Summary
Background and objectives
Plasma phosphate levels display considerable intraindividual variability. The phosphatonin fibroblast growth factor 23 is a central regulator of plasma phosphate levels, and it has been postulated to be a more stable marker than conventional CKD–mineral and bone disorder parameters. Thus, fibroblast growth factor 23 has been hypothesized to reflect time-averaged plasma phosphate levels in CKD patients.
Design, setting, participants, & measurements
Among 40 patients from the outpatient dialysis center, serial measurements of plasma calcium and phosphate (before every dialysis session) as well as C-terminal fibroblast growth factor 23, parathyroid hormone, and alkaline phosphatase (one time weekly) were performed over a study period of 4 weeks in November and December of 2011. Intraindividual variability of repeated plasma fibroblast growth factor 23 measurements compared with other CKD–mineral and bone disorder markers was tested, and the association of a single plasma fibroblast growth factor 23 measurement with time-averaged plasma phosphate levels was analyzed.
Results
Against expectations, intraindividual variability of fibroblast growth factor 23 (median coefficient of variation=27%; interquartile range=20–35) was not lower than variability of plasma phosphate (median coefficient of variation=15%; interquartile range=10–20), parathyroid hormone (median coefficient of variation=24%; interquartile range=15–39), plasma calcium (median coefficient of variation=3%; interquartile range=2–4), or alkaline phosphatase (median coefficient of variation=5%; interquartile range=3–10). Moreover, the correlation between the last fibroblast growth factor 23 measurement after 4 weeks and time-averaged plasma phosphate did not surpass the correlation between the last fibroblast growth factor 23 measurement and a single plasma phosphate value (r=0.67, P<0.001; r=0.76, P<0.001, respectively).
Conclusions
Surprisingly, fibroblast growth factor 23 was not more closely associated to time-averaged plasma phosphate levels than a single plasma phosphate value, and it did not show a lower intraindividual variability than other tested markers of CKD–mineral and bone disorder. Thus, fibroblast growth factor 23 should not be used in clinical practice as a reflector of time-averaged plasma phosphate levels.
Introduction
Elevated phosphate burden is considered to be an important driver of excess cardiovascular morbidity and mortality in CKD (1). Evidence from epidemiologic studies associated hyperphosphatemia with adverse outcome in dialysis (CKD 5D) patients (2–5), and experimental data revealed pathophysiological pathways for phosphate-induced vascular calcification (6). Subsequently, hyperphosphatemia has been acknowledged as a central therapeutic target in CKD, and recent Kidney Disease Improving Global Outcomes (KDIGO) guidelines on CKD–mineral and bone disorder (CKD-MBD) recommended prescription of oral phosphate binders to patients with elevated plasma phosphate, aiming to lower plasma phosphate to physiologic levels (7).
Of note, large intraindividual variability in plasma phosphate levels exists in hemodialysis patients: plasma phosphate levels follow a circadian rhythm (8), and high biologic variability persists even when drawing blood samples at the same time of day in CKD 5D patients (9).
Therefore, a single monthly plasma phosphate measurement (as suggested by current KDIGO guidelines) may be suboptimal for assessing time-averaged plasma phosphate levels in individual dialysis patients, and a more reliable marker is eagerly awaited.
Fibroblast growth factor 23 (FGF-23) is a central regulator of plasma phosphate levels. FGF-23 is secreted from osteoblasts and osteocytes on stimulation by calcitriol (10), parathyroid hormone (11), and (yet incompletely identified) other mechanisms. FGF-23 increases urinary phosphate excretion by reducing expression of sodium phosphate cotransporters in the proximal tubules (12) and lowers circulating calcitriol levels by decreasing renal expression of 1α hydroxylase, thus decreasing intestinal phosphate absorption (13,14).
Because of the central role of FGF-23 in keeping phosphate levels within physiologic boundaries, FGF-23 has been hypothesized to serve as a marker for time-averaged plasma phosphate levels (15), although no study has ever proven this idea.
Against this background, we initiated our Dialysis in Homburg evaluation (DIAL HOMe) study and measured CKD-MBD parameters repeatedly over a period of 28 days. Our aim was twofold: first, compare intraindividual variability of distinct parameters of CKD-MBD and second, analyze whether a single FGF-23 measurement drawn at the end of this study period (day 28) adequately reflects averaged plasma phosphate levels during the preceding 4 weeks.
Materials and Methods
The DIAL HOMe study was conducted over a 4-week period between November 28, 2011 and December 24, 2011. The local ethics committee approved the study design, and all participants gave their written informed consent before beginning of the study. The authors adhered to the Declaration of Helsinki.
