Abstract
Background and objectives
Since 1998, 35% of kidney transplants in the United States have been derived from living donors. Research suggests minimal long-term health consequences after donation, but comprehensive studies are limited. The primary objective was to evaluate trends in comorbidity burden and complications among living donors.
Design, setting, participants, & measurements
The National Inpatient Sample (NIS) was used to identify donors from 1998 to 2010 (n=69,117). Comorbid conditions, complications, and length of stay during hospitalization were evaluated. Outcomes among cohorts undergoing appendectomies, cholecystectomies and nephrectomy for nonmetastatic carcinoma were compared, and sample characteristics were validated with the Scientific Registry of Transplant Recipients (SRTR). Survey regression models were used to identify risk factors for outcomes.
Results
The NIS captured 89% (69,117 of 77,702) of living donors in the United States. Donor characteristics were relatively concordant with those noted in SRTR (mean age, 40.1 versus 40.3 years [P=0.18]; female donors, 59.0% versus 59.1% [P=0.13]; white donors, 68.4% versus 69.8% [P<0.001] for NIS versus SRTR). Incidence of perioperative complications was 7.9% and decreased from 1998 to 2010 (from 10.1% to 7.6%). Men (adjusted odds ratio [AOR], 1.37; 95% confidence interval [CI], 1.20 to 1.56) and donors with hypertension (AOR, 3.35; 95% CI, 2.24 to 5.01) were more likely to have perioperative complications. Median length of stay declined over time (from 3.7 days to 2.5 days), with longer length of stay associated with obesity, depression, hypertension, and pulmonary disorders. Presence of depression (AOR, 1.08; 95% CI, 1.04 to 1.12), hypothyroidism (AOR, 1.07; 95% CI, 1.04 to 1.11), hypertension (AOR, 1.38; 95% CI, 1.27 to 1.49), and obesity (AOR, 1.07; 95% CI, 1.03 to 1.11) increased over time. Complication rates and length of stay were similar for patients undergoing appendectomies and cholecystectomies but were less than those with nephrectomies for carcinoma.
Conclusions
The NIS is a representative sample of living donors. Complications and length of stay after donation have declined over time, while presence of documented comorbid conditions has increased. Patients undergoing appendectomy and cholecystectomy have similar outcomes during hospitalization. Monitoring the health of living donors remains critically important.
Introduction
Living kidney donors have constituted 45% of donors and 35% of kidney transplants in the United States since 1988 (1). Kidney transplantation provides an important survival benefit to patients relative to dialysis (2–4). Living-donor kidney transplantation is an increasingly important option for candidates because of expanding waiting times to receive a deceased donation (5,6). Studies evaluating long-term safety of living donation suggest minimal long-term risks, including no increased mortality for living donors compared with matched controls, but relative increases in surgical mortality among African Americans, Hispanics, men, and donors with hypertension (7). Among living donors with private health insurance, African Americans, and Hispanics have increased risk of hypertension and diabetes subsequent to donation, but the incidence of ESRD is <1% with 8-year follow-up (8). Studies identified no increased risk of fractures, cardiovascular events, or cancers among donors relative to matched controls (9–11).
Although the principal findings on risks for living donors are reassuring, continued vigilance regarding their safety is critical. Recommendations from the 2010 living-donor follow-up writing group stated the need to capture information from data sources beyond the Organ Procurement and Transplant Network (OPTN) forms to include information of psychosocial assessment and to improve surveillance of comorbid conditions (12). Particularly given evidence that screening criteria for living donation vary among centers and may have become more liberal in recent years, monitoring short- and long-term health of donors from all sources is important (13–16).
To examine the presence of comorbid conditions and evaluate short-term health of living donors beyond what is collected by the OPTN, we undertook a study using national hospitalization databases. The Agency for Healthcare Research and Quality (AHRQ) maintains research files compiled for hospitalized patients across the United States. As opposed to standard transplant forms, these files include all diagnoses and procedure codes that occur during hospitalization. The aims of the present study were to evaluate (1) the external validity of samples derived from national hospitalization files for living donors in the United States, (2) the incidence of and risk factors for procedure-related complications and length of stay (LOS) for living donors, (3) secular trends in comorbidity burden among living donors, and (4) outcomes of living donation compared with other common and relatively low-risk abdominal surgeries.
Materials and Methods
Two data sources were used for this study: the National Inpatient Sample (NIS) developed by AHRQ and the Scientific Registry of Transplant Recipients (SRTR). The NIS is a stratified clustered sample of all hospitalizations in the United States (17). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of OPTN, and has been described elsewhere (18). The Health Resources and Services Administration, U.S. Department of Health and Human Services, oversees the activities of the OPTN and SRTR contractors. SRTR data were used to compare and externally validate the composition of the NIS sample. We incorporated data for adult patients (age ≥18 years) from both data sources between 1998 and 2010.
