Summary
Background and objectives
According to the cost of health care utilization systems, there may be regional differences in the relative strength of association of income and education-based socioeconomic status measures with CKD. This study investigated the relative strength of the association of income and education with CKD in a United States and a Dutch population.
Design, setting, participants, & measurements
This cross-sectional study examined individuals who participated in the 1999–2002 National Health and Nutritional Examination Survey (NHANES) and in Prevention of Renal and Vascular End-stage Disease (PREVEND 1997–1998), general population-based cohorts in the United States and The Netherlands, respectively. The main outcome was CKD, defined as estimated GFR <60 ml/min per 1.73 m2 (using creatinine) or albuminuria ≥30 mg/24 hours or albumin-to-creatinine ratio ≥30 mg/g.
Results
In NHANES (n=6428), income was strongly associated with CKD (adjusted odds ratio, 2.34 [95% confidence interval (CI), 1.68 to 3.27]; P for trend<0.001) but education was not (adjusted odds ratio, 1.62 [95% CI, 0.87 to 2.25]; P for trend=0.05]. In contrast, in PREVEND (n=7983), low income was weakly associated with CKD whereas low education had a strong association. The fit of the logistic regression model estimating association of income and education with CKD was significantly improved only after income was added in NHANES (P<0.001) and education was added in PREVEND (P=0.01). Sensitivity analyses that used other CKD-defining variables and restricted analyses to participants <65 years of age resulted in similar findings.
Conclusion
In the United States, where access to health care is traditionally income dependent, income appeared more strongly associated with CKD than in The Netherlands, where education showed a stronger association.
Introduction
The incidence and prevalence of CKD are increasing, and CKD has been recognized as a global public health problem (1). Many patients with CKD are not aware of having this disease and consequently are not treated. This has led to the recommendation that high-risk populations, such as people with known diabetes or hypertension, should be screened (2). Although some have argued against screening for CKD, the prevailing guidelines for CKD recommend that high-risk individuals should be screened (3). Lower socioeconomic status (SES) has been suggested to be associated with CKD, and some have additionally recommended screening this high-risk group (4). SES can be measured by several individual and neighborhood characteristics, but income and education are among the important defining components for SES. This brings forward the question of identifying a measure of SES that is the strongly linked with CKD.
The strength of income and education, in terms of determining health outcomes such as CKD, varies according to regional factors (5,6). We hypothesize that one of the regional factors can be the cost of accessing health care. Therefore, in a formal comparison of income and education in the United States, where access to health care is traditionally income dependent (7), income might have a stronger association with CKD than does education. Similarly, income might be less strongly associated with CKD in populations where access to health care is relatively income independent; for example, in The Netherlands, access to preventive and primary health care is not fundamentally different between the rich and poor (8–10). Education also exhibits a significant role in living a healthy lifestyle and using preventive health care services (11,12). In The Netherlands, compared with income, education might have a stronger association with CKD. To date, evidence testing these hypotheses is lacking.
Therefore, this study sought to formally investigate the relative strength of association of income and education with CKD prevalence in a United States and a Dutch population.
Materials and Methods
Study Design and Population
We used baseline data of the National Health and Nutritional Examination Survey (NHANES) and the Prevention of Renal and Vascular ENd-stage Disease (PREVEND) study participants.
Briefly, the NHANES surveys are cross-sectional, multistage, stratified, clustered probability samples of the noninstitutionalized civilian population in the United States (details can be found at http://www.cdc.gov/nchs/nhanes.htm). For the current study, data of participants aged 28–75 years from 1999–2000 and 2001–2002 NHANES surveys with measured serum creatinine were combined (n=7402) (13).
The PREVEND study is a prospective cohort study initiated in 1997 when 40,856 people aged 28–75 years were screened from the general population of Groningen, The Netherlands. From these participants, a cohort (n=8952) was formed that was screened in detail between 1997 and 1998 and followed up over time. PREVEND has been shown to be representative of the Dutch population (14,15); details have been published elsewhere (16,17).
Of the 7402 NHANES participants and 8592 PREVEND participants, we excluded those lacking information on education and income (n=93 in NHANES and n=527 in PREVEND), serum creatinine (n=882 in NHANES and n=67 in PREVEND), and albumin (n=597 in NHANES and n=18 in PREVEND), leaving 6428 and 7983 participants for analysis in NHANES and PREVEND, respectively. Characteristics of missing participants were similar to those of final study participants.
Measurements and Definitions
Income and Education.