We included CKD 5D patients who underwent three times weekly hemodialysis treatment at our outpatient dialysis center (Saarland University Medical Center, Homburg, Germany) if they had 3 months or more of dialysis vintage. Patients who were hospitalized and/or suffered from acute systemic infection at the time point of study initiation were excluded.
Forty-two adult hemodialysis patients met the inclusion criteria and gave their informed consent. One patient died during the study period, and another one patient withdrew his informed consent 1 week after beginning of the study. Thus, 40 participants completed the study, and their main baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of Dialysis in Homburg evaluation (DIAL HOMe) study participants
| Variable | Value |
|---|---|
| Calcium (mg/dl) | 9.2±0.8 |
| Phosphate (mg/dl) | 5.2±1.7 |
| Alkaline phosphatase (U/ml) | 93.7±50.4 |
| Parathyroid hormone (pg/ml) | 127 (66–267) |
| C-terminal FGF-23 (rU/ml) | 2785 (1386–9715) |
| 25-OH-vitamin D (ng/ml) | 32.0±10.1 |
| Glucose (mg/dl) | 128.7±44.9 |
| Plasma protein (g/L) | 70.0±5.1 |
| Hemoglobin (g/dl) | 11.3±1.5 |
| Ferritin (ng/ml) | 557 (346–744) |
| C-reactive protein (mg/L) | 4.4 (2.2–13.4) |
| Systolic BP (mmHg) | 137±25 |
| Diastolic BP (mmHg) | 70±13 |
| Body mass index (kg/m2) | 28.4±6.2 |
| Coronary artery disease (%) | 14 (35) |
| Cerebrovascular disease (%) | 8 (20) |
| Diabetes mellitus (%) | 20 (50) |
| Active smoking (%) | 6 (15) |
| Iron supplements (%) | 21 (53) |
| Phosphate binder intake (%) | 33 (83) |
| Cinacalcet intake (%) | 6 (15) |
| Active vitamin D intake (%) | 24 (60) |
| Self-reported diuresis>500 ml/24 h (%) | 24 (60) |
Continuous data are shown as mean ± SD or median (interquartile range) as appropriate; categorical variables are depicted as number of patients (percentage). FGF-23, fibroblast growth factor 23.
No modifications in any therapeutic factors affecting calcium-phosphate homeostasis during the 4-week study phase (namely dialysate calcium content, prescription of oral phosphate binders, active vitamin D, cholecalciferol, and calcimimetics) were allowed unless vitally indicated. Moreover, no changes in iron substitution, hemodialysis dose and/or frequency, anticoagulation, type, and surface of the dialyzer membrane, and dialysate flow rate were allowed throughout the study period.
The 4-week study comprised a total of 12 consecutive dialysis sessions; plasma samples were obtained from arteriovenous fistula needles or central venous catheters for dialysis immediately before each dialysis session. We measured predialysis plasma phosphate and calcium at each dialysis session. Plasma levels of C-terminal FGF-23, parathyroid hormone (PTH), and alkaline phosphatase (AP) were quantified one time weekly before each week’s third dialysis session. Finally, we assessed 25-OH-vitamin D levels 6 weeks before study initiation and 1 week after study finalization, and these two values were averaged to yield an individual’s 25-OH-vitamin D level.
Plasma samples were processed within 15 minutes, with the samples being centrifuged at 4,000 rpm for 5 minutes at room temperature. Supernatants were stored in aliquots at −80°C until further use. C-terminal FGF-23 levels were determined by ELISA (low cutoff value=1.5 rU/ml, high cutoff value=1500 rU/ml [samples with FGF-23>1500 rU/ml were measured after dilution]; Immutopics, San Clemente, CA). Levels of AP, calcium, and phosphate were determined by standard laboratory methods. PTH levels were measured by a second generation electrochemiluminescent immunoassay (normal range=15–65 pg/ml; Hoffmann-La Roche, Bale, Switzerland).
To minimize the influence of diurnal variation, time of blood collection was standardized for individual study participants, and patients were asked not to switch from morning to afternoon dialysis sessions or vice versa (in fact, only one patient temporarily changed from morning to afternoon dialysis sessions for logistic reasons).
After completion of the DIAL HOMe study period, we additionally aimed to assess variation of FGF-23 levels during a single week. Therefore, we recruited 11 of the original 40 DIAL HOMe participants in March of 2013, and we measured C-terminal FGF-23 before each of the three dialysis sessions (e.g., Monday, Wednesday, and Friday or Tuesday, Thursday, and Saturday as appropriate).
