Summary
Cardiorenal syndromes (CRSs) with bidirectional heart-kidney signaling are increasingly being recognized for their association with increased morbidity and mortality. In acute CRS, recognition of the importance of worsening kidney function complicating management of acute decompensated heart failure has led to the examination of this specific outcome in the context of acute heart failure clinical trials. In particular, the role of fluid overload and venous congestion has focused interest in the most effective use of diuretic therapy to relieve symptoms of heart failure while at the same time preserving kidney function. Additionally, many novel vasoactive therapies have been studied in recent years with the hopes of augmenting cardiac function, improving symptoms and patient outcomes, while maintaining or improving kidney function. Similarly, recent advances in our understanding of the pathophysiology of chronic CRS have led to reanalysis of kidney outcomes in pivotal trials in chronic congestive heart failure, and newer trials are including changes in kidney function as well as kidney injury biomarkers as prospectively monitored and adjudicated outcomes. This paper provides an overview of some new developments in the pharmacologic management of acute and chronic CRS, examines several reports that illustrate a key management principle for each subtype, and discusses opportunities for future research.
Acute Cardiorenal Syndrome
Acute cardiorenal syndrome (CRS) is simply defined as acute worsening of cardiac function leading to renal dysfunction (1). More specifically, it is the development of AKI in the setting of hospitalization for acute decompensated heart failure or cardiogenic shock, and clinical phenotypes range from acute pulmonary edema with hypertension through severe peripheral fluid overload to cardiogenic shock and hypotension (2,3). AKI is itself defined as an increase in serum creatinine of ≥0.3 mg/dl (≥26.4 μmol/L), an increase in creatinine to ≥1.5- to 2-fold from baseline, and/or urine output <0.5 ml/kg per hour for >6 hours (4). Although many previous heart failure studies have not used this specific definition, the majority have used the term “worsening renal function,” with a cutoff of increase in serum creatinine of ≥0.3 mg/dl, a value at which the odds ratio for mortality significantly increases (5).
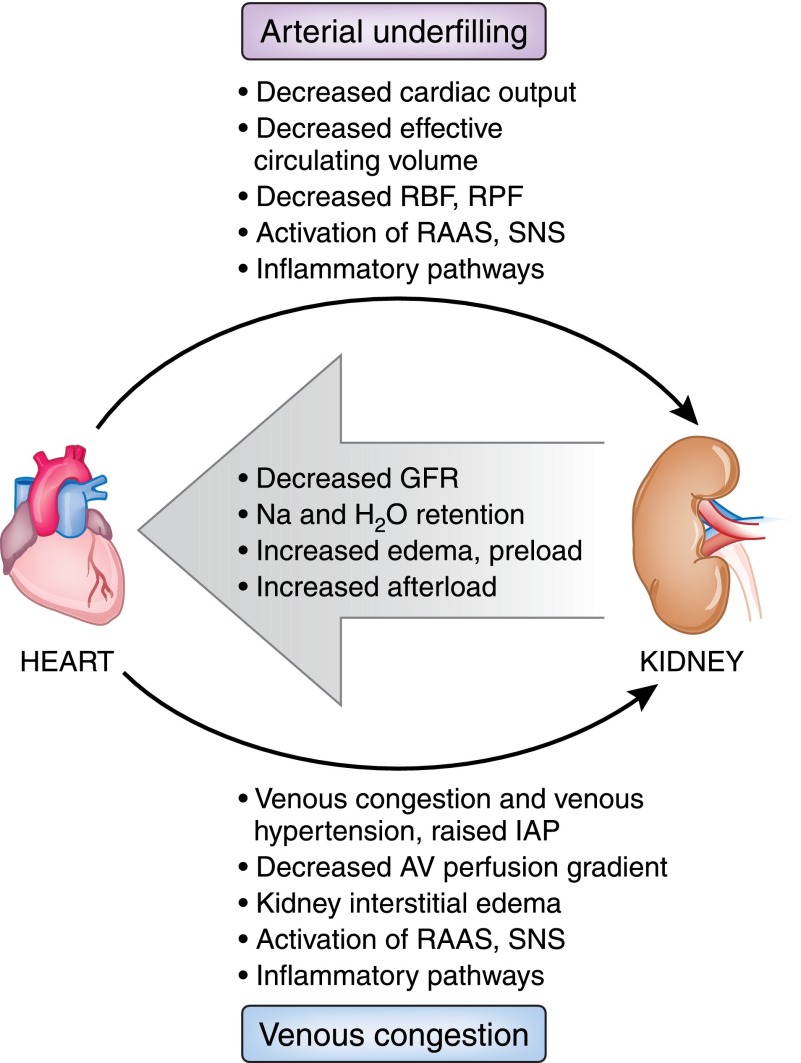
The conventional explanation for acute CRS has revolved around hypotension with decreased cardiac output, neurohormonal activation, and renal hypoperfusion. With the observation that many patients hospitalized with evidence of acute CRS have preserved or even elevated BP and normal left ventricular ejection fraction (6), some studies have noted the importance of renal congestion or renal venous hypertension and raised intra-abdominal pressure (7,8). Others have identified changes in systolic BP rather than cardiac index or right atrial pressure as being predictive of changes in kidney function (9). Figure 1 presents a simple schematic depicting these hemodynamic relationships.