For NHANES and PREVEND, income was recorded as poverty income ratio (PIR). The PIR was calculated by dividing the family income by the country-dependent poverty threshold specific for family size and year (18). The PIR was divided into quintiles. In PREVEND, income was not recorded as a continuously distributed variable but had a discrete distribution (classes); as a result, not all quintiles covered exactly 20%. For NHANES, quintiles 1 to 5 ranged from 4.12 to 5.00, from 2.39 to 4.11, from 1.38 to 3.38, from 0.80 to 1.37, and from 0 to 0.79, respectively. For PREVEND, quintiles 1–5 ranged from 1.62 to 3.70, from 1.88 to 2.21, from 1.24 to 1.59, from 0.78 to 1.23, and from 0 to 0.77, respectively. In NHANES and PREVEND, education levels were categorized according to the International Standard Classification of Education (ISCED) (19). Educational levels concern bachelor, master or doctorate graduate (level 1), postsecondary or nontertiary or short-cycle tertiary education (level 2), upper secondary education (level 3), lower secondary education (level 4), and primary or below primary education (level 5). With the ISCED, the association of education level with CKD can be compared between both regions.
Definition of CKD
For both PREVEND and NHANES, GFR was estimated with the CKD-Epidemiology Collaboration equation using serum creatinine as a marker of renal function (20). Albuminuria was expressed as the albumin-to-creatinine ratio in NHANES and as average urinary albumin excretion in PREVEND. CKD was defined as estimated GFR (eGFR) < 60 ml/min per 1.73 m2 or albuminuria ≥ 30 mg/24 hours (30 mg/g), or both, according to prevailing guidelines (21).
Serum Creatinine.
Serum creatinine was measured by the kinetic alkaline picrate method in NHANES and by dry chemistry (Eastman Kodak, Rochester, NY) in PREVEND.
Urinary Albumin and Creatinine.
In NHANES, urinary albumin and creatinine concentrations were measured from spot urine samples by the modified kinetic Jaffe method with a Synchron AS/Astra Analyzer (Beckman Coulter). In PREVEND, urinary albumin concentration was measured in two 24-hour urine collections by nephelometry (BNII; Dade Behring Diagnostic, Marburg, Germany).
Other Covariates.
During the physical examination, weight, height, and BP were measured. Body mass index was calculated as weight (kg) divided by the square of height (m2), and BP was measured in accordance with the recommendation of the American Heart Association (22). Information on cardiovascular disease history and smoking was obtained from questionnaires; information on diabetes was obtained from questionnaires as well as objective measurements (13,15). Current smokers were defined as those who had smoked at least 100 cigarettes during their lifetime and, at the time of the interview, reported current smoking or smoking in the year before the baseline measurement (23). Diabetes was defined on a single fasting plasma glucose level ≥126 mg/dl, a nonfasting glucose level ≥200 mg/dl, or the use of oral antidiabetic drugs/insulin or self-report (24).
Statistical Analyses
To compare the strength of association of income and education with CKD, we performed logistic regression analysis. We ran two parallel models with education and income separately. Model 1 adjusted for age, sex, and race. Income and education were added simultaneously in the subsequent model 2 to explore their relative strength of association with CKD. Interaction terms were also investigated. Further, we performed likelihood ratio tests to compare the fit of models with education and income.
For NHANES, to address population representativeness, we used recommended 4-year sampling weights from NHANES 1999–2002 (13). For PREVEND, a specific weighing factor was used to account for the overrepresentation of participants with higher levels of albuminuria.
We performed several sensitivity analyses. First, we redefined CKD for PREVEND by using the albumin-to-creatinine ratio instead of 24-hour urinary albumin excretion and repeated the analyses. Second, we conducted stratified analyses by age (in both cohorts) and race (in NHANES), accounting for higher prevalence of poverty and CKD in participants ≥65 years of age and the higher number of non-Hispanic blacks and Mexican whites in the NHANES compared with the PREVEND study (25). Third, we additionally tested the association between SES measures and CKD after adjusting for other covariates (smoking, diabetes, BP, body mass index [BMI], and cardiovascular disease). Fourth, we also tested associations for low renal function and albuminuria, separately. Fifth, we tested the observed differences in association between the two countries by introducing the interaction between SES and cohorts versus CKD prevalence in the combined data. Finally, we tested the association between income and CKD in NHANES and PREVEND using absolute income levels (expressed per family member) to explore the robustness of results against a differential classification of income levels. A P value <0.05 was considered to indicate statistical significance. All analyses were conducted using Stata software, version 11 (Stata Corp., College Station, TX).
Results
Mean age (47.3 years versus 48.9 years), percentage of men (48% versus 49%), and percentage of whites (69% versus 96%) were lower in NHANES than in PREVEND. The prevalence of CKD was 14% in NHANES and 9% in PREVEND. Mean serum creatinine ± SD was 0.83±0.37 mg/dl in NHANES and 0.95±0.18 mg/dl in PREVEND. Baseline characteristics of the NHANES and PREVEND cohorts are presented in Tables 1 and 2, respectively. Percentage distribution of income in education levels and education in income levels for NHANES and PREVEND are shown in Supplemental Figures 1 and 2, respectively.