History of smoking, current drug intake, and cardiovascular comorbidity were assessed by standardized questionnaires and verified by chart review. Cardiovascular risk factors and comorbidity were defined as described previously (16,17). Echocardiography data on systolic left ventricular function were available in all patients.
Liver disease was defined by elevated liver enzymes (alanine/aspartate aminotransferase≥100 U/L), laboratory cholestasis (direct bilirubin≥1.0 mg/dl), prevalent chronic hepatitis B/ C, and/or histologic evidence of liver damage. Body weight and resting BP were recorded before and after each hemodialysis session.
In addition, we assessed information on food composition in 35 of 40 study participants by interview during the study period. Daily caloric and phosphate intakes were estimated using the German Bundeslebensmittelschlüssel provided by the Max-Rubner-Institute, Karlsruhe, Germany (www.mri.bund.de), which provides detailed information on nutritional compositions of a wide range of German foods.
Statistical analyses were performed with PASW Statistics 18, and the level of significance was set at P value<0.05. Categorical variables are presented as percentage of patients and compared by chi-squared test. Continuous data are expressed as mean ± SD or median (interquartile range [IQR]) as appropriate based on distribution properties and were compared by Mann–Whitney test, t test, or ANOVA for trend.
To evaluate the intraindividual stability of FGF-23, calcium, phosphate, AP, and PTH, we calculated percentage coefficients of variation (CVs; SD divided by the mean×100) (10) from the four measurements taken at the last dialysis day of each week.
Correlation coefficients between continuous variables were calculated according to Pearson.
Results
Population Characteristics
Forty patients, of whom 16 (40%) patients were women, completed the 4-week study period. Mean age of our patients was 69±14 years, and mean dialysis vintage was 3.1 (IQR=1.7–6.1) years. Two patients had undergone parathyroidectomy before study initiation. All participants underwent dialysis three times per week. Mean dialysis session length was 246±26 minutes; 30 patients were treated in the morning shift, and 10 patients were treated in the afternoon shift.
Mean daily phosphate intake was 2362±1292 mg, and mean daily caloric intake was 1438±525 kcal; phosphate intake was strongly correlated with caloric intake (r=0.87, P<0.001) but not mean plasma phosphate levels (r=0.15, P=0.40). Of note, there was no correlation between FGF-23 at week 4 and daily oral phosphate intake (r=0.21, P=0.23).
Comedication targeting CKD-MBD parameters comprised active vitamin D (24 patients), cinacalcet (6 patients), and various classes of phosphate binders (lanthanum carbonate [18 patients], calcium acetate [16 patients], sevelamer [15 patients], aluminum hydroxide [2 patients], and magnesium carbonate [2 patients]), and no changes in this medication occurred during the study period. Other main baseline characteristics are shown in Table 1.
Dialysis filter and dialysis dose were kept constant during the study period for all patients, with the exception of a single patient, in whom session time was reduced from 5 to 4 hours in the last 2 study weeks. Dialysate calcium concentration was kept stable at 3.0 mEq/L throughout the study period in all but one patient.
Intraindividual Variability of Calcium-Phosphate Metabolism Markers
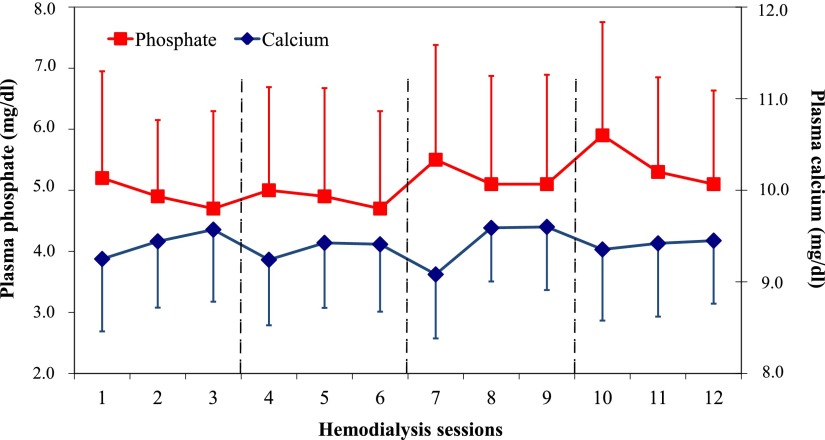
Baseline measurements of CKD-MBD parameters are depicted in Table 1. During the 4-week study period, we observed infradian rhythms in plasma phosphate levels, with peak values before the first dialysis session of each week, followed by a progressive decrease before the second and third sessions of each week (Figure 1).