Figure 1.
Dual hemodynamic pathways for acute cardiorenal syndrome. In this schematic, impaired forward flow and decreased effective circulating volume, as would be seen in severe systolic heart failure or cardiogenic shock, leads to arterial underfilling and activation of neurohormonal and inflammatory pathways. Autoregulation of GFR fails and kidney function declines, leading to worsening fluid retention, preload, and afterload. In a separate process, venous congestion and high right-sided pressure predominate, as would be seen in heart failure with preserved ejection fraction or isolated right heart failure. This also leads to decreased kidney function, worsening of fluid retention, and increased preload and afterload. These pathways are not mutually exclusive and often coexist in the same patient to varying degrees. AV, arteriovenous; IAP, intra-abdominal pressure; RAAS, renin-angiotensin-aldosterone system; RBF, renal blood flow; RPF, renal plasma flow; SNS, sympathetic nervous system.
Studies on early management and prevention of acute CRS have focused on strategies to reduce congestion using boluses or infusions of intravenous diuretics and the use of vasodilators, with somewhat mixed results. Table 1 (adapted from House et al. [10]) lists some of the more commonly used agents in both acute and chronic CRS, along with indications, expected actions, and potential problems. Therapies listed under categories for both acute and chronic CRS are not meant to be mutually exclusive because patients often move between these categories.
Table 1.
Treatments in management of acute and chronic cardiorenal syndromes
| Drug | Indication | Intended Action and Effects | Potential Adverse Effects and Problems |
| Diuretics | |||
| Loop diuretics | Acute CRS | Natriuresis; decrease in fluid overload, sodium, and water elimination | Neurohormonal activation, electrolyte imbalance, and worsening kidney function |
| Chronic CRS | Control of hypertension, diuresis, and extracellular fluid volume | Worsening kidney function, hyperuricemia, electrolyte imbalance, diuretic resistance | |
| ACE inhibitors or ARBs | Acute CRS | Management of acute low cardiac output, afterload and preload reduction | Decrease in GFR and increase in serum creatinine, hypotension, hyperkalemia |
| Chronic CRS | Management of CHF, increase in cardiac output (in low-output states) | Hypotension, hyperkalemia, increase in serum creatinine; often held if creatinine increased by >30% | |
| Aldosterone blockade | |||
| Spironolactone, eplerenone | Acute and chronic CRS | Control of hypertension, diuresis, and extracellular fluid volume | Hyperkalemia (especially with ACE inhibitors or ARBs), worsening kidney function |
| β-Blockers | Acute CRS | Not recommended or extreme caution required in low-cardiac-output state | Cardiogenic shock, worsening kidney function |
| Chronic CRS | Control of hypertension or arrhythmias, management of ischemic heart disease, possible favorable renal effects (carvedilol) | Toxic effects due to accumulation of certain agents in patients with decreased GFR (atenolol, sotalol) | |
| Vasodilators | |||
| Nitroglycerin | Acute CRS | Venodilation, decreased cardiac ischemia, decreased afterload | Hypotension, tolerance |
| Chronic CRS | With hydralazine, used as an alternative to RAAS blockade | Hypotension, tolerance | |
| Nitroprusside | Acute CRS | Arterial and venous dilation, decreased preload, afterload | Hypotension, thiocyanate toxicity (particularly with decreased GFR) |
| Nesiritide | Acute CRS | Decrease in preload, afterload, and pulmonary vascular resistance; increase in cardiac output in ADHF | Possible decrease in kidney function, hypotension |
| Inotropes | Acute and chronic CRS | Extreme cases of low cardiac output, increase cardiac output | Possible increase in myocardial injury, arrhythmias, worsened outcomes |
| Levosimendan (not available in North America) | Acute and chronic CRS | Increase cardiac output, improve hemodynamics, renal perfusion, and diuresis | Hypotension, unclear efficacy |
Adapted from reference 10, with permission. CRS, cardiorenal syndrome; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CHF, congestive heart failure; RAAS, renin-angiotensin-aldosterone system; ADHF, acute decompensated heart failure.