Table 1.
Baseline characteristics of the National Health and Nutrition Examination Survey study population according to income quintiles and education levels
| Characteristics | Income Levelsa | Education Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile1 (Highest) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 (Lowest) | Level 1 (Highest) | Level 2 | Level 3 | Level 4 | Level 5 (Lowest) | |
| Patients (n) | 1161 | 1158 | 1165 | 1162 | 1171 | 1336 | 1528 | 1425 | 1115 | 1024 |
| Age (yr) | 48.2±12.1 | 46.2±13.3 | 47.2±14.1 | 46.9±14.4 | 46.2±14.2 | 46.3±13.0 | 46.6±12.9 | 47.1±13.8 | 48.5±13.9 | 52.5±13.6 |
| Men (%) | 51 | 49 | 50 | 47 | 42 | 52 | 45 | 48 | 48 | 49 |
| Whites (%) | 88 | 79 | 71 | 63 | 45 | 83 | 75 | 77 | 55 | 32 |
| BMI (kg/m2) | 27.7±5.7 | 28.0±5.7 | 28.5±6.3 | 29.1±6.4 | 29.5±6.8 | 27.1±5.5 | 28.8±6.4 | 28.8±6.3 | 29.2±6.7 | 28.4±5.4 |
| Smoking (%) | 12 | 17 | 23 | 29 | 34 | 7.6 | 20 | 28 | 37 | 23 |
| Diabetes mellitus | 4.5 | 6.3 | 8.9 | 11 | 12 | 4.3 | 7.5 | 7.9 | 13 | 15 |
| Hypertension (%) | 15 | 17 | 18 | 21 | 25 | 14 | 17 | 19 | 24 | 25 |
| Education level (%) | ||||||||||
| Level 1 (highest) | 51 | 30 | 19 | 9.2 | 8.5 | |||||
| Level 2 | 28 | 31 | 30 | 24 | 19 | |||||
| Level 3 | 17 | 27 | 32 | 32 | 26 | |||||
| Level 4 | 3.6 | 8.9 | 15 | 23 | 29 | |||||
| Level 5 (lowest) | 1.0 | 2.2 | 4.7 | 12 | 18 | |||||
| Income level (%) | ||||||||||
| Level 1 (highest) | 47 | 23 | 15 | 5.9 | 1.9 | |||||
| Level 2 | 21 | 22 | 19 | 10 | 4.9 | |||||
| Level 3 | 14 | 22 | 23 | 19 | 14 | |||||
| Level 4 | 6.5 | 14 | 19 | 26 | 29 | |||||
| Level 5 (lowest) | 4.9 | 11 | 16 | 27 | 37 | |||||
| CKD (%) | 8.8 | 9.7 | 14 | 17 | 20 | 8.6 | 13 | 12 | 19 | 20 |
| Serum creatinine (mg/dl) | 0.82±0.36 | 0.83±0.28 | 0.82±0.39 | 0.83±0.40 | 0.84±0.41 | 0.84±0.32 | 0.82±0.37 | 0.83±0.41 | 0.81±0.42 | 0.84±0.45 |
| eGFR (ml/min per 1.73 m2) | 103±20.5 | 101±20.6 | 99.6±22.2 | 97.8±22.5 | 96.4±23.8 | 99.9±21.4 | 100±21.5 | 99.3±22.1 | 98.7±24.3 | 98.4±21.1 |
| eGFR < 60 ml/min per 1.73 m2 (%) | 2.1 | 2.5 | 3.4 | 3.7 | 5.0 | 2.1 | 3.1 | 3.2 | 5.4 | 5.0 |
| ACR (mg/g) | 6.3 (3.2–12.1) | 6.9 (3.7–13.3) | 8.1 (4.3–16.6) | 8.1 (4.2–18.3) | 9.0(4.5–21.4) | 6.2 (3.2–11.8) | 7.2 (4.0–15.6) | 7.9 (4.1–15.9) | 8.9 (4.6–22.2) | 8.9 (4.4–19.2) |
| ACR ≥ 30 mg/g (%) | 6.7 | 7.8 | 11 | 14 | 18 | 7.0 | 11 | 11 | 16 | 15 |
Continuous variables are presented as mean ± SD or as median and interquartile range in case of skewed distributions, and categorical variables are presented as percentages. BMI, body mass index; eGFR, estimated GFR; ACR, albumin-to-creatinine ratio.
The total of 611 participant were missing information on income only. These participants were relatively older and consisted of more men than the rest of the study population.
Table 2.