Figure 1.
Plasma phosphate and calcium levels throughout the study period. Note that measurements 1, 4, 7, and 10 refer to the first measurements of each week; the 4 study weeks are separated by dashed lines. Phosphate and calcium levels are depicted as means and SDs.
In contrast, no infradian rhythms were observed in C-terminal FGF-23 levels among a subgroup of 11 study participants recruited after our original DIAL HOMe study period (Supplemental Figure 1).
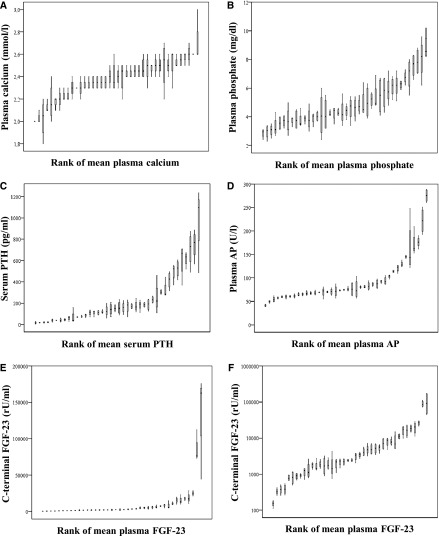
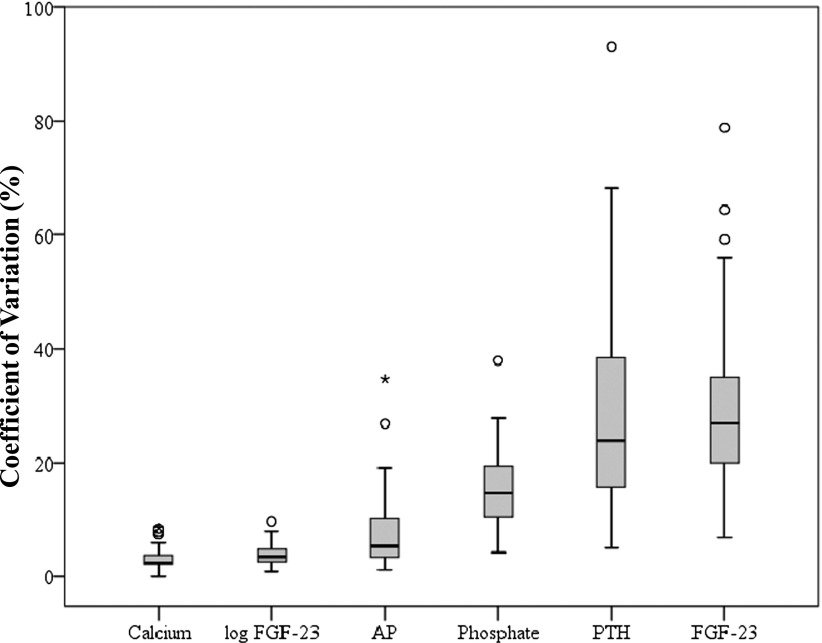
For evaluating the stability of CKD-MBD parameters during the 4-week study period, we first examined the intraindividual median and range of variation for plasma phosphate, calcium, PTH, and FGF-23 measured at the end of the first, second, third, and fourth study weeks (Figure 2; patients rank ordered by individual participants’ mean and FGF-23 depicted on both linear [Figure 2E] and logarithmic scales [Figure 2F]). By visual inspection, the overall range of intraindividual variation seems less pronounced for FGF-23 compared with PTH and phosphate. Nonetheless, when calculating the CV for each CKD-MBD parameter from the same four measurements, FGF-23 (median CV=27% [IQR=20–35]) and PTH (median CV=24% [IQR=15–39]) showed the highest CV values followed by phosphate (median CV=15% [IQR=10–20]), AP (median CV=5% [IQR=3–10]), and calcium (median CV=3% [IQR=2–4]) (Figure 3). If log-transferred FGF-23 levels were used for calculating CV, then FGF-23 becomes a seemingly more stable CKD-MBD parameter than plasma phosphate (median CV=4% [IQR=3–5]).
Figure 2.
Range of variation in CKD-MBD parameters. Depicted are medians, interquartile ranges, and minimum and maximum blood values of (A) calcium, (B) phosphate, (C) parathyroid hormone, (D) alkaline phosphatase, (E) C-terminal FGF-23 on a linear scale, and (F) C-terminal FGF-23 on a logarithmic scale. Values for each individual patient are plotted in rank order of their individual means. AP, alkaline phosphatase; CKD-MBD, CKD–mineral and bone disorder; FGF-23, fibroblast growth factor 23; PTH, parathyroid hormone.