Since the early 1960s (11), loop diuretics such as furosemide have been recognized as potent natriuretic agents and venodilators and have served as a mainstay of therapy for the patient presenting with signs and symptoms of acute decompensated heart failure and congestion, despite a dearth of clinical trial evidence for safety and efficacy. With use of diuretics, one strives to deplete the intravascular component of the extracellular fluid compartment at a rate that allows for adequate refilling from the interstitial space to avoid precipitous decreases in blood pressure and tissue hypoperfusion. This rate probably varies widely but was found in one study to be in excess of 600–700 ml per hour in the first hours of aggressive management of patients with severe congestion receiving ultrafiltration and high-dose diuretics for decompensated heart failure (12). The complexity of this relationship between decongestion and acute CRS is evident in studies such as that of Testani et al., who found that hemoconcentration during management of decompensated heart failure was associated with not only worsening renal function but also improved 180-day survival (13). With the uncertainty about diuretic strategies raised by previous observational studies, it is only recently that clinical trials in patients with acute heart failure have been conducted to examine the most effective and safe way to use these agents, in terms of dose and administration as bolus versus infusion.
In the Diuretic Optimization Strategies Evaluation (DOSE-HF) study, Felker and colleagues (14) enrolled 308 patients with acute decompensated heart failure and a serum creatinine level <3.0 mg/dl (265.2 μmol/L) and used a 2×2 factorial design comparing low-dose versus high-dose furosemide, given as an intravenous bolus (every 12 hours) or a continuous infusion. Low dose was considered as the usual prehospitalization oral dose given intravenously, and high dose was approximately 2.5-fold greater. On the basis of the primary outcome of the study, the authors concluded that there were no significant differences in patients’ global assessment of their symptoms or in the change in renal function when the dose or administration strategy was compared. Although on the surface it appears to be a negative or neutral trial, based on the co-primary endpoints, the data warrant deeper scrutiny. It is true that the global visual analogue scale score did not differ between dosages, but there was a trend toward better symptom control in the high-dose group (P=0.06). In addition, looking specifically at several secondary endpoints, the area under the curve for dyspnea scores at 72 hours, weight change at 72 hours, and net fluid loss at 72 hours improved significantly, with a numerically greater change in N-terminal pro-brain natriuretic peptide (NT-proBNP) (P=0.06), all in favor of the high-dose group. Furthermore, the low-dose group was much less likely to be able to convert from intravenous to oral formulation at 48 hours (17% versus 31%; P<0.001) and was more likely to require a 50% increase in dose at 48 hours (24% versus 9%; P=0.003). In terms of bolus versus continuous infusion, the bolus group received a median dose of study medication of 518 mg versus 406 mg at 72 hours (P=0.008) and was twice as likely to require a dose increase as the infusion group (21% versus 11%; P=0.01).
Of note, these improved clinical outcomes did not seem to come at the expense of renal function, as measured by change in creatinine from baseline to 72 hours as a co-primary endpoint, although a secondary outcome of increase in serum creatinine of ≥0.3 mg/dl was observed with greater frequency in the high-dose group, which does meet the definition of AKI. However, on inspection of the supplementary data (Figure 2, adapted from [14]) for changes in serum creatinine and cystatin C over 60 days (all P>0.05), there seems to be no appreciable difference in kidney function change between any of the groups.