Baseline characteristics of the Prevention of Renal and Vascular End-stage Disease study population according to income quintiles and education levels
| Characteristics | Income Levelsa | Education Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 (Highest) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 (Lowest) | Level 1 (Highest) | Level 2 | Level 3 | Level 4 | Level 5 (Lowest) | |
| Patients (n) | 1143 | 1649 | 1411 | 2025 | 1398 | 946 | 1599 | 2099 | 2068 | 1271 |
| Age (yr) | 44.8±10.7 | 47.3±12.8 | 48.7±12.7 | 49.6±12.9 | 48.1±13.1 | 42.6±10.7 | 44.9±11.2 | 45.8±12.4 | 51.6±12.1 | 57.2±11.5 |
| Men (%) | 55 | 48 | 48 | 45 | 38 | 57 | 44 | 49 | 44 | 39 |
| Whites (%) | 97 | 98 | 98 | 96 | 92 | 96 | 98 | 95 | 94 | 96 |
| BMI (kg/m2) | 25.1±3.5 | 25.2±3.7 | 25.6±3.9 | 25.9±4.0 | 25.9±4.7 | 24.5±3.2 | 24.7±3.6 | 25.7±4.1 | 26.5±4.3 | 27.7±4.5 |
| Smoking (%) | 27 | 33 | 36 | 40 | 41 | 24 | 30 | 36 | 38 | 42 |
| Diabetes mellitus | 1.5 | 1.5 | 3.0 | 3.1 | 3.2 | 1.0 | 1.0 | 1.9 | 3.2 | 6.2 |
| Hypertension (%) | 22 | 25 | 26 | 29 | 27 | 15 | 17 | 25 | 34 | 44 |
| Education level (%)a | ||||||||||
| Level 1 (highest) | 26 | 20 | 7.5 | 4.5 | 7.8 | |||||
| Level 2 | 34 | 32 | 19 | 14 | 12 | |||||
| Level 3 | 25 | 25 | 32 | 28 | 25 | |||||
| Level 4 | 13 | 18 | 30 | 32 | 26 | |||||
| Level 5 (lowest) | 3.0 | 4.7 | 12 | 21 | 29 | |||||
| Income level (%)a | ||||||||||
| Level 1 (highest) | 37 | 30 | 17 | 11 | 4.0 | |||||
| Level 2 | 32 | 29 | 19 | 16 | 7.0 | |||||
| Level 3 | 10 | 16 | 23 | 23 | 15 | |||||
| Level 4 | 4.9 | 10 | 18 | 23 | 25 | |||||
| Level 5 (lowest) | 12 | 11 | 19 | 23 | 42 | |||||
| CKD (%) | 6.5 | 9.5 | 8.7 | 11 | 11 | 5.9 | 5.7 | 6.9 | 9.2 | 15 |
| Serum creatinine (mg/dl) | 0.96±0.15 | 0.95±0.16 | 0.94±0.17 | 0.95±0.19 | 0.91±0.23 | 0.96±0.24 | 0.93±0.15 | 0.95±0.17 | 0.94±0.19 | 0.94±0.35 |
| eGFR (ml/min per 1.73 m2) | 85.9±14.0 | 84.1±14.8 | 84.2±15.4 | 82.7±15.7 | 85.7±16.4 | 87.6±13.9 | 86.7±14.5 | 85.7±15.6 | 81.6±15.9 | 78.5±15.2 |
| eGFR < 60 ml/min per 1.73 m2 (%) | 2.6 | 4.1 | 5.2 | 4.5 | 4.9 | 1.2 | 2.5 | 4.5 | 5.9 | 9.5 |
| UAE (mg/24 hr) | 8.9 (6.3–15.2) | 8.9 (6.4–15.3) | 9.7 (6.3–19.3) | 9.9 (6.4–18.5) | 9.1 (6.1–18.4) | 8.5 (6.3–14.5) | 8.7 (6.1–14.1) | 8.9 (6.2–16.2) | 10 (6.4–19.6) | 12 (6.8–28.2) |
| UAE ≥ 30 mg/24 hr (%) | 4.4 | 5.3 | 6.5 | 6.9 | 6.7 | 5.2 | 3.4 | 5.4 | 7.5 | 9.7 |
Continuous variables are presented as mean ± SD and categorical variables as percentages, and skewed variables are presented as median and interquartile range. BMI, body mass index; eGFR, estimated GFR; UAE, urinary albumin excretion.
The total of 357 participant were missing information on income only. These participants were relatively older than the rest of the study population.