Figure 3.
Intraindividual variation of CKD-MBD parameters. For each individual patient, coefficients of variation were calculated from four measurements (at the end of the first, second, third, and fourth weeks). Depicted are medians, interquartile ranges, and outliers from individual coefficients of variation across the study cohort of 40 hemodialysis patients. AP, alkaline phosphatase; CKD-MBD, CKD–mineral and bone disorder; FGF-23, fibroblast growth factor 23; PTH, parathyroid hormone.
Of note, calculating CVs from all 12 phosphate measurements (e.g., on different days of the weeks; CV=14% [IQR=11–18]) or the first dialysis day of each week (e.g., Monday or Tuesday; CV=13% [IQR=8–19]) rather than from four measurements on Fridays or Saturdays, respectively, yielded virtually identical findings.
Correlations between a Single FGF-23 Measurement and Plasma Phosphate Levels
To further analyze how far a single FGF-23 measurement reliably indicates average plasma phosphate levels within the preceding weeks, we compared the correlation of the FGF-23 levels at study end with either a single plasma phosphate measurement obtained at the same day or time-averaged plasma phosphate levels over the 4-week study period.
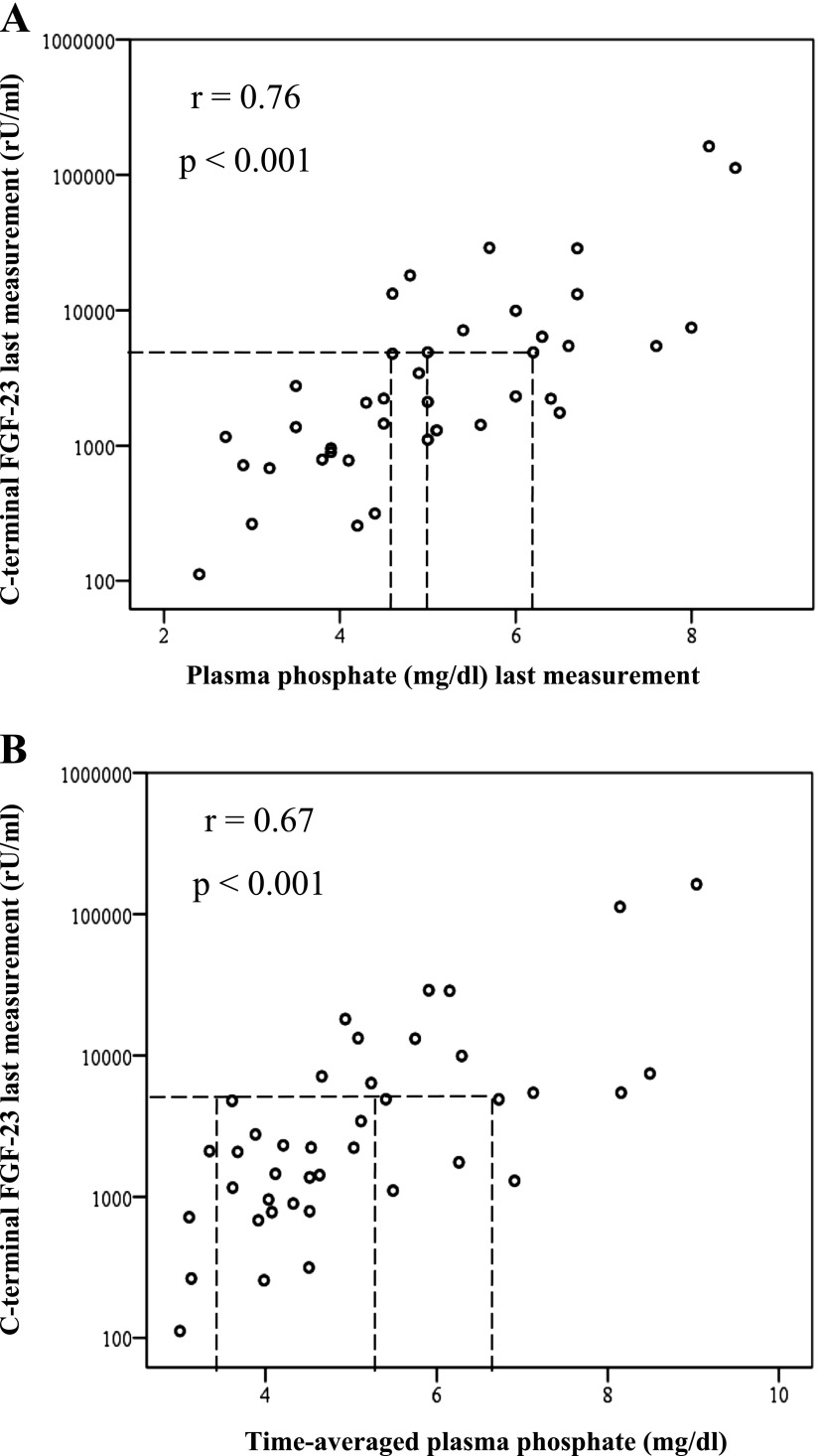
Table 2 depicts correlation coefficients between CKD-MBD parameters measured at the end of the 4-week study period; expectedly, a significant correlation between FGF-23 and plasma phosphate was found (Figure 4A).
