF was used as rescue therapy after failure of medical management | 63 | Mean time between admission and initiation of UF was 8 d. Mean duration of UF was 3±2 d. UF rate of 100–400 ml/h at the discretion of the physician | No significant change in serum creatinine level or BUN after UF therapy in the whole cohort, but significant deterioration in renal function in those who began UF >48 h after admission. 59% required dialysis | Significant weight loss as well as improvement in hemodynamic variables achieved with UF. Overall poor outcomes with 30% mortality |
UF, ultrafiltration; eGFR, estimated GFR; LVAD, left ventricular assist device.
Overall, there is a paucity of vigorous clinical trials in the field of UF therapy for HF. Most of these second-generation studies have severe shortcomings such as small patient populations and short follow-ups. Moreover, significant variation exists with regard to clinical characteristics of the included patients (e.g., HF class), study endpoints, and the protocol for use of UF or medical therapy, potentially limiting the applicability of their results. Of note, the two largest trials in this field (i.e., UNLOAD and CARRESS-HF) have excluded patients with more severe renal dysfunction (serum creatinine level of >3 and >3.5 mg/dl, respectively).
The Study of Heart Failure Hospitalizations after Aquapheresis Therapy Compared with Intravenous Diuretic Treatment (AVOID-HF) is an ongoing randomized controlled trial recruiting >800 patients (NCT01474200). The trial is intended to determine whether patients will have fewer HF events after receiving UF therapy compared with intravenous diuretics. It is expected that the results of this study can more precisely define the effect of ultrafiltration on HF events.
UF Therapy: Concerns and Questions
Although some of the proposed theoretical advantages of UF over diuretic therapy have been supported by clinical trials, there exist a number of concerns and unanswered questions that could potentially hinder its widespread use.
Effect on Renal Function
It was hypothesized that UF could positively affect renal hemodynamics and function through a number of mechanisms including reduction in venous congestion (backward flow) as well as improvement in cardiac output and renal perfusion (forward flow). However, studies so far have failed to demonstrate any protective effects for UF (44). In the UF study from the Mayo Clinic, not only did the investigators not observe any significant change in the mean serum creatinine levels, but 45% of the patients experienced WRF and 5 of them required dialysis on the same or subsequent admission (45). This is similar to a more recent report by Bartone et al. that retrospectively studied 75 patients and compared UF to conventional therapy with or without nesiritide (46). Those patients in the UF group experienced significant WRF with an increase of >0.5 mg/dl in serum creatinine level in 44% of patients compared with 24% and 20% in the diuretics and nesiritide groups, respectively. Experiences such as this illustrate the need for customized UF regimens and highlight the difficulty in determining the ideal UF volume and rate. In a substudy of the UNLOAD trial, Rogers et al. evaluated the effect of UF on renal parameters through using iothalamate and para-aminohippurate to precisely measure GFR, filtration fraction, and renal plasma flow (47). In line with previous studies, they found that UF and furosemide portend similar adverse effects on renal function (reduction in GFR of 3.4 and 3.6 ml/min, respectively). Finally, as previously mentioned, UF therapy resulted in worse renal outcomes compared with diuretics in the CARRESS-HF trial (40). The findings of the recent clinical trials regarding renal function are summarized in Tables 3 and 4. The apparent discrepancies in the results of studies regarding the effect of UF on renal function can in part be attributable to the differences in the delicate balance between the UF rate and plasma refill rate in the study populations. No widely accepted consensus guidelines for the use of UF in this setting currently exist; the ideal rate of fluid removal, optimal monitoring measures, and the practical criteria to determine the best timing for discontinuation of UF therapy are still unknown. Monitoring the changes in hematocrit via online hematocrit sensors can be used as a surrogate for the plasma refill rate, although it has not been shown to be superior to any other method. Echocardiographic monitoring of the changes in inferior vena cava and left atrial diameters, electrocardiography modifications (amplitude of QRS complexes) related to variations in intravascular volume, impedance cardiography, and biomarkers are among other potential options.
Cost
In the current era of financial containment, the expenditure associated with the use of UF has understandably been a major concern. It was initially suggested that the substantial upfront cost of this therapy could be offset over time due to lower rates of readmission and shorter length of stay (27). However, a decision model analysis evaluating cost-effectiveness of UF from various payer perspectives revealed that UF had a high probability of being more expensive from societal and hospital perspectives, whereas it could be cost-saving only from a Medicare payer perspective (48). Of note, those estimations were based on the use of newer devices using proprietary supplies. Conceivably, UF could be financially more attractive if already existing nephrology resources such as dialysis machines, disposable supplies, and nursing staff are used (49,50).