Three criteria were used to identify living donors in the NIS database. First, patients had an International Classification of Diseases, Ninth Revision (ICD-9), diagnosis code indicating kidney donation (V59.4). Second, patients must have received a procedure code for nephroureterectomy (5551). Finally, we only included admissions coded as elective (as opposed to emergent). For comparison purposes, we also identified adult patients age 18–65 years undergoing other abdominal surgical interventions. The methods for identifying each of these cohorts were complied from other studies using NIS as follows: Patients undergoing appendectomies had primary ICD-9 procedure codes of 47.01 or 47.09 along with ICD-9 diagnosis codes of 540.0, 540.1, 540.09, 541.×, or 542.×. Patients who had cholecystectomy were identified by an ICD-9 diagnosis code of 574.×, 575.×, or 576.×, along with an ICD-9 primary procedure code of 51.22 or 51.23. Patients undergoing nephrectomy for nonmetastatic carcinoma were identified by an ICD-9 procedure code of 189.1 or 189.2, excluding diagnosis codes of 197.0, 197.7, or 198.× (indicating metastases), along with ICD-9 procedure codes of 55.51 or 54.21 (19–21). Procedure-related complications were defined by ICD-9 diagnosis codes related to the primary procedure for the given hospitalization. These ICD-9 codes (996.×–999.×) have been used to characterize surgical complications in several studies (22–24).
The NIS data sample contains weights representing patient and hospital characteristics and the applicable composition of the United States population. These weights are important to incorporate in statistical analyses, and they affect the variability of estimates. As such, we used survey regression procedures designed to incorporate weights for descriptive statistics and multivariable models. In the present study, univariable comparisons of categorical variables were made with the Rao-Scott chi-squared test, and multivariable survey binary logistic and linear regression models were used to evaluate independent factors associated with outcomes. We also used NIS severity files to extract comorbid conditions of patients that were established by AHRQ; algorithms for identifying conditions are available online (25). Comorbid conditions that were rare (<0.5% of the sample) or that were not considered clearly characterized as baseline factors (present on admission) as opposed to hospital-acquired were ignored for the purpose of this study. Of note, "private pay" insurance patients are considered synonymous with those with commercial insurance in these data. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul, as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The study was approved by the Cleveland Clinic Institutional Review Board. All analyses were conducted using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Study Population
The number of adult living kidney donors in the United States from 1998 to 2010 identified with the NIS was 69,117. This represented 89% (69,117 of 77,702) of living donors compared with the same time period from SRTR data (Table 1). Average age was approximately 40 years; 70% of donors were white, and 59% were female. The composition of the NIS sample was very similar to that of the SRTR, although the SRTR data contained a statistically significant greater proportion of white persons (P<0.001).
Table 1.
Comparison of study population characteristics with national Scientific Registry of Transplant Recipients data
| Characteristic | SRTR Data (n=77,702) | Nationwide Inpatient Sample (n=69,117) | P Value |
|---|---|---|---|
| Mean age ± SEM (yr) | 40.3±0.04 | 40.1±0.09a | 0.18 |
| Age > 50 yr (%) | 21.6 | 21.4 | 0.97 |
| Race/ethnicity (%) | <0.001 | ||
| Whiteb | 69.8 | 68.4 | |
| African Americanb | 13.1 | 11.6 | |
| Hispanicb | 12.7 | 13.2 | |
| Women (%)b | 59.1 | 59.0 | 0.13 |
SRTR, Scientific Registry of Transplant Recipients.
SDs not directly estimable because of sampling design; the SEM estimate includes variability derived from sampling design.
Limited to nonmissing values; n=4078 missing values for race/ethnicity and n=459 missing values for gender from the National Inpatient Sample data.
Presence of Comorbid Conditions among Living Donors
The overall proportion of documented comorbid conditions was relatively low (<5%); however, several conditions have increased substantially in recent years, including diagnoses of hypertension, depression, and hypothyroidism, which more than tripled over the period (Figure 1). As indicated in the figure, changes in documented comorbidity burden were not uniform; for example, the proportion of patients with hypertension has increased dramatically in the past few years, while the proportion of donors with depression and hypothyroidism has increased steadily since approximately 2000.
Figure 1.
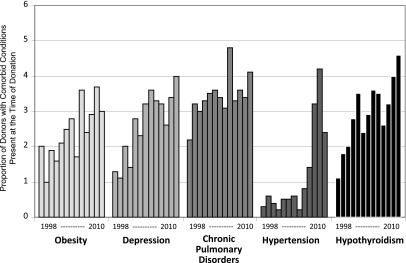
Proportion of living donors with comorbid conditions listed at discharge by year. Results based on National Inpatient Sample (n=69,117).
Factors Associated with Presence of Comorbid Conditions
On the basis of multivariable models, the annual likelihood of diagnoses of obesity, depression, hypertension, and hypothyroidism all increased significantly over the study period (Table 2). In addition, several factors were associated with diagnosis of these conditions. Depression was more common among older donors; women; white persons; and patients who had diagnoses of obesity, chronic pulmonary disorders, and hypothyroidism. Obesity was associated with being female, being African American, and having diagnoses of hypertension and chronic pulmonary disease. Hypertension was associated with older age, African American ethnicity, donors with Medicare as primary payer, and presence of obesity and chronic pulmonary disorders. Finally, hypothyroidism was associated with older age, being female, being white, and diagnosis of depression.
Table 2.