Income Levels
Participants in the highest income group were 7.1 times and 3.3 times richer than participants in the lowest income group in NHANES and in PREVEND, respectively. In NHANES, people with lower income levels had a higher prevalence of impaired eGFR, increased albuminuria, and CKD. A similar trend was observed for hypertension, smoking, high BMI, and diabetes in NHANES. In NHANES and PREVEND, prevalence rates of smoking were higher with lower income levels. The prevalence of hypertension was also highest in people with the lowest income level, but the pattern was less clear in PREVEND than in NHANES. Similarly, there was no clear pattern regarding increased albuminuria in PREVEND.
Education Levels
In NHANES and PREVEND, 16% of the study population had primary education. Participants with lower education levels were older in both cohorts; they had a higher BMI in PREVEND but not in NHANES. The lower education levels had higher proportions of patients with high albuminuria, impaired eGFR, and CKD in PREVEND. Similar trends were observed in NHANES. In NHANES, rates of hypertension and diabetes were higher in participants with lower education levels, but for smoking the rate was not highest in the lowest education level. In PREVEND, rates of hypertension, diabetes mellitus, and smoking were higher in participants with lower education levels.
Association of Income and Education Levels with CKD
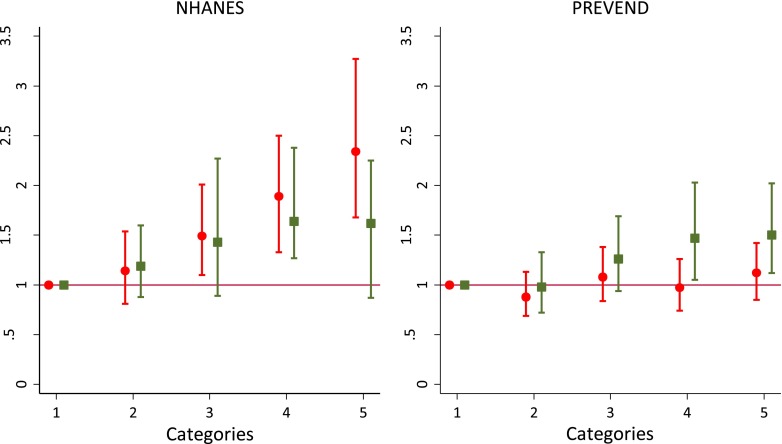
The adjusted odds ratios for the income and education levels with CKD, for both NHANES and PREVEND, are presented in Tables 3 and 4, respectively. In model 1 (adjusted for age, sex, and race), income and education levels were independently associated with CKD. In NHANES, after simultaneous adjustment in model 2, only income level showed a graded and significant association with CKD. In contrast, in PREVEND, education showed a stronger significant association with CKD. Figure 1 shows odds ratios for income and education levels adjusted for age, sex, race, and education (for income)/income (for education) in NHANES (left panel) and in PREVEND (right panel). In NHANES, adding income to the model with education improved the fit of the model more strongly (P<0.001) than did adding education to the model with income (P=0.07). In contrast, in PREVEND, the addition of education to the model with income resulted in a statistically stronger improvement in the fit of the model (P=0.01) than did adding income to the model with education (P=0.05). The interactions of age and race with education and income for CKD prevalence were not statistically significant in either NHANES or PREVEND.
Table 3.
Association of income quintiles with CKD prevalence in the National Health and Nutrition Examination Survey and in the Prevention of Renal and Vascular End-Stage Disease study
| Modela | Odds Ratio per Income Quintiles (95% Confidence Interval) | P Value for Trend | ||||
|---|---|---|---|---|---|---|
| Quintile 1 (Highest) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 (Lowest) | ||
| 1 | ||||||
| NHANES | 1 | 1.18 (0.87 to 1.59) | 1.65 (1.25 to 2.19) | 2.10 (1.58 to 2.79) | 2.74 (2.04 to 3.68) | <0.001 |
| PREVEND | 1 | 0.95 (0.74 to 1.21) | 1.21 (0.95 to 1.53) | 1.12 (0.88 to 1.43) | 1.29 (1.02 to 1.64) | 0.006 |
| 2 | ||||||
| NHANES | 1 | 1.10 (0.81 to 1.50) | 1.49 (1.10 to 2.01) | 1.82 (1.33 to 2.50) | 2.34 (1.68 to 3.27) | <0.001 |
| PREVEND | 1 | 0.88 (0.69 to 1.13) | 1.08 (0.84 to 1.38) | 0.97 (0.74 to 1.26) | 1.12 (0.85 to 1.42) | 0.30 |
Shown are odds ratios of CKD for income levels in logistic regression models. Results were similar when only age and sex were included as adjustment variables. NHANES, National Health and Nutrition Examination Survey; PREVEND, Prevention of Renal and Vascular End-stage Disease.
Model 1: age plus sex plus race. Model 2: model 1 plus education.
Table 4.