Figure 2.
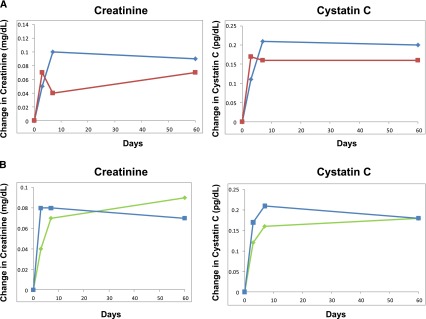
Changes in renal function over time. (A) For intermittent furosemide injection every 12 hours (blue line) versus continuous furosemide infusion (red line). (B) For high-dose furosemide (blue line) versus low-dose furosemide (green line). Modified from reference 14, with permission. P>0.05 for all timepoints.
Additional insights into effective use of diuretics can be found in the recently published comparison of a stepped pharmacologic approach versus ultrafiltration in patients with acute decompensated heart failure and worsening kidney function (15). In this study, called the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), the diuretic strategy yielded similar outcomes in terms of fluid loss and symptom control but had better renal outcomes. At 96 hours, the mean change in the serum creatinine level was a decrease of 0.04±0.53 mg/dl (3.5±46.9 μmol/L) in the stepped pharmacologic therapy group, compared with an increase of 0.23±0.70 mg/dl (20.3±61.9 μmol/L) in the ultrafiltration group (P=0.003). Mortality or rehospitalizations did not differ, but the ultrafiltration group experienced more serious adverse events.
The specifics of the stepped pharmacologic approach may be found in the supplementary materials provided with that report, but in brief, patients were given a bolus followed by infusion of intravenous furosemide according to their usual prehospitalization dose, with the total daily dose at least two-fold greater than baseline. The investigators then targeted urine output of approximately 3–5 L per day, and amounts greater or less than this target would allow the dosage of diuretic to be decreased or increased, respectively, according to a fairly simple grid. Metolazone was added to augment diuresis. Vasodilators or inotropes could be added on the basis of failure to achieve clinical targets and depending on BP and ejection fraction.
Putting the results of these trials into perspective, it would seem that a strategy for care of patients with acute decompensated heart failure of accelerating loop diuretic dose, preferentially using high-dose infusion over bolus administration, and adding the potent thiazide metolazone, while reserving vasodilators, inotropes, and salvage ultrafiltration for more refractory cases, would be expected to improve symptoms and decongest patients without necessarily compromising kidney function any further. Noteworthy is the fact that only approximately half of the patients in the CARRESS-HF study were receiving angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers before hospitalization. These agents, by virtue of their preferential effects on the efferent arteriole, are often reduced or withheld during the management of acute decompensated heart failure or acute CRS because they impair the maintenance of GFR, particularly in states of decreased effective circulating volume, and may also contribute to hyperkalemia. Continuing these agents, where possible, with close monitoring of renal function and potassium would be desirable to reap their proven benefits in terms of cardiovascular outcomes and mortality.
Because an important pathophysiologic component to reduction in GFR in relation to heart failure involves the persistence of renal vasoconstriction related to tubuloglomerular feedback (16) and the action of vasoactive peptides, such as adenosine and endothelin (17,18), as well as a diminished response to endogenous natriuretic peptides, there has been great interest in using pharmacologic agents to target these maladaptive renal responses. Several novel approaches have shown promising results in preclinical or early clinical trials in terms of clinical outcomes, including acute CRS. Unfortunately, larger-scale randomized controlled trials have been disappointing for agents that target receptors for endothelin (19–21), adenosine (22), and vasopressin (23). Likewise, use of pharmacologic doses of the natriuretic peptide nesiritide in acute decompensated heart failure failed to provide significant clinical benefits and did not prevent acute CRS (24). When patients have low BP and poor renal perfusion, positive inotropes, such as dobutamine or phosphodiesterase inhibitors, are advocated (3), although limited data demonstrate efficacy and safety for prevention or treatment of acute CRS. In fact, the use of inotropes may accelerate some harmful processes, such as ischemia or arrhythmia; milrinone, for example, had a higher incidence of hypotension, more arrhythmias, and no benefit on mortality or hospitalization in patients with acute decompensated heart failure (25). Levosimendan, a phosphodiesterase inhibitor with calcium sensitizing activity, has shown inconsistent results for prevention and treatment of acute CRS (26–28) and is currently unavailable in North America.