Long-Term Outcomes
So far no study has evaluated the effect of UF on long-term outcomes of patients with HF (51). Clinical trials have also failed to demonstrate any positive effect on established surrogates of outcomes such as serum sodium level and BUN (52). Therefore, it is not clear whether this costly modality could have any beneficial effect on the long-term outcomes of these patients. Moreover, certain benefits of UF proposed by earlier studies are not fully supported by more recent investigations. For example, although UF use resulted in more efficient fluid removal and shorter hospital length of stay compared with conventional therapy in a randomized controlled trial on 36 patients, there was no significant difference between the two groups in terms of hospital readmissions, emergency department visits, changes in inflammatory markers (e.g., IL-6), or N-terminal fragment of B-type natriuretic peptide levels (53).
Practical Aspects
The potential complications of UF (e.g., air emboli) are similar to other extracorporeal therapies and were reviewed elsewhere (51). Although the new UF devices are marketed with the ability of using peripherally inserted catheters, most studies have failed to report the number of patients who actually needed placement of a central venous access. Patients with HF tend to have compromised peripheral blood vessels due to their advanced age, fluid overload, and poor cardiac output. In addition to the potential complications associated with placement of a central venous catheter, this is of particular concern in this patient population with a high prevalence of kidney disease in which there might be a need for a permanent vascular access in the future. Finally, due to a relative paucity of data on the practical aspects of UF therapy in the specific setting of ADHF (e.g., optimal patient population, timing of initiation and discontinuation of therapy, and optimal rate of fluid removal), the guidelines provided by professional societies are often vague (Table 5), possibly contributing to significant variations in the delivery of this modality (49,54). The potential disadvantages of UF therapy are summarized in Table 2.
Table 5.
Current guidelines on the use of ultrafiltration in heart failure
| Reference | Guidelines |
| American College of Cardiology/American Heart Association (2009) (57) | Ultrafiltration is reasonable for patients with refractory congestion not responding to medical therapy; new recommendation |
| If all diuretic strategies are unsuccessful, ultrafiltration or another renal replacement strategy may be reasonable | |
| Consultation with a kidney specialist may be appropriate before opting for any mechanical strategy to affect diuresis | |
| Canadian Cardiovascular Society (2012) (58) | Venovenous ultrafiltration may be of benefit in relieving congestion particularly in diuretic-resistant patients |
| Patients with persistent congestion despite diuretic therapy, with or without impaired renal function, may, under experienced supervision, receive continuous venovenous ultrafiltration | |
| European Society of Cardiology (2012) (59) | Venovenous isolated ultrafiltration is sometimes used to remove fluid in patients with heart failure, although is usually reserved for those unresponsive or resistant to diuretics |
| If doubling the dose of loop diuretics and infusion of dopamine do not result in an adequate diuresis and the patient remains in pulmonary edema, venovenous isolated ultrafiltration should be considered |
In conclusion, UF has emerged as an appealing option for management of ADHF based on the recent advances in our understanding of the pathophysiologic mechanisms underlying ADHF and cardiorenal syndrome as well as suboptimal efficacy and safety of conventional therapies. Although studies have generally reported favorable results in this setting, a number of unresolved questions and concerns do exist, especially with regard to the effect of UF on renal function and long-term outcomes. Future studies are needed to further define the role of UF therapy for management of heart failure and/or cardiorenal syndrome and to shed light on its less well characterized aspects such as indications, patient selection, and timing of initiation and discontinuation.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wier LM, Pfuntner A, Maeda J, Stranges E, Ryan K, Jagadish P, Collins Sharp B, Elixhauser A: HCUP Facts and Figures: Statistics on Hospital-based Care in the United States, 2009. Rockville, MD: Agency for Healthcare Research and Quality, 2011. Available at: http://www.hcup-us.ahrq.gov/reports.jsp Accessed March 6, 2013
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research: Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 123: 933–944, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators: Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149: 209–216, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA: Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 360: 1418–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Fang J, Mensah GA, Croft JB, Keenan NL: Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 52: 428–434, 2008 [DOI] [PubMed] [Google Scholar]
- 8.111th US Congress: The Patient Protection and Affordable Care Act, 2010 Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm Accessed March 6, 2013