Association of education levels with CKD in the National Health and Nutrition Examination Survey and in the Prevention of Renal and Vascular End-Stage Disease study
| Modelsa | Odds Ratio per Education Levels (95% Confidence Intervals) | P Value for Trend | ||||
|---|---|---|---|---|---|---|
| Level 1 (Highest) | Level 2 | Level 3 | Level 4 | Level 5 (Lowest) | ||
| 1 | ||||||
| NHANES | 1 | 1.46 (1.12 to 1.90) | 1.63 (1.25 to 2.12) | 2.36 (1.81 to 3.08) | 1.78 (1.29 to 2.46) | <0.001 |
| PREVEND | 1 | 0.93 (0.70 to 1.21) | 1.21 (0.94 to 1.56) | 1.43 (1.12 to 1.84) | 1.53 (1.18 to 1.99) | <0.001 |
| 2 | ||||||
| NHANES | 1 | 1.19 (0.88 to 1.60) | 1.43 (0.89 to 2.27) | 1.74 (1.27 to 2.38) | 1.62 (0.87 to 2.25) | 0.05 |
| PREVEND | 1 | 0.98 (0.72 to 1.33) | 1.26 (0.94 to 1.69) | 1.47 (1.05 to 2.03) | 1.50 (1.12 to 2.02) | <0.001 |
Shown are odds ratios of CKD for education levels in logistic regression models. Results were similar when only age and sex were included as adjustment variables. NHANES, National Health and Nutrition Examination Survey; PREVEND, Prevention of Renal and Vascular End-stage Disease.
Model 1: age plus sex plus race. Model 2: model 1 plus education.
Figure 1.
Association of income quintiles and education levels with CKD in the National Health and Nutrition Examination Survey (NHANES) (left panel) and in Prevention of Renal and Vascular End-stage Disease study (right panel). Presented are odds ratios (95% confidence intervals) for income quintiles (circles and lines) and education levels (squares and lines) adjusted for age, sex, race, and education (for income) or income (for education). Reference categories (category 1) were highest income quintile and highest education level, respectively.
Sensitivity Analyses
In PREVEND, the results from redefining CKD by using an albumin-to-creatinine ratio of ≥30 mg/g instead of 24-hour urinary albumin excretion were essentially similar to the earlier results. Stratified analyses by race in NHANES showed similar stronger associations of income levels with CKD prevalence than of education in all racial groups (Supplemental Table 1). Results for participants age <65 years were essentially similar to the overall results (Supplemental Table 2). In participants age ≥ 65 years, the association of income and education levels with CKD was not statistically significant in either NHANES or PREVEND. After adjustment for smoking, diabetes, BP, BMI, and cardiovascular disease along with age, sex, and race, the association of CKD with income was stronger in NHANES (Supplemental Table 3) while the association with education was stronger in PREVEND (Supplemental Table 4). Results for the association of low renal function and albuminuria with SES measures are presented in Supplemental Tables 5 and 6, respectively. Analysis of combined data from both studies showed a significant interaction of cohort with income (P=0.01) and education (P=0.01) versus CKD prevalence. The associations of CKD with absolute income values also showed stronger income-CKD associations in NHANES than in PREVEND (Supplemental Figure 3).
Discussion
This study examined income and education for the relative strength of their association with CKD prevalence in the United States and The Netherlands. In NHANES, low income was more strongly associated with the likelihood of having CKD than was low education. In contrast, in PREVEND, low education was more strongly associated with CKD prevalence. Furthermore, the association of income with CKD in the United States cohort was stronger than the association of education with CKD in the Dutch cohort.
The stronger association of income with CKD in the United States cohort compared with the Dutch cohort might be due to two factors. First, access to health care in the United States is income dependent. Therefore, along with affecting health lifestyle choices (e.g., access to healthy diet), low income additionally affects health care access in the United States and ultimately affects the prevalence of CKD (26). Second, in the United States income inequalities are bigger (27), as also reflected by the higher PIR between the fifth and first quintiles in our study. In the Dutch cohort, disparities in CKD prevalence are more strongly determined by education, but not as strongly as by income in the United States. This finding suggests that the overall role of SES in The Netherlands is less marked than in the United States. In this study, analysis of combined NHANES and PREVEND data also showed differential associations of SES measures with CKD in both cohorts.
We used PIR instead of absolute income levels. Poverty thresholds in the United States and The Netherlands are measured from similar parameters of an individual’s purchasing power and financial ability for social inclusion that are country specific (25). Hence, levels of poverty are likely to be different in both countries. We believe that using the PIR (calculated from regional poverty threshold) enables a more valid comparison between countries than using absolute income levels. We performed a sensitivity analysis with absolute income levels (expressed per family member), which showed that the association of income with CKD was still stronger in the United States than in The Netherlands. This illustrates that a differential classification of income levels in NHANES and PREVEND from PIR is not responsible for the country differences.