Multivariable logistic models evaluating patient and hospital characteristics associated with comorbid factors among living donors
| Characteristics (Reference Group) | Adjusted Odds Ratio for Comorbid Condition (95% CI) | |||
|---|---|---|---|---|
| Depression | Obesity | Hypertension | Hypothyroidism | |
| Age (per 10 years) | 1.17 (1.07 to 1.28) | 0.99 (0.98 to 1.00) | 1.10 (1.08 to 1.12) | 1.05 (1.04 to 1.06) |
| Female sex (male)a | 2.32 (1.78 to 3.02) | 1.32 (1.04 to 1.67) | 0.62 (0.45 to 0.84) | 4.82 (3.52 to 6.60) |
| African American race (white) | 0.22 (0.11 to 0.45) | 1.47 (1.02 to 2.12) | 2.55 (1.51 to 4.30) | 0.30 (0.14 to 0.61) |
| Other race (white)a | 0.33 (0.21 to 0.52) | 0.77 (0.52 to 1.14) | 0.70 (0.39 to 1.24) | 0.60 (0.41 to 0.88) |
| Lowest quartile income (highest) | 0.80 (0.58 to 1.12) | 1.26 (0.91 to 1.75) | 1.28 (0.82 to 2.00) | 0.75 (0.54 to 1.05) |
| Second quartile income (highest) | 0.78 (0.58 to 1.06) | 1.15 (0.84 to 1.57) | 1.17 (0.76 to 1.81) | 0.68 (0.51 to 0.91) |
| Third quartile income (highest)a | 0.93 (0.71 to 1.22) | 1.31 (0.98 to 1.75) | 1.19 (0.80 to 1.79) | 0.91 (0.71 to 1.17) |
| Primary payer Medicare (private) | 1.09 (0.77 to 1.53) | 1.50 (1.05 to 2.15) | 2.10 (1.38 to 3.18) | 1.07 (0.76 to 1.49) |
| Primary payer Medicaid (private) | 1.53 (0.61 to 3.81) | 0.31 (0.04 to 2.20) | –b | 0.89 (0.29 to 2.75) |
| Primary payer other (private) | 1.14 (0.90 to 1.44) | 1.79 (1.39 to 2.30) | 1.08 (0.76 to 1.52) | 1.25 (0.99 to 1.56) |
| Depression diagnosis at living donation (no) | – | 1.91 (1.17 to 3.14) | 1.53 (0.78 to 3.02) | 2.53 (1.72 to 3.73) |
| Obesity diagnosis at living donation (no) | 2.01 (1.24 to 3.28) | – | 2.92 (1.61 to 5.31) | 1.08 (0.59 to 1.97) |
| Hypertension diagnosis at living donation (no) | 1.31 (0.65 to 2.62) | 2.83 (1.58 to 5.08) | – | 1.03 (0.51 to 2.10) |
| Hypothyroidism diagnosis at living donation (no) | 2.51 (1.71 to 3.69) | 1.03 (0.56 to 1.88) | 1.12 (0.55 to 2.26) | – |
| Chronic pulmonary disorder diagnosis at living donation (no) | 2.04 (1.34 to 3.10) | 2.93 (1.99 to 4.32) | 1.94 (1.07 to 3.52) | 0.86 (0.49 to 1.50) |
| Hospital size small/medium (large) | 0.84 (0.60 to 1.19) | 0.82 (0.57 to 1.18) | 1.18 (0.75 to 1.88) | 0.77 (0.55 to 1.08) |
| Hospital control private (public) | 1.25 (0.26 to 5.94) | 0.74 (0.34 to 1.59) | 0.71 (0.26 to 1.94) | 1.03 (0.57 to 1.85) |
| Year of donation (per year) | 1.08 (1.05 to 1.11) | 1.07 (1.03 to 1.10) | 1.30 (1.22 to 1.39) | 1.08 (1.05 to 1.11) |
| C-statistic | 0.73 | 0.67 | 0.85 | 0.78 |
Results indicate multivariable odds ratios for presence of comorbid condition; results based on National Inpatient Sample (n=69,117). CI, confidence interval.
Missing levels of characteristics not displayed.
No cases in the study population for this variable level.
Procedure-Related Complications
The proportion of patients who experienced procedure-related complications was 7.9%. As displayed in Figure 2, the proportion of living-donor complications declined over the study period. Among donors experiencing a complication, the most common reported types included digestive disease (32%), respiratory (14%), accidental puncture or laceration (13%), urinary (11%), hemorrhage or hematoma (11%), infectious (9%), and cardiac (4%). Each of these specific types of complications declined over the study period. Complications were higher among men than women (9.6% versus 7.2%; P<0.001), among African Americans (10.4%) and whites (8.7%) compared with other race groups (6.3%; P<0.001), among donors without private pay insurance (8.5%) compared with those who had private pay (7.3%; P=0.002), and donors with hypertension (17.7%) compared with patients without hypertension (7.9%; P<0.001). The multivariable model indicated that being male, having nonprivate insurance, and donors with hypertension were independently associated with procedure-related complications (Table 3). In contrast, more proximate year and race groups not categorized because being African American or white was associated with lower likelihood of complications.