- 9.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA, Stevenson LW: Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J 140: 840–847, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, Massie BM, O’Connor CM, Ponikowski P, Teerlink JR, Voors AA, Cotter G: Dyspnoea in patients with acute heart failure: An analysis of its clinical course, determinants, and relationship to 60-day outcomes in the PROTECT pilot study. Eur J Heart Fail 12: 499–507, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G: Dyspnoea and worsening heart failure in patients with acute heart failure: Results from the Pre-RELAX-AHF study. Eur J Heart Fail 12: 1130–1139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Filippatos G: Reassessing treatment of acute heart failure syndromes: The ADHERE Registry. Eur Heart J 7[suppl B]: B13–B19, 2005 [Google Scholar]
- 13.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW; ESCAPE Investigators and ESCAPE Study Coordinators: Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 294: 1625–1633, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Hata N, Yokoyama S, Shinada T, Kobayashi N, Shirakabe A, Tomita K, Kitamura M, Kurihara O, Takahashi Y: Acute kidney injury and outcomes in acute decompensated heart failure: Evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 12: 32–37, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM: Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, Adams KF, Jr: Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial. Eur J Heart Fail 9: 1064–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mielniczuk LM, Tsang SW, Desai AS, Nohria A, Lewis EF, Fang JC, Baughman KL, Stevenson LW, Givertz MM: The association between high-dose diuretics and clinical stability in ambulatory chronic heart failure patients. J Card Fail 14: 388–393, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kazory A, Ross EA: Emerging therapies for heart failure: Renal mechanisms and effects. Heart Fail Rev 17: 1–16, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Guazzi MD, Agostoni P, Perego B, Lauri G, Salvioni A, Giraldi F, Matturri M, Guazzi M, Marenzi G: Apparent paradox of neurohumoral axis inhibition after body fluid volume depletion in patients with chronic congestive heart failure and water retention. Br Heart J 72: 534–539, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P: Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol 38: 963–968, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Agostoni P, Marenzi G, Lauri G, Perego G, Schianni M, Sganzerla P, Guazzi MD: Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: Failure of furosemide to provide the same result. Am J Med 96: 191–199, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini GF, Valente S, Ciaccheri M, Castelli G, Romano SM: Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure: The ULTRADISCO study. Eur J Heart Fail 13: 337–346, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Giglioli C, Landi D, Gensini GF, Valente S, Cecchi E, Scolletta S, Chiostri M, Romano SM: Cardiac efficiency improvement after slow continuous ultrafiltration is assessed by beat-to-beat minimally invasive monitoring in congestive heart failure patients: A preliminary report. Blood Purif 29: 44–51, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Bart BA: Treatment of congestion in congestive heart failure: Ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failure. Circ Heart Fail 2: 499–504, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators: Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 297: 1319–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, Mackedanz S, Sobotka PA, Schollmeyer M, Goldsmith SR: Ultrafiltration versus usual care for hospitalized patients with heart failure: The Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol 46: 2043–2046, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA; UNLOAD Trial Investigators: Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 49: 675–683, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Costanzo MR, Saltzberg MT, Jessup M, Teerlink JR, Sobotka PA; Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) Investigators: Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: Results from UNLOAD. J Card Fail 16: 277–284, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Agostoni PG, Marenzi GC, Pepi M, Doria E, Salvioni A, Perego G, Lauri G, Giraldi F, Grazi S, Guazzi MD: Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol 21: 424–431, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Pepi M, Marenzi GC, Agostoni PG, Doria E, Barbier P, Muratori M, Celeste F, Guazzi MD: Sustained cardiac diastolic changes elicited by ultrafiltration in patients with moderate congestive heart failure: Pathophysiological correlates. Br Heart J 70: 135–140, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winton FR: The influence of venous pressure on the isolated mammalian kidney. J Physiol 72: 49–61, 1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL: Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53: 582–588, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Guglin M, Rivero A, Matar F, Garcia M: Renal dysfunction in heart failure is due to congestion but not low output. Clin Cardiol 34: 113–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, Tang WH: Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail 14: 508–514, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Silverstein ME, Ford CA, Lysaght MJ, Henderson LW: Treatment of severe fluid overload by ultrafiltration. N Engl J Med 291: 747–751, 1974 [DOI] [PubMed] [Google Scholar]