Table 2.
Correlation coefficients between CKD–mineral and bone disorder parameters
| CKD-MBD Parameter | Phosphate | AP | Log PTH | Log FGF-23 | Time-Averaged Phosphate |
|---|---|---|---|---|---|
| Calcium | r=−0.17, P=0.31 | r=0.17, P=0.29 | r=−0.06, P=0.71 | r=0.05, P=0.77 | r=−0.35, P=0.03 |
| Phosphate | r=0.13, P=0.42 | r=0.45, P=0.004 | r=0.76, P<0.001 | r=0.87, P<0.001 | |
| AP | r=0.40, P=0.01 | r=0.25, P=0.12 | r=0.08, P=0.61 | ||
| Log PTH | r=0.41, P=0.009 | r=0.34, P=0.03 | |||
| Log FGF-23 | r=0.67, P<0.001 |
Because of non-normal distribution, FGF-23 and PTH were log-transformed for this analysis. AP, alkaline phosphatase; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; r, Pearson correlation coefficient; P, level of significance.
Figure 4.
Correlation of FGF-23 measured at study end with (A) a single plasma phosphate measurement obtained at study end or (B) plasma phosphate measurements averaged across the 4-week study period. As a representative example illustrating wide ranges of individual plasma phosphate measurements in patients with similar FGF-23 levels, lines are drawn for patients with FGF-23 of ∼8.000 rU/ml. Note that FGF-23 levels are depicted on a logarithmic scale. FGF-23, fibroblast growth factor 23.
Notably, a correlation analysis between FGF-23 at study end and time-averaged plasma phosphate levels (Figure 4B) yielded a numerically lower correlation coefficient than the correlation analysis between FGF-23 and a single phosphate measurement (Figure 4A). In a linear regression analysis, a single phosphate measurement even outperformed time-averaged plasma phosphate levels as a predictor for log-transformed FGF-23 at study end (coefficient of regression [B], 95% confidence interval [95% CI], and level of significance [P] for a single phosphate measurement and time-averaged plasma phosphate, respectively: B=0.27, 95% CI=0.08 to 0.46, P=0.007; B=0.08, 95% CI=−0.11 to 0.27, P=0.41).
Moreover, despite the seemingly high correlation coefficient between FGF-23 and both single and time-averaged plasma phosphate levels, individuals with very similar FGF-23 measurement may have widely differing plasma phosphate levels (as depicted in Figure 4 for a plasma FGF-23 of ∼8,000 rU/ml), further arguing against the use of FGF-23 as a reliable indicator of plasma phosphate levels.
We aimed to better characterize those patients with high FGF-23 levels (arbitrarily defined as ≥2000 rU/ml at week 4) and normophosphatemia during the 4-week study (time-averaged plasma phosphate≤4.5 mg/dl). Five patients fulfilled these criteria; they did not significantly differ in daily dietary phosphate (2312±529 mg) or caloric intake (1571±262 kcal) from the remaining patients (daily dietary phosphate intake=2370±1385 mg, daily caloric intake=1416±557 kcal; P=0.93 and P=0.55, respectively). Moreover, we excluded a significant difference in dialysis vintage (FGF-23≥2000 rU/ml and normophosphatemia=4.4±2.4 years; remaining patients=3.7±2.4 years; P=0.87). Finally, compared with the remaining 35 patients, 5 patients with plasma phosphate levels within the normal range and FGF-23 levels≥2000 rU/ml did not differ in the prevalence of heart failure (P=0.69), diabetes mellitus (P=0.63), liver disease (P=0.26), or residual renal function (defined as self-reported diuresis≥500 ml/24 hours).
Discussion
In our DIAL HOMe study, we set out to test the use of FGF-23 as a stable reflector of time-averaged plasma phosphate levels during the preceding 4 weeks. Such use of FGF-23 was suggested earlier (15) on the basis of the central physiologic role of FGF-23 in phosphate regulation (18).