One pharmacologic agent that has generated recent enthusiasm for the role of vasoactive drugs in the management of acute decompensated heart failure is a recombinant human relaxin-2 called serelaxin. Relaxin is an endogenous peptide that plays a role in the maternal circulatory changes associated with pregnancy and has effects on arterial compliance, cardiac output, and renal blood flow that could counterbalance the maladaptive changes seen in acute CRS. In the RELAXin in Acute Heart Failure (RELAX-AHF) trial (29), investigators compared standard care plus 48-hour intravenous infusions of placebo or serelaxin (30 μg/kg per day) within 16 hours of presentation with acute heart failure in >1100 patients. They demonstrated statistically significant improvement in visual analogue scale area under the curve dyspnea scores and fewer deaths at 180 days (hazard ratio, 0.63; P=0.019), although the latter was a secondary outcome. More patients receiving placebo had adverse events related to renal impairment compared with serelaxin (placebo, 51 patients [9%]; serelaxin, 32 [6%]; P=0.03).
In a subsequent publication (30), the investigators assessed kidney function in the study in more detail. Blood was sampled at days 2, 5, and 14 for creatinine and cystatin-C, and acute CRS was defined as increases in serum creatinine and plasma cystatin-C values of 0.3 mg/dl and 0.3 mg/L, respectively. Relative changes from baseline for both analytes were more favorable in the serelaxin group, and fewer patients receiving serelaxin reached the aforementioned thresholds. Specifically, at day 2, 19.8% treated with placebo versus 10.9% treated with serelaxin had experienced an increase in serum creatinine ≥0.3 mg/dL (P<0.001).
Notwithstanding improvements in our understanding and management of acute heart failure and acute CRS, the aforementioned studies also highlight a discouraging reality, as eloquently described in a recent editorial by Fonarow (31). He observed that patients with heart failure being cared for in clinical trial centers with highly experienced clinicians and staff still experience unacceptably high rates of death, rehospitalization, or emergency department visits, irrespective of treatment assignment, and concluded that “clearly, there is a crucial need to develop new agents and effective strategies for this patient population” (31).
Chronic Cardiorenal Syndrome
Chronic CRS is defined as chronic abnormalities in cardiac function leading to renal dysfunction (1). Examples of this would include progressive CKD resulting from congenital or acquired chronic congestive heart failure or from repeated episodes of acute CRS. Animal studies and human observational evidence demonstrate that chronic heart failure leads to varying degrees of albuminuria/proteinuria, progressive decline in GFR, and expression of renal injury biomarkers (32,33). Key to the pathophysiology is chronic activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system as a result of arterial underfilling and/or venous congestion. Inflammatory pathways are activated, leading to progressive glomerulosclerosis and tubular fibrosis and atrophy (34).
Despite our current recognition of the importance of the RAAS in progression of CKD, unfortunately many of the pivotal trials of angiotensin blockade (with ACE inhibitors or ARBs) for congestive heart failure examined renal function as a safety endpoint, and there is a paucity of information on clinically important long-term renal endpoints in these studies. An additional shortcoming of the existing data is the difficulty in using changes in the surrogate outcome of serum creatinine (or estimated GFR) because hemodynamic (and potentially reversible) changes in GFR are a known feature of angiotensin blockade, due to the role of efferent arteriolar vasoconstriction in the regulation of glomerular pressure and filtration.
In the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), randomization to enalapril was associated with a marked reduction in mortality and improvement in symptoms (35). A post hoc analysis of the data (36) showed that patients treated with enalapril had an early rise in serum creatinine to about 10%–15% above baseline (typically within the first 2 weeks). After this, the rate of increase was much slower and similar to that seen with placebo, consistent with the aforementioned hemodynamic effect. Doubling of creatinine was reported in 11% of enalapril recipients compared with 3% of controls, although intercurrent illness or hypotension was the explanation for most of these instances. In most of these patients, the creatinine decreased to within 30% of baseline; this includes many patients who continued to receive enalapril, albeit at a reduced dose.