- 36.Sharma A, Hermann DD, Mehta RL: Clinical benefit and approach of ultrafiltration in acute heart failure. Cardiology 96: 144–154, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Canaud B, Leblanc M, Leray-Moragues H, Delmas S, Klouche K, Beraud JJ: Slow continuous and daily ultrafiltration for refractory congestive heart failure. Nephrol Dial Transplant 13[Suppl 4]: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT: Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 9: 227–231, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Costanzo MR, Saltzberg M, O’Sullivan J, Sobotka P: Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol 46: 2047–2051, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E; Heart Failure Clinical Research Network: Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367: 2296–2304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dev S, Shirolkar SC, Stevens SR, Shaw LK, Adams PA, Felker GM, Rogers JG, O’Connor CM: Reduction in body weight but worsening renal function with late ultrafiltration for treatment of acute decompensated heart failure. Cardiology 123: 145–153, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Patarroyo M, Wehbe E, Hanna M, Taylor DO, Starling RC, Demirjian S, Tang WH: Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 60: 1906–1912, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Barsuk JH, Gordon RA, Cohen ER, Cotts WG, Malkenson D, Yancy CW, Williams MV: A diuretic protocol increases volume removal and reduces readmissions among hospitalized patients with acute decompensated heart failure. Congest Heart Fail 19: 53–60, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Kazory A, Ross EA: Ultrafiltration for decompensated heart failure: Renal implications. Heart 95: 1047–1051, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Liang KV, Hiniker AR, Williams AW, Karon BL, Greene EL, Redfield MM: Use of a novel ultrafiltration device as a treatment strategy for diuretic resistant, refractory heart failure: Initial clinical experience in a single center. J Card Fail 12: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Bartone C, Saghir S, Menon SG, Brosmer J, Kereiakes DJ, Mazur W, Chung ES: Comparison of ultrafiltration, nesiritide, and usual care in acute decompensated heart failure. Congest Heart Fail 14: 298–301, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Rogers HL, Marshall J, Bock J, Dowling TC, Feller E, Robinson S, Gottlieb SS: A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail 14: 1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Bradley SM, Levy WC, Veenstra DL: Cost-consequences of ultrafiltration for acute heart failure: A decision model analysis. Circ Cardiovasc Qual Outcomes 2: 566–573, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kazory A, Ejaz AA, Ross EA: Ultrafiltration for heart failure: How fast should we move? Am Heart J 157: 205–207, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Kazory A, Bellamy FB, Ross EA: Ultrafiltration for acute decompensated heart failure: Financial implications. Int J Cardiol 154: 246–249, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Kazory A, Ross EA: Contemporary trends in the pharmacological and extracorporeal management of heart failure: A nephrologic perspective. Circulation 117: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Kazory A: Ultrafiltration does not affect certain predictors of outcome in heart failure. Int J Cardiol 143: 1–3, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Hanna MA, Tang WH, Teo BW, O’Neill JO, Weinstein DM, Lau SM, Van Lente F, Starling RC, Paganini EP, Taylor DO: Extracorporeal ultrafiltration vs. conventional diuretic therapy in advanced decompensated heart failure. Congest Heart Fail 18: 54–63, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Kazory A: Need for a unified decision-making tool for ultrafiltration therapy in heart failure; call for action. Am Heart J 159: 505–507, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Dahle TG, Blake D, Ali SS, Olinger CC, Bunte MC, Boyle AJ: Large volume ultrafiltration for acute decompensated heart failure using standard peripheral intravenous catheters. J Card Fail 12: 349–352, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Jaski BE, Romeo A, Ortiz B, Hoagland PM, Stone M, Glaser D, Thomas L, Walsh C, Smith SC, Jr: Outcomes of volume-overloaded cardiovascular patients treated with ultrafiltration. J Card Fail 14: 515–520, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW: 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: e391–e479, 2009 [DOI] [PubMed] [Google Scholar]
- 58.McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, Estrella-Holder E, Giannetti N, Grzeslo A, Harkness K, Howlett JG, Kouz S, Leblanc K, Mann E, Nigam A, O’Meara E, Rajda M, Steinhart B, Swiggum E, Le VV, Zieroth S, Arnold JM, Ashton T, D’Astous M, Dorian P, Haddad H, Isaac DL, Leblanc MH, Liu P, Rao V, Ross HJ, Sussex B: The 2012 Canadian Cardiovascular Society heart failure management guidelines update: Focus on acute and chronic heart failure. Can J Cardiol 29: 168–181, 2013 [DOI] [PubMed] [Google Scholar]
- 59.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines: ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33: 1787–1847, 2012 [DOI] [PubMed] [Google Scholar]