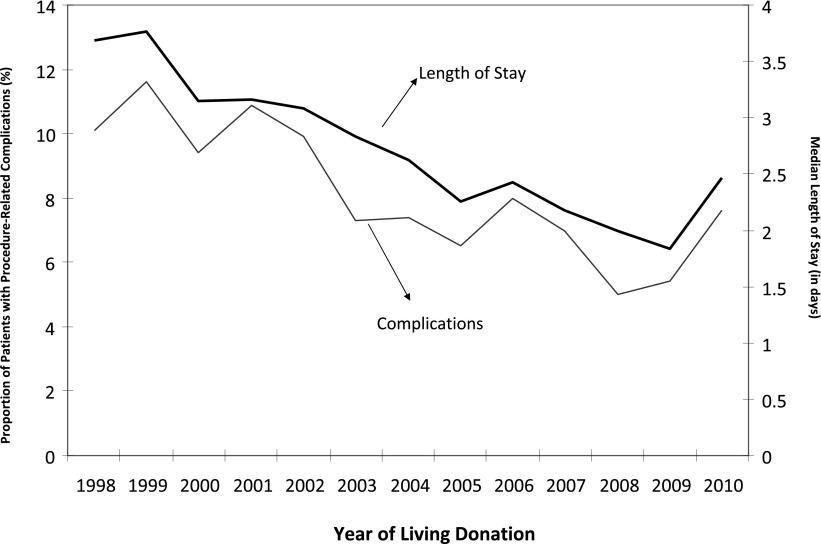
Figure 2.
Secular trends in length of stay and procedure-related complications. Results based on National Inpatient Sample data (n=69,117).
Table 3.
Multivariable logistic model for likelihood of any procedure-related complication and length of stay during initial hospitalization
| Characteristics (Reference Group) | Adjusted Odds Ratio for Procedure-Related Complication (95% CI) | Length of Staya | |
|---|---|---|---|
| Estimate | P Value | ||
| Year of donation (per year) | 0.94 (0.92 to 0.95) | −0.16 | <0.001 |
| Age (per 10 yr) | 1.05 (0.99 to 1.11) | 0.04 | 0.001 |
| Maleb (female) | 1.38 (1.21 to 1.57) | −0.02 | 0.60 |
| Medicare as primary insurance (private) | 1.26 (1.03 to 1.54) | −0.26 | 0.005 |
| Medicaid as primary insurance (private) | 1.05 (0.59 to 1.86) | −0.06 | 0.53 |
| Other primary insurance (private) | 1.19 (1.04 to 1.37) | 0.07 | 0.46 |
| African American race (white) | 1.21 (0.98 to 1.50) | 0.11 | 0.05 |
| Other race (white) | 0.74 (0.60 to 0.90) | −0.04 | 0.31 |
| Obesity (no) | 1.26 (0.87 to 1.83) | 0.18 | 0.03 |
| Hypothyroidism (no) | 0.93 (0.62 to 1.38) | −0.003 | 0.95 |
| Depression (no) | 1.31 (0.90 to 1.89) | 0.18 | 0.005 |
| Hypertension (no) | 2.70 (1.81 to 4.02) | 0.28 | 0.006 |
| Chronic pulmonary disease (no) | 1.08 (0.77 to 1.50) | 0.27 | <0.001 |
| Lowest income quartile (highest) | 1.03 (0.85 to 1.24) | 0.10 | 0.005 |
| Second income quartile (highest) | 1.09 (0.92 to 1.30) | 0.10 | 0.02 |
| Third income quartile (highest) | 0.94 (0.80 to 1.11) | 0.13 | 0.01 |
| Hospital size small/medium (large) | 1.01 (0.84 to 1.22) | 0.17 | <0.001 |
| Hospital control private (public) | 1.19 (0.85 to 1.65) | 0.21 | 0.001 |
CI, confidence interval.
Estimate reflects the number of increased/decreased length of stay days associated with the applicable parameter; results based on National Inpatient Sample data (n=69,117).
Missing levels not shown; results based on National Inpatient Sample data (n=69,117).
LOS
The average ± SEM and median LOS for living donors during initial hospitalization was 3.3 ± 0.02 days and 2.7 days, respectively. As depicted in Figure 2, LOS declined over the period; median LOS was 3.69 days in 1998 compared with 2.46 days in 2010. On the basis of the multivariable linear regression model, factors associated with shorter LOS included more proximate year and Medicare primary insurance (Table 3). In contrast, older age, presence of obesity, depression, hypertension and chronic pulmonary disease, lower median income, smaller hospital size, and privately controlled hospitals were associated with longer LOS.