In Studies of Left Ventricular Dysfunction (SOLVD) (37), enalapril decreased the incidence of symptomatic heart failure and hospitalizations. However, the investigators demonstrated a 33% greater likelihood of experiencing an increase in creatinine of ≥0.5 mg/dl (44 μmol/L) from baseline in the enalapril group (P=0.003) and found in multivariable analysis that age, decreased ejection fraction, diabetes, and diuretics were also associated with higher risk, whereas β-blockers appeared to be protective (38). The study provided no detail on more serious outcomes, such as doubling of creatinine or ESRD. A more recent post hoc analysis of SOLVD study data using estimated GFR found that in 22% of all patients, regardless of treatment assignment, estimated GFR decreased by at least 20% in a follow-up period just short of 3 years, indicating how common progressive CKD is in this population (39). In the multivariable analysis, enalapril was not associated with rapid decline of GFR, but the analysis did not indicate whether RAAS blockade was protective for important long-term renal outcomes.
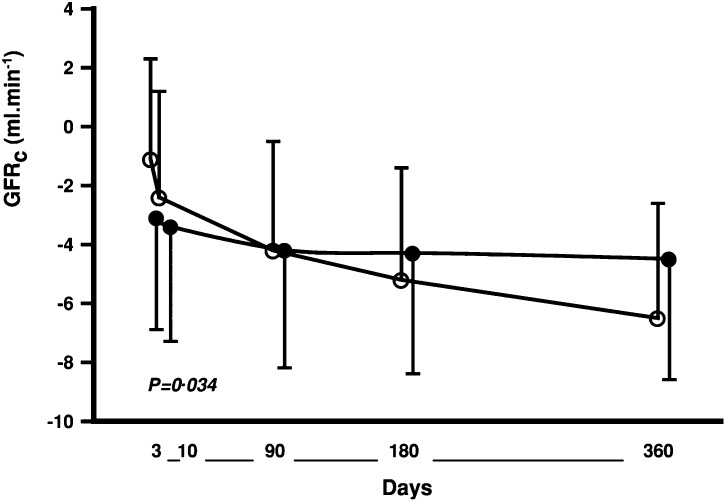
The Captopril and Thrombolysis Study (CATS) randomly assigned 298 patients with a first anterior-wall myocardial infarction to captopril or placebo, initiated immediately upon completion of the streptokinase infusion (40). This study, although not in patients with heart failure per se, did demonstrate a more rapid decline in GFR in the subsequent year after infarction than would ordinarily be expected, as well as the development of heart failure in a significant proportion of patients. The investigators found an abrupt early decline of GFR in the captopril group that was greater than in the control group; subsequently, the rate of decline leveled in the captopril group but continued to steadily decrease in the control group (Figure 3 from reference 40). This resulted in a GFR decline of 5.5 ml/min per 1.73 m2 in the first year in the placebo group, compared with a decrease of only 0.5 ml/min per 1.73 m2 in the ACE inhibitor group (P<0.05). The study did not report clinically important long-term renal outcomes.
Figure 3.
Loss of calculated GFR (GFRc) for groups treated with placebo (open circle; n=104) or captopril (closed circle; n=121) versus baseline GFRc (day 0) over the first year after myocardial infarction. Values are expressed in mean ± SEM. Reproduced from reference 40, with permission.
Similar difficulties in the interpretation of aldosterone antagonism in heart failure and its effect on renal function may be found in the literature. For instance, the Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) demonstrated that the addition of the low-dose mineralocorticoid receptor antagonist eplerenone to standard medical therapy for patients with acute myocardial infarction and heart failure with reduced ejection fraction improved mortality by 15%. Patients assigned to eplerenone had a decline in estimated GFR of 1.4 ml/min per 1.73 m2 compared with placebo (P=0.0001), a difference that emerged within the first month and persisted throughout the study, with similar GFR slopes after the first month (41). Although eplerenone was associated with early worsening of GFR, there was no significant interaction between worsening GFR and the beneficial effect of eplerenone on cardiovascular death or hospitalization. The study provided no data on doubling of creatinine or ESRD.