In a separate analysis for participants age ≥65 years, we found no significant associations between SES measures and CKD. This may be due to lower power but could also be due to the fact that in developed economies, elderly patients usually receive state financial benefits and aid in accessing health care.
Income can affect CKD incidence, prevalence, and progression via several biologic, environmental, or behavioral mechanisms. Strong links of low income with poor food and lifestyle choices can lead to diabetes mellitus, obesity, and hypertension, which are established risk factors for CKD (28). Along with poor food and lifestyle choices, in a gene-environmental effect, certain genes (e.g., APOLI) linked to kidney disease are known to have more adverse effect among individuals living in poor neighborhoods (29).
For education, the factors linking low education to CKD are complex. Education can influence health and health-related behavior in many ways, including an individual’s cognitive ability to resolve adverse health issues, self-control, uptake of information, and use of health care resources; effective patient-physician communication; and food and lifestyle choices (30–32).
Strengths of this study are the use of detailed baseline information for relevant covariates from two large community-based study populations. We used PIR as measure of income. Along with household income, PIR accounts not only for household size but also for family composition, which has been a limitation in several previous studies of health disparities (33,34).
This study also had limitations. We studied general population cohorts with participants especially in the earlier stages of CKD. In these stages, CKD is unlikely to affect earning potential. However, because data on exposure and outcome are measured at one point in time and reverse causation cannot be excluded, results should be interpreted with caution. Second, the studies used different laboratory methods to measure CKD markers. However, because conclusions are mainly drawn on within-study differences in associations of SES measures with CKD, this will not affect our results. Third, PREVEND predominantly comprises whites, whereas NHANES also comprises non-Hispanic blacks and Mexican Americans, groups known to have a higher CKD prevalence. Stratified analysis on race in NHANES, however, resulted in essentially similar findings in all racial subgroups (Supplemental Table 1). Fourth, some might consider the lack of adjustment for the associations of SES measures with CKD prevalence for health (behavior) factors to be a limitation. These risk factors are more prevalent in persons with lower SES but should not be considered confounders because they are probably situated in the causal pathway by which low SES leads to CKD (35). However, to test whether the associations change after accounting for these factors, we additionally adjusted for smoking, diabetes, obesity, BP, and cardiovascular disease along with age, sex, and race. Essentially similar results were obtained. Finally, although we tried to keep the time period in which the NHANES and PREVEND studies were performed as similar as possible, a relatively small difference exists (1999–2002 and 1997–1998, respectively), which should be taken into account in interpreting our results.
In wake of recent developments in health care organization in the United States and Western Europe, this study may have important public health implications. The results indicate that in the United States disparities in health care access dependent on income may have negative consequences for health and consequently favor ensuring affordable care irrespective of income. In The Netherlands, improvement of education attainment might be one of the correct policy responses if, for example, there were no alternative (or cheaper) methods for addressing CKD-related health inequalities among different socioeconomic classes. Furthermore, our findings indicate that the definition of low SES as high-risk group to screen for CKD may differ between societies dependent on the organization of reimbursement of care. Some experts suggest there is insufficient evidence of improved health outcomes in asymptomatic adults from early identification of CKD (36). However, prevailing guidelines for CKD suggest screening for target populations (3). Research consistently demonstrates that taking into account income and education is important in screening for chronic diseases (37). Similarly, suggesting either education- or income-based screening for CKD might improve rates of participation in CKD screening. It might also help in effective use of limited resources. In the current economic scenario, reorganization of health care from a socioeconomic perspective might benefit from future research comparing the role of SES measures in overall health disparities in the United States and Western Europe.
In conclusion, income has a stronger association with CKD in the United States population than in a Dutch population. Screening strategies addressing social inequalities in CKD prevalence should therefore be region specific and use income-based social stratification in the United States and education-based social stratification in The Netherlands.
Disclosure
None
Supplementary Material
Acknowledgments
We thank NHANES and PREVEND study participants and the staff who helped in data collection.
This study did not receive any specific funding. All authors received funding from their respective institutes. NHANES is funded by the National Center for Health Statistics and PREVEND is funded by the Dutch Kidney Foundation.
The funding bodies had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
The abstract of this manuscript was published in the abstract supplement of the American Society of Nephrology Kidney Week 2012.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12521212/-/DCSupplemental.