Outcomes Compared with Other Surgery Populations
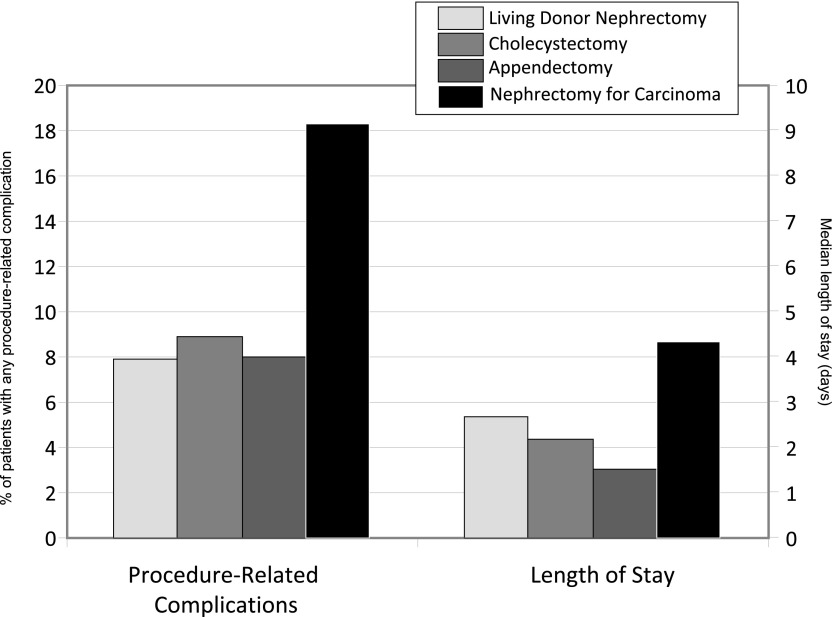
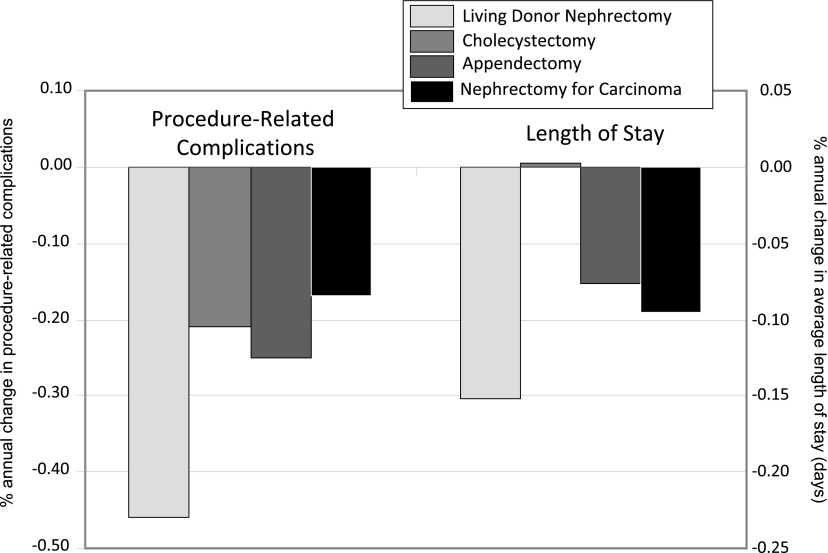
During the same study period, there were 3,011,628 cholecystectomies, 2,262,065 appendectomies, and 13,461 nephrectomies for nonmetastatic carcinoma. The rates of nephrectomies for carcinoma (P=0.97) and appendectomies (P=0.06) did not change over time, whereas cholecystectomies increased over time (2% per year; P=0.01). The proportion of procedure-related complications was similar between each of the groups, with the exception of nephrectomy patients for nonmetastatic carcinoma, which exceeded 18% of cases (Figure 3). Median LOS was 2.06 days for patients who had cholecystectomy, 1.43 days for those having appendectomy, and 4.16 days for those undergoing nephrectomy for carcinoma. Figure 4 depicts the annual rate of change in complications and LOS. As indicated, each of the groups had an annual decline in complications, but the decline was most pronounced among the living-donor population compared with all other groups (P<0.001). Similarly, LOS declined most dramatically among living donors (P<0.001), and there was no change among patients with cholecystectomy. Mortality rates during hospitalizations for the respective interventions were 0.17% among living donors, 0.15% among patients having cholecystectomy, 0.40% among those undergoing appendectomy, and 0.42% among those undergoing nephrectomy for carcinoma.
Figure 3.
Proportion of patients with procedure-related complications and median length of stay between surgical interventions. Sample sizes for study groups derived from the National Inpatient Sample between 1998 and 2010 are as follows: living-donor nephrectomy, n=69,117; cholecystectomy, n=3,011,628; appendectomy, n=2,262,065; and nephrectomy for carcinoma, n=13,461.
Figure 4.
Change in annual rates of complications and length of stay over time between surgical interventions. Sample sizes for study groups derived from the National Inpatient Sample between 1998 and 2010 are as follows: living-donor nephrectomy, n=69,117; cholecystectomy, n=3,011,628; appendectomy, n=2,262,065; and nephrectomy for carcinoma, n=13,461.
The proportion of comorbid conditions among each surgical group stratified by era (1998–2001, 2002–2006, and 2007–2010) are displayed in Table 4. As depicted in the table, the overall level of comorbid conditions was generally lower among living donors in each era. In addition, there was a relative increase in documented comorbid conditions for each of the surgery groups. In the most recent era, the proportion of the comorbid conditions among living donors ranged between 3% and 3.5%.
Table 4.