We a priori postulated that any stable marker of time-averaged plasma phosphate levels should fulfill two requirements. First, such a marker should have a lower intraindividual variability than plasma phosphate itself. Second, in a given individual, a single measurement of this marker should be more closely associated with mean plasma phosphate levels averaged from repeated measurements than a random single phosphate value.
Intraindividual Variation of CKD-MBD Parameters
First, we could confirm that plasma phosphate levels undergo substantial infradian rhythm, with highest levels after the long interdialytic interval at the beginning of the week on Mondays/Tuesdays. In contrast, we found no such infradian rhythm for FGF-23 levels.
Because circadian rhythms in plasma phosphate levels have been described earlier (9,19,20), we deliberately set out a study protocol, in which patients were not considered to change from morning to afternoon dialysis sessions and vice versa.
Respecting these infradian rhythms of plasma phosphate, we decided to calculate CVs on the basis of a single weekly phosphate measurement (on the same days on which FGF-23, AP, and PTH were measured; i.e., Fridays or Saturdays) rather than all 12 phosphate values for comparing intraindividual stability of CKD-MBD parameters. In line with clinical practice, most dialysis units perform routine laboratory tests on the same days of the week. Notably, calculating CVs from all 12 measurements or the first dialysis day of each week rather than 4 measurements on Fridays or Saturdays, respectively, yielded virtually identical findings.
Against our expectations, we found that repeatedly measured FGF-23 displayed higher rather than lower CVs compared with plasma phosphate measurements. After log transformation, FGF-23 values seemingly become more stable.
Notably, the only other epidemiologic study that assessed stability of FGF-23 in CKD patients revealed similar findings: among 44 peritoneal dialysis patients who underwent three monthly FGF-23 measurements, FGF-23 became a seemingly stable parameter only after log transformation (21).
Admittedly, high stability after log transfer of a biologic variable is of limited clinical relevance, because nephrologists will be confronted with crude FGF-23 values rather than log-transferred values in their clinical practice.
Association between FGF-23 and Time-Averaged Plasma Phosphate
As expected, we found a strong correlation between FGF-23 levels and plasma phosphate levels at any given time point, which was shown before among individuals with intact renal function (22,23) and in multiple cohort studies across the spectrum of CKD (24–28).
This close association between FGF-23 and plasma phosphate levels and the high intraindividual variability of plasma phosphate measurements fueled the idea that FGF-23 might mirror time-averaged plasma phosphate levels, similar to glycosylated hemoglobin in glucose metabolism (29). Interestingly, the idea of using FGF-23 as such a marker for time-averaged plasma phosphate levels has never been proven by adequate clinical data.
We now found that the correlation between FGF-23 levels and time-averaged phosphate did not surpass the correlation between FGF-23 levels and a single phosphate measurement. Moreover, we exemplarily illustrate that CKD patients with similar FGF-23 levels may have dramatically different plasma phosphate levels: patients who are in KDIGO target ranges for plasma phosphate and patients with massively elevated plasma phosphate levels may share virtually identical FGF-23 levels. Although various factors beyond CKD-MBD parameters may affect FGF-23 (30–32), we did not identify single factors explaining the coexistence of very high FGF-23 levels and normophosphatemia in individual patients, which may result from the limited patient size of our cohort.
In summary, on the basis of our data, no single FGF-23 level can reliably separate patients with adequate plasma phosphate values from patients with poor plasma phosphate control.
Limitations
We cannot rule out that FGF-23 measurement may reveal information on phosphate balance that is not reflected by immediate changes in plasma phosphate levels. Of note, the largest amount of phosphate exists in other compartments outside the intravascular space, and harmful extraosseous phosphate deposition with vascular calcification may occur even before plasma phosphate levels are overtly elevated. Nonetheless, epidemiologic data on the association between FGF-23 levels and vascular calcification revealed discrepant findings (14,33).
Moreover, we arbitrarily defined a study period of 4 weeks for assessing average plasma phosphate levels, and it may be argued that an even longer observation period would have yielded discrepant results.
Additionally, in CKD 5 patients who have very high FGF-23 levels, serial dilutions are necessary for measuring C-terminal FGF-23 levels. These dilutions could increase analytic variability and hence, CVs in our sample. Of note, CVs did not differ between patients with the lowest (CV=26% [IQR=20–39]) and highest (CV=27% [IQR=19–35]) FGF-23 levels.