References
- 1.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, Dekker FW: Screening strategies for chronic kidney disease in the general population: Follow-up of cross sectional health survey. BMJ 333: 1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 4.van der Velde M, de Jong PE, Gansevoort RT: Comparison of the yield of different screening approaches to detect chronic kidney disease. Nephrol Dial Transplant 25: 3222–3230, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S: Socioeconomic status in health research: One size does not fit all. JAMA 294: 2879–2888, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gilthorpe MS, Wilson RC: Rural/urban differences in the association between deprivation and healthcare utilisation. Soc Sci Med 57: 2055–2063, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, Billings J, Stewart A: Preventable hospitalizations and access to health care. JAMA 274: 305–311, 1995 [PubMed] [Google Scholar]
- 8.van Kippersluis H, Van Ourti T, O’Donnell O, van Doorslaer E: Health and income across the life cycle and generations in Europe. J Health Econ 28: 818–830, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Mackenbach JP : An analysis of the role of health care in reducing socioeconomic inequalities in health: The case of the Netherlands. Int J Health Serv 33: 523–541, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Schrijvers CT, Coebergh JW, van der Heijden LH, Mackenbach JP: Socioeconomic variation in cancer survival in the southeastern Netherlands, 1980-1989. Cancer 75: 2946–2953, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Stirbu I, Kunst AE, Mielck A, Mackenbach JP: Inequalities in utilisation of general practitioner and specialist services in 9 European countries. BMC Health Serv Res 11: 288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry R, Kann L, Collins JL, Kolbe LJ: The effect of socioeconomic status on chronic disease risk behaviors among US adolescents. JAMA 276: 792–797, 1996 [PubMed] [Google Scholar]
- 13.National Health and Nutrition Examination Survey (NHANES): Analytic and reporting guidelines. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2006. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf Accessed August 14, 2012
- 14.Alssema M, Newson RS, Bakker SJ, Stehouwer CD, Heymans MW, Nijpels G, Hillege HL, Hofman A, Witteman JC, Gansevoort RT, Dekker JM: One risk assessment tool for cardiovascular disease, type 2 diabetes, and chronic kidney disease. Diabetes Care 35: 741–748, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbesma N, Jansen DF, Heymans MW, Stolk RP, de Jong PE, Gansevoort RT, PREVEND Study Group : Development and validation of a general population renal risk score. Clin J Am Soc Nephrol 6: 1731–1738, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJL, de Jong PE, Gansevoort RT, PREVEND Study Group : Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 168: 897–905, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J, Prevention of Renal and Vascular End-stage Disease (PREVEND) Study Group : Microalbuminuria and risk of venous thromboembolism. JAMA 301: 1790–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 18.The Netherlands Institute for Social Research. Institute for Social Research. Available at: http://www.scp.nl/english Accessed June 9, 2012
- 19.UNESCO Institute for Statistics: International Standard Classification of Education, ISCED 2011. Paris, France, UNESCO, 2011 [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ: Recommendations for blood pressure measurement in humans and experimental animals: part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005 [DOI] [PubMed] [Google Scholar]
- 23.O’Connor RJ, Giovino GA, Kozlowski LT, Shiffman S, Hyland A, Bernert JT, Caraballo RS, Cummings KM: Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. Am J Epidemiol 164: 750–759, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Resnick HE, Foster GL, Bardsley J, Ratner RE: Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: The National Health and Nutrition Examination Survey. Diabetes Care 29: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bassuk SS, Berkman LF, Amick BC. 3rd: Socioeconomic status and mortality among the elderly: Findings from four US communities. Am J Epidemiol 155: 520–533, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Agrawal V, Jaar BG, Frisby XY, Chen SC, Qiu Y, Li S, Whaley-Connell AT, McCullough PA, Bomback AS, KEEP Investigators : Access to health care among adults evaluated for CKD: Findings from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 59[Suppl 2]: S5–S15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H: Do people die from income inequality of a decade ago? Soc Sci Med 75: 36–45, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Seligman HK, Laraia BA, Kushel MB: Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr 140: 304–310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi AI, Weekley CC, Chen SC, Li S, Tamura MK, Norris KC, Shlipak MG: Association of educational attainment with chronic disease and mortality: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 58: 228–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkleby MA, Jatulis DE, Frank E, Fortmann SP: Socioeconomic status and health: How education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 82: 816–820, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G: Indicators of socioeconomic position (part 1). J Epidemiol Community Health 60: 7–12, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR: Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55: 992–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews DC, McClellan WM, Shoham DA, Gao L, Warnock DG, Judd S, Muntner P, Miller ER, Powe NR: Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis 60: 779–786, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Qaoud TM, Nitsch D, Wells J, Witte DR, Brunner EJ: Socioeconomic status and reduced kidney function in the Whitehall II Study: Role of obesity and metabolic syndrome. Am J Kidney Dis 58: 389–397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glassock RJ, Winearls C: Screening for CKD with eGFR: Doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC: Progress in cancer screening practices in the United States: Results from the 2000 National Health Interview Survey. Cancer 97: 1528–1540, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.