Presence of comorbid conditions by surgical intervention and era
| Comorbid Condition per Surgical Intervention | Overall Percentage of Patients with Condition | Percentage of Patients with Comorbid Condition by Era | ||
|---|---|---|---|---|
| 1998–2001 | 2002–2006 (Percentage Change from 1998 to 2001) | 2007–2010 (Percentage Change from 1998 to 2001) | ||
| Living-donor nephrectomy (n=69,117) | ||||
| Obesity | 2.4 | 1.6 | 2.6 (63) | 3.1 (94) |
| Depression | 2.6 | 1.5 | 3.1 (107) | 3.0 (100) |
| Chronic pulmonary disorders | 3.4) | 3.0 | 3.7 (23) | 3.4 (13) |
| Hypertension | 1.2 | 0.4 | 0.5 (25) | 3.1 (675) |
| Hypothyroidism | 2.8 | 1.9 | 3.2 (68) | 3.3 (74) |
| Cholecystectomy (n=3,011,628) | ||||
| Obesity | 11.7 | 7.3 | 11.1 (52) | 16.7 (129) |
| Depression | 5.4 | 2.8 | 5.7 (104) | 7.7 (175) |
| Chronic pulmonary disorders | 9.0 | 6.9 | 9.3 (35) | 10.8 (57) |
| Hypertension | 24.4 | 19.3 | 24.8 (28) | 28.7 (49) |
| Hypothyroidism | 5.0 | 3.9 | 5.1 (31) | 6.0 (54) |
| Appendectomy (n=2,262,065) | ||||
| Obesity | 4.5 | 2.4 | 4.1 (71) | 6.7 (179) |
| Depression | 2.8 | 1.3 | 2.8 (131) | 3.9 (200) |
| Chronic pulmonary disorders | 4.9 | 3.5 | 4.9 (40) | 6.1 (74) |
| Hypertension | 11.5 | 7.4 | 11.5 (55) | 15.0 (103) |
| Hypothyroidism | 2.5 | 1.5 | 2.5 (67) | 3.2 (113) |
| Nephrectomy for nonmetastatic carcinoma (n=13,461) | ||||
| Obesity | 6.7 | 5.3 | 4.9 (−7.5) | 10.4 (96) |
| Depression | 4.9 | 2.7 | 4.7 (−74) | 7.1 (163) |
| Chronic pulmonary disorders | 15.4 | 11.9 | 16.0 (34) | 18.4 (55) |
| Hypertension | 40.4 | 7.4 | 11.5 (55) | 15.0 (103) |
| Hypothyroidism | 4.7 | 4.2 | 4.9 (17) | 4.9 (17) |
Discussion
The primary findings of the study were (1) that the NIS provides a highly representative sample of living donors in the United States and that, on the basis of these data, (2) the proportion of procedure-related complications and LOS have declined over the past decade, (3) documented comorbidity burden has increased, and (4) complication rates among living donors are similar to patients undergoing cholecystectomies and appendectomies. Cumulatively, these data confirm that short-term risks associated with living donation are relatively modest, but the long-term impact of complications and comorbid conditions may be particularly important to evaluate in coming years.
Monitoring the health of living donors is an important responsibility of the transplant community. However, the precise evaluation of donor outcomes with standard data sources has been a significant challenge because of incomplete follow up (26,27). In addition, determining a cohort that may serve as an accurate control group for evaluating the health of living donors is complex given screening processes and underlying characteristics of donors. This study evaluated factors that contribute to short-term complications, which may be a proxy for long-term health, and places findings into perspective among other relatively low-risk abdominal surgical interventions. It is noteworthy that factors associated with short-term complications identified in this study (older age, being male, and hypertension) also persist with longer-term follow-up (7–9). Results of this study may be useful to disseminate to donors and donor advocates for the purpose of informed consent and treatment planning after the procedure (28).
One of the prominent purposes of this study was to describe the level of comorbidity burden among living donors. The findings indicate that the proportion of living donors with selected comorbid conditions has been 3%–3.5% in recent years. Thus, the magnitude of these comorbid conditions is relatively low but also should be placed in the context that they are rising and represent average rates that are likely to vary among centers (16). For example, Rodrigue et al. reported significant center-level variability of psychosocial criteria for living donation, indicating that the observed levels of depression may not be uniform across all centers (29). A recent study also documented increased trends with acceptance of donors of older age, obese donors, and those with glucose intolerance (30). Combined with the current findings, these studies improve our understanding of current practice with acceptance criteria of living donors and potentially inform whether certain conditions could or should be incorporated into living donor guidelines. Clearly there is also an important need to continue to assess whether these conditions manifest into any differences in long-term outcomes for the living-donor population.
While relatively rare, several studies have evaluated short-term complications and morbidity for living donors. Patel et al. reported a 10% overall complication rate after living donation, including a 4% major complication rate (31). On the basis of more than 6000 cases from the NIS between 1999 and 2005, Friedman et al. reported an 18% complication rate after donation, with higher risks among older and obese donors (32). The higher rate reported in the Friedman study did not incorporate the weights derived from the complex survey design of the NIS but was also based on the selection of specific complications that were not classified as procedure-related used in the present study. Columbo et al. reported only a 0.6% complication rate among living donors using NIS data through 2006 (33). However, the complications considered were relatively narrowly defined and excluded most procedure-related complications. We specifically selected to use criteria that indicated that complications were related to surgery and provided comparable rates using other relatively low-risk surgical interventions. Although this method may ignore certain complications that were coded by other diagnoses, the complications that were documented are likely to be accurate, less subject to interpretation as to whether they were truly “procedure-related,” and comparable to other surgical groups and over time. One of the findings of the study was a 0.17% mortality rate among living donors. This rate is significantly higher than reported in other studies, in which estimates are approximately 0.03% (7,34). It is certainly possible the mortality data reported in this study may be influenced by a few cases of patients misspecified as living donors and should be interpreted with caution given that the limitations for identifying donors with certainty using ICD-9 coding.