As another limitation, we focused our cohort study on C-terminal FGF-23 levels, because many nephrologists use C-terminal FGF-23 rather than intact FGF-23 measurements in clinical practice for logistic and economical reasons. Moreover, because of higher instability of intact FGF-23 in the preanalytic phase, the measurement of C-terminal FGF-23 is recommended, especially for samples from CKD patients (34).
In our DIAL HOMe study, we investigated two aspects: first, the intraindividual stability of FGF-23 in case of repeated measurements and second, its value as a marker for time-averaged plasma phosphate levels over 4 weeks. Against our expectations, plasma FGF-23 had a higher intraindividual variation than plasma phosphate, and plasma FGF-23 failed to provide specific information on time-averaged plasma phosphate levels during the preceding weeks.
Disclosures.
S.S. and K.S.R. have received speaker fees from Sanofi. G.L., P.E., L.H.F., A.L.-G., and M.Z. have no conflict of interest to declare. M.K. has received speaker fees from Baxter, Abbot, and Fresenius. D.F. has been a board member and additionally, received speaker fees from Shire, Amgen, Sanofi-Genzyme, and FMC. G.H.H. has received travel grants and speaker fees from Shire.
Supplementary Material
Acknowledgments.
We thank Marie-Theres Blinn and Martina Wagner for their excellent assistance work.
Footnotes
S.S. and G.L. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13021212/-/DCSupplemental.
References
- 1.Ketteler M, Wolf M, Hahn K, Ritz E: Phosphate: A novel cardiovascular risk factor. Eur Heart J 34: 1099–1101, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Wald R, Sarnak MJ, Tighiouart H, Cheung AK, Levey AS, Eknoyan G, Miskulin DC: Disordered mineral metabolism in hemodialysis patients: An analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis 52: 531–540, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Ring T, Sanden AK, Hansen HH, Halkier P, Nielsen C, Fog L: Ultradian variation in serum phosphate concentration in patients on haemodialysis. Nephrol Dial Transplant 10: 59–63, 1995 [PubMed] [Google Scholar]
- 9.Levitt H, Smith KG, Rosner MH: Variability in calcium, phosphorus, and parathyroid hormone in patients on hemodialysis. Hemodial Int 13: 518–525, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y: Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18: 2683–2688, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Baum M, Schiavi S, Dwarakanath V, Quigley R: Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int 68: 1148–1153, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Seiler S, Heine GH, Fliser D: Clincial relevance of FGF-23 in chronic kidney disease. Kidney Int Suppl 114: S34–S42, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Heine GH, Seiler S, Fliser D: FGF-23: The rise of a novel cardiovascular risk marker in CKD. Nephrol Dial Transplant 27: 3072–3081, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH: CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 32: 84–92, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH: CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 60: 1512–1520, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Ketteler M, Petermann AT: Phosphate and FGF23 in early CKD: On how to tackle an invisible foe. Nephrol Dial Transplant 26: 2430–2432, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Perry HM, 3rd, Province MA, Droke DM, Kim GS, Shaheb S, Avioli LV: Diurnal variation of serum calcium and phosphorus in postmenopausal women. Calcif Tissue Int 38: 115–118, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Ishida M, Seino Y, Yamaoka K, Tanaka Y, Satomura K, Kurose Y, Yabunchi H: The circadian rhythms of blood ionized calcium in humans. Scand J Clin Lab Invest Suppl 165: 83–86, 1983 [PubMed] [Google Scholar]
- 21.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M: Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol 6: 2688–2695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC: Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J 161: 956–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semba RD, Fink JC, Sun K, Cappola AR, Dalal M, Crasto C, Ferrucci L, Fried LP: Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol 7: 85–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH: FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant 25: 3983–3989, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24: 2792–2796, 2009 [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association : Standards of medical care in diabetes—2009. Diabetes Care 32[Suppl 1]: S13–S61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hryszko T, Rydzewska-Rosolowska A, Brzosko S, Koc-Zorawska E, Mysliwiec M: Low molecular weight iron dextran increases fibroblast growth factor-23 concentration, together with parathyroid hormone decrease in hemodialyzed patients. Ther Apher Dial 16: 146–151, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M, Chronic Renal Insufficiency Cohort : Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol 7: 1155–1162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS, Scheller B, Böhm M, Fliser D, Heine GH: The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J 32: 2688–2696, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fassbender WJ, Brandenburg V, Schmitz S, Sandig D, Simon SA, Windolf J, Stumpf UC: Evaluation of human fibroblast growth factor 23 (FGF-23) C-terminal and intact enzyme-linked immunosorbent-assays in end-stage renal disease patients. Clin Lab 55: 144–152, 2009 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.