These examples highlight the difficulty of examining a surrogate, such as change in creatinine or estimated GFR, to assess whether interruption of RAAS is able to block the pathophysiologic changes that lead to progressive CKD complicating heart failure. They also demonstrate the limitations of post hoc analysis because many of the renal data were incomplete. With increasing recognition of the importance of the emergence or progression of CKD in this population, ongoing clinical trials are capturing renal data prospectively, and some are incorporating injury biomarkers in their analysis.
Although it is unethical for investigators to ever study angiotensin blockade against placebo in heart failure trials, given the overwhelming evidence of mortality and morbidity benefit, an ongoing trial will certainly shed much-needed light on this issue. The PARADIGM-HF study (clinicaltrials.gov identifier NCT01035255) is comparing the efficacy and safety of LCZ696 versus enalapril on morbidity and mortality in 8000 patients with chronic heart failure (New York Heart Association class II–IV and ejection fraction ≤35%), with a primary outcome of cardiovascular death or heart failure hospitalization over 4 years of study. The investigators are prospectively collecting renal function data with time to renal dysfunction as a predefined secondary outcome. Because LCZ696 combines the ARB actions of valsartan with the neprilysin inhibitor prodrug AHU377 in a single molecule (enhancing the actions of endogenous BNP), this provides an excellent opportunity to examine this combined approach of RAAS blockade and vasodilator against RAAS blockade alone, while furthering our understanding of chronic CRS.
A smaller phase 2 study of LCZ696 versus valsartan alone in patients with heart failure and preserved ejection fraction, the Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT), found that LCZ696 reduced NT-proBNP to a greater extent, reduced left atrial size and improved remodeling, lowered blood pressure, and improved heart failure symptoms (42). At the same time, the investigators showed preservation of estimated GFR to a greater extent, with a 36-week decline in the LCZ696 group of 1.6 ml/min per 1.73 m2 versus 5.2 ml/min per 1.73 m2 in the valsartan group (P=0.007).
Finally, a small study of BAY 94–8862 in patients with heart failure and CKD is taking the investigation of chronic CRS a step further by examining the effects of this nonsteroidal, mineralocorticoid receptor antagonist on biomarkers of cardiac and renal function and injury (43). The MinerAlocorticoid Receptor Antagonist Tolerability Study (ARTS) will be measuring BNP, NT-proBNP, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, cystatin-C and other biomarkers and should inform future chronic CRS trials.
Summary
Recent focus on the importance of abrupt changes in kidney function during episodes of acute decompensated heart failure (acute CRS) and the role of chronic congestive heart failure as a contributor to progressive CKD (chronic CRS) is leading to improved diagnostic tools and pharmacologic treatments for these conditions. Whether it is the development of novel therapies, such as recombinant relaxin or neprilysin inhibition, or a better understanding of optimal use of existing therapies, such as diuretics and RAAS blockade, the last few years have seen major steps forward in the management of CRS, and future studies hold significant promise.