Reduction in LOS has been reported for living donors and is partially ascribed to more frequent use of laparoscopic procedures (19,35). Since 2003, the SRTR has captured data about the type of surgical procedure for living donation, and in 2003 approximately 26% of living donors underwent open procedures compared with 5% in 2009. Consistent with past findings, the current study confirms that age, obesity, and hypertension are associated with longer LOS. Of note, this study also demonstrated that diagnosis of depression, chronic pulmonary disease, and payer status were associated with LOS. Also of interest was that both complication rates and average LOS declined more rapidly among the living-donor population compared with other surgical comparative groups. This may reflect improvement in surgical practice and changes in practice specific to donation despite indicators that donors may be more medically complex in recent years.
Evaluation of donor outcomes using the hospitalization files has several important advantages. First, these data capture all diagnoses and procedure codes associated with hospitalizations that are not available in standard forms. Second, compared with studies using single-payer administrative data (e.g., Medicare), these files make up the largest all-payer hospitalization database in the United States. Third, these data allow comparison of other surgical conditions using equivalent methods.
However, these data also have important limitations. These data lack comprehensive long-term follow-up information, and ICD coding can be limited with respect to underreporting of conditions that are not used for administrative purposes. A good example of this in the present study is the diagnosis of obesity, which is clearly underreported according to diagnosis claims (in this study, the proportion of patients with obesity in 2009 was 3.7%) compared with United Network for Organ Sharing data, which indicates 22% of donors have a body mass index >30 kg/m2 (12). Conversely, there may be some reluctance to document comorbid conditions among some patients because of the deleterious effect on insurance eligibility. In addition, the incidence of complications can be sensitive to the specific codes that are considered. We limited complications to those defined as procedure-related, but other conditions may be important to further delineate severity (31,36,37). It is also possible that more rigorous evaluation of donors has led to improved documentation of comorbid conditions over time rather than actual changes in acuity. Given that documented comorbid conditions increased in each of the surgical groups, coding for comorbid conditions may have uniformly increased over time. This may be related to variations in coding specialists by institutions and an increase in the rigor of practice. Thus, although it is unlikely that documented comorbid conditions that are reported are inaccurate (i.e., sensitivity is probably high), there may be a lack of specificity in coding. Finally, these data lack important information specific to transplantation, such as donor information, laboratory values, and medication use.
In summary, this study has several principal findings. First, the procedure-related complication rate for living donors is approximately 8%, which has declined over the past decade and is similar to the rate in patients undergoing appendectomy or cholecystectomy. LOS has declined in recent years and is significantly associated with demographic characteristics, payer status, and presence of comorbid conditions. Documented comorbid conditions have significantly increased in recent years, which may be a product of increased complexity of donors and more rigorous documentation of conditions. Further ongoing efforts to enhance data collection and critically evaluate the long-term health of the living donor population are needed.
Disclosures
None.
Acknowledgments
We would like to thank Songhua Lin, MS, for her contribution with assistance with statistical programming for this study.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. government.
The study examined outcomes for living kidney donors using discharge data from the NIS, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.
This research is supported, in part, by R01DK085185 (J.R., D.M., J.S.) and National Institutes of Health/National Institutes of Mental Health (P60MD00265; J.S.). The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Better Understanding Live Donor Risk through Big Data,” on pages 1645–1647.
References
- 1.Organ Procurement and Transplantation Network: Donors Recovered in the U.S. by Donor Type. 2012. Available at: http://optn.transplant.hrsa.gov/latestData/rptData.asp Accessed August 14, 2012
- 2.Gill JS, Tonelli M, Johnson N, Kiberd B, Landsberg D, Pereira BJ: The impact of waiting time and comorbid conditions on the survival benefit of kidney transplantation. Kidney Int 68: 2345–2351, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B: Survival improvement among patients with end-stage renal disease: Trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol 12: 1293–1296, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segev DL, Gentry SE, Montgomery RA: Association between waiting times for kidney transplantation and rates of live donation. Am J Transplant 7: 2406–2413, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, McBride MA, Montgomery RA: Perioperative mortality and long-term survival following live kidney donation. JAMA 303: 959–966, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, Davis CL, Abbott KC, Brennan DC: Racial variation in medical outcomes among living kidney donors. N Engl J Med 363: 724–732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg AX, Prasad GV, Thiessen-Philbrook HR, Ping L, Melo M, Gibney EM, Knoll G, Karpinski M, Parikh CR, Gill J, Storsley L, Vlasschaert M, Mamdani M, Donor Nephrectomy Outcomes Research (DONOR) Network : Cardiovascular disease and hypertension risk in living kidney donors: An analysis of health administrative data in Ontario, Canada. Transplantation 86: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Garg AX, Pouget J, Young A, Huang A, Boudville N, Hodsman A, Adachi JD, Leslie WD, Cadarette SM, Lok CE, Monroy-Cuadros M, Prasad GV, Thomas SM, Naylor K, Treleavan D, Donor Nephrectomy Outcomes Research (DONOR) Network : Fracture risk in living kidney donors: A matched cohort study. Am J Kidney Dis 59: 770–776, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Vijayan