Disclosures
A.A.H. reports receiving honoraria from Gambro and research support from Pfizer.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.House AA, Anand I, Bellomo R, Cruz D, Bobek I, Anker SD, Aspromonte N, Bagshaw S, Berl T, Daliento L, Davenport A, Haapio M, Hillege H, McCullough P, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Ronco C; Acute Dialysis Quality Initiative Consensus Group: Definition and classification of Cardio-Renal Syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 25: 1416–1420, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW; American College of Cardiology Foundation; American Heart Association: 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53: e1–e90, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K; ESC Committee for Practice Guidelines (CPG): ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 10: 933–989, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL: Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J Card Fail 13: 599–608, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW: Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol 101: 1151–1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL: Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9: 872–878, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH: Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J Am Coll Cardiol 51: 300–306, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, Tang WH: Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: The importance of blood pressure reduction during treatment. Eur J Heart Fail 15: 433–440, 2013 [DOI] [PubMed] [Google Scholar]
- 10.House AA, Haapio M, Lassus J, Bellomo R, Ronco C: Therapeutic strategies for heart failure in cardiorenal syndromes. Am J Kidney Dis 56: 759–773, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kleinfelder H: Experimental studies and clinical trials of a new diuretic. Dtsch Med Wochenschr 88: 1695–1702, 1963 [DOI] [PubMed] [Google Scholar]
- 12.Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P: Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol 38: 963–968, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP: Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122: 265–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM; NHLBI Heart Failure Clinical Research Network: Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364: 797–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E; Heart Failure Clinical Research Network: Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367: 2296–2304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margulies KB, Burnett JC, Jr: Atrial natriuretic factor modulates whole kidney tubuloglomerular feedback. Am J Physiol 259: R97–R101, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Neuhofer W, Pittrow D: Role of endothelin and endothelin receptor antagonists in renal disease. Eur J Clin Invest 36[Suppl 3]: 78–88, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Vallon V, Miracle C, Thomson S: Adenosine and kidney function: Potential implications in patients with heart failure. Eur J Heart Fail 10: 176–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ: The endothelin system as a therapeutic target in cardiovascular disease: Great expectations or bleak house? Br J Pharmacol 153: 1105–1119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coletta AP, Cleland JG: Clinical trials update: highlights of the scientific sessions of the XXIII Congress of the European Society of Cardiology—WARIS II, ESCAMI, PAFAC, RITZ-1 and TIME. Eur J Heart Fail 3: 747–750, 2001 [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I; VERITAS Investigators: Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 298: 2009–2019, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O’Connor CM, Givertz MM: Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). J Am Coll Cardiol 57: 1899–1907, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators: Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA 297: 1332–1343, 2007 [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM: Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365: 32–43, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M; Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators: Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 287: 1541–1547, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Landoni G, Biondi-Zoccai G, Greco M, Greco T, Bignami E, Morelli A, Guarracino F, Zangrillo A: Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Crit Care Med 40: 634–646, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M; SURVIVE Investigators: Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE Randomized Trial. JAMA 297: 1883–1891, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I: Levosimendan improves renal function in patients with acute decompensated heart failure: Comparison with dobutamine. Cardiovasc Drugs Ther 21: 431–435, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M; RELAXin in Acute Heart Failure (RELAX-AHF) Investigators: Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 381: 29–39, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR; RELAX-AHF Investigators: Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol 61: 196–206, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC: Comparative effectiveness of diuretic regimens. N Engl J Med 364: 877–878, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A: Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 125: 1402–1413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haapio M, House AA, de Cal M, Cruz DN, Lentini P, Giavarina D, Fortunato A, Menghetti L, Salgarello M, Lupi A, Soffiati G, Fontanelli A, Zanco P, Ronco C: Heart-kidney biomarkers in patients undergoing cardiac stress testing. Int J Nephrol 2011: 425923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka M, Yoshida H, Furuhashi M, Togashi N, Koyama M, Yamamoto S, Yamashita T, Okazaki Y, Ishimura S, Ota H, Hasegawa T, Miura T: Deterioration of renal function by chronic heart failure is associated with congestion and oxidative stress in the tubulointerstitium. Intern Med 50: 2877–2887, 2011 [DOI] [PubMed] [Google Scholar]
- 35.The CONSENSUS Trial Study Group: Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 316: 1429–1435, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Ljungman S, Kjekshus J, Swedberg K: Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial). Am J Cardiol 70: 479–487, 1992 [DOI] [PubMed] [Google Scholar]
- 37.The SOLVD Investigators: Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med 327: 685–691, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Knight EL, Glynn RJ, McIntyre KM, Mogun H, Avorn J: Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: Results from the studies of left ventricular dysfunction (SOLVD). Am Heart J 138: 849–855, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, Sarnak MJ, Levin A: Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol 17: 244–253, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Hillege HL, van Gilst WH, van Veldhuisen DJ, Navis G, Grobbee DE, de Graeff PA, de Zeeuw D; CATS Randomized Trial: Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: The CATS randomized trial. Eur Heart J 24: 412–420, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, Lamiral Z, Dobre D, Pitt B, Zannad F: Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: Insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation 125: 271–279, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators: The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 380: 1387–1395, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, Nowack C, Kolkhof P, Kim SY, Zannad F: Rationale and design of ARTS: A randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail 14: 668–675, 2012 [DOI] [PubMed] [Google Scholar]