A, Xiao H, Schnitzler MA, Davis CL, Garg AX, Axelrod D, Abbott KC, Brennan DC: Cancer diagnoses after living kidney donation: Linking U.S. Registry data and administrative claims. Transplantation 94: 139–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leichtman A, Abecassis M, Barr M, Charlton M, Cohen D, Confer D, Cooper M, Danovitch G, Davis C, Delmonico F, Dew MA, Garvey C, Gaston R, Gill J, Gillespie B, Ibrahim H, Jacobs C, Kahn J, Kasiske B, Kim J, Lentine K, Manyalich M, Medina-Pestana J, Merion R, Moxey-Mims M, Odim J, Opelz G, Orlowski J, Rizvi A, Roberts J, Segev D, Sledge T, Steiner R, Taler S, Textor S, Thiel G, Waterman A, Williams E, Wolfe R, Wynn J, Matas AJ, Living Kidney Donor Follow-Up Conference Writing Group : Living kidney donor follow-up: state-of-the-art and future directions, conference summary and recommendations. Am J Transplant 11: 2561–2568, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Davis CL: Evaluation of the living kidney donor: current perspectives. Am J Kidney Dis 43: 508–530, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Davis CL: Variable evaluation and selection criteria for living kidney donors: Have we gotten the message yet? Am J Transplant 7: 2219–2220, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hall EC, James NT, Garonzik Wang JM, Berger JC, Montgomery RA, Dagher NN, Desai NM, Segev DL: Center-level factors and racial disparities in living donor kidney transplantation. Am J Kidney Dis 59: 849–857, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Reese PP, Feldman HI, McBride MA, Anderson K, Asch DA, Bloom RD: Substantial variation in the acceptance of medically complex live kidney donors across US renal transplant centers. Am J Transplant 8: 2062–2070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality: HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project 2012 Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp Accessed August 15, 2012 [Google Scholar]
- 18.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE: Analytical methods and database design: Implications for transplant researchers, 2005. Am J Transplant 6: 1228–1242, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hamidi V, Andersen MH, Oyen O, Mathisen L, Fosse E, Kristiansen IS: Cost effectiveness of open versus laparoscopic living-donor nephrectomy. Transplantation 87: 831–838, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hanna N, Sun M, Trinh QD, Hansen J, Bianchi M, Montorsi F, Shariat SF, Graefen M, Perrotte P, Karakiewicz PI: Propensity-score-matched comparison of perioperative outcomes between open and laparoscopic nephroureterectomy: A national series. Eur Urol 61: 715–721, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Masoomi H, Mills S, Dolich MO, Ketana N, Carmichael JC, Nguyen NT, Stamos MJ: Comparison of outcomes of laparoscopic versus open appendectomy in adults: Data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg 15: 2226–2231, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Livingston EH: Procedure incidence and in-hospital complication rates of bariatric surgery in the United States. Am J Surg 188: 105–110, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Memtsoudis SG, Ma Y, González Della Valle A, Mazumdar M, Gaber-Baylis LK, MacKenzie CR, Sculco TP: Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology 111: 1206–1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarze ML, Shen Y, Hemmerich J, Dale W: Age-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001-2006. J Vasc Surg 50: 722–729, e2, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality: HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp Accessed August 15, 2012
- 26.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, Hanto DW, Rodrigue JR: The medical evaluation of living kidney donors: A survey of US transplant centers. Am J Transplant 7: 2333–2343, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Mandelbrot DA, Pavlakis M, Karp SJ, Johnson SR, Hanto DW, Rodrigue JR: Practices and barriers in long-term living kidney donor follow-up: A survey of U.S. transplant centers. Transplantation 88: 855–860, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Smith SW, Nazione S, LaPlante C, Clark-Hitt R, Park HS, Sung R, Leichtman A: Living kidney donor decision making and communication. J Health Commun 16: 870–888, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Rodrigue JR, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, Hanto DW, Mandelbrot DA: Evaluating living kidney donors: relationship types, psychosocial criteria, and consent processes at US transplant programs. Am J Transplant 7: 2326–2332, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Taler SJ, Messersmith EE, Leichtman AB, Gillespie BW, Kew CE, Stegall MD, Merion RM, Matas AJ, Ibrahim HN, RELIVE Study Group : Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant 13: 390–398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S, Cassuto J, Orloff M, Tsoulfas G, Zand M, Kashyap R, Jain A, Bozorgzadeh A, Abt P: Minimizing morbidity of organ donation: Analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation 85: 561–565, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Friedman AL, Cheung K, Roman SA, Sosa JA: Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg 145: 356–362, discussion 362, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Colombo B, Singla A, Li Y, Tseng JF, Saidi RF, Bozorgzadeh A, Shah SA: Current trends and short-term outcomes of live donor nephrectomy: A population-based analysis of the nationwide inpatient sample. World J Surg 34: 2985–2990, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Davis CL, Cooper M: The state of U.S. living kidney donors. Clin J Am Soc Nephrol 5: 1873–1880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troppmann C, Ormond DB, Perez RV: Laparoscopic (vs open) live donor nephrectomy: A UNOS database analysis of early graft function and survival. Am J Transplant 3: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Mjøen G, Øyen O, Holdaas H, Midtvedt K, Line PD: Morbidity and mortality in 1022 consecutive living donor nephrectomies: Benefits of a living donor registry. Transplantation 88: 1273–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Permpongkosol S, Link RE, Su LM, Romero FR, Bagga HS, Pavlovich CP, Jarrett TW, Kavoussi LR: Complications of 2,775 urological laparoscopic procedures: 1993 to 2005. J Urol 177: 580–585, 2007 [DOI] [PubMed] [Google Scholar]