Abstract
Background
Second generation antipsychotics (SGAs) are thought to have a lower likelihood of inducing extrapyramidal symptoms (EPS) than first generation antipsychotics (FGAs). Clinical observations suggest that younger patients may be more sensitive to SGA-associated EPS than adults and require therapy with anticholinergic agents, which are known to impair cognitive performance. The scope of anticholinergic use in this patient population and differences in utilization across SGAs (as well as FGAs) has not been extensively studied.
Objective
The primary objective of this study was to determine the proportion of patients five to 18 years of age who received anticholinergic therapy during the initial stages of antipsychotic treatment. A secondary objective was to compare anticholinergic use across patients receiving aripiprazole, risperidone, and quetiapine, SGAs previously identified to be the most commonly prescribed at the academic institution studied.
Methods
Patients five to 18 years of age initiating a trial of an antipsychotic between January 1, 2005 and September 1, 2008 were identified in a retrospective review of prescription and medical records. Demographic characteristics, antipsychotic and anticholinergic utilization, indications, diagnoses, and concomitant medications and doses were collected from the electronic medical record. For patients who received more than one therapeutic course of an antipsychotic during the identified timeframe, only the first course identified in the medical record was used for analyses. Anticholinergic utilization at antipsychotic initiation and after 30 days was assessed. Variables associated with anticholinergic use and differences across agents were identified.
Results
A total of 235 antipsychotic treatment courses were identified. Of these, 152 patients met our inclusion criteria and represented the first documented use of an antipsychotic at the present institution. Anticholinergic use at any time during the first 30 days of treatment was identified in 32 patients (21%) while EPS was documented for 12 patients (7.8%). FGA or polypharmacy defined as simultaneous use of ≥1 scheduled antipsychotic versus SGA (OR=18.98; 95% CI:4.74 – 75.95) was the primary characteristic significantly associated with anticholinergic use 30 days after initiation. Risperidone, quetiapine, and aripiprazole were the three most commonly prescribed antipsychotics. Of these SGAs, anticholinergic use was most frequently prescribed with risperidone treatment (p=0.03).
Conclusion
Anticholinergic use exceeded the incidence of EPS as documented in the electronic medical record (21% vs 8%) and differed across individual medications as well as antipsychotic class. Use of an FGA or polypharmacy was a key predictor of anticholinergic use.
Keywords: Anticholinergic, Antipsychotic, Pediatric, Adolescent, Extrapyramidal symptoms
Introduction
The use of antipsychotic medications in pediatric patients has increased exponentially over the last decade.1 This is likely in part due to an increased recognition and diagnosis of psychiatric disorders in younger patient populations. Currently, risperidone, aripiprazole, and olanzapine are the second generation antipsychotic (SGA) agents approved for use by the U.S. Food and Drug Administration (FDA) in children and adolescents with irritability associated with autism, bipolar mania, or schizophrenia. However, off-label use of SGAs including paliperidone, quetiapine, and ziprasidone has been described in pediatric patients.2 First generation antipsychotics with indications for children and adolescents include haloperidol, pimozide, and thioridazine. Patients with disruptive behavioral disorders, mental retardation, tic disorders, as well as numerous other childhood psychiatric conditions are also prescribed antipsychotic agents in selected cases.3 Long-term treatment with first-generation antipsychotics (FGA) has been associated with movement disorders such as tardive dyskinesia and extrapyramidal symptoms (EPS).4 This has been well documented in both adult and child psychiatric patients. Tardive dyskinesia occurs in approximately one in three children taking FGAs long term.1
Adult studies demonstrate improvement in clinical symptoms and fewer movement-related side effects with the use of SGAs versus FGAs.5 More specifically, adult studies indicate that SGAs are associated with fewer EPS and may confer more negative symptom improvement in schizophrenia for some patients.6 Whether this data can be extrapolated to the pediatric population is unknown. A number of studies investigating the safety and usage of SGAs in children and adolescents have been conducted. In adolescent patients with schizophrenia, the incidence of side effects, particularly EPS, appears to be higher in younger patients compared with adults.7 Similarly, the results of a pilot study of antipsychotics in psychotic youth implicated more prevalent and severe EPS in pediatric patients compared to adults.8
EPS are thought to be related to dose-dependent dopamine type-2 (D2) receptor antagonism in the nigrostriatal dopamine pathway. Significant D2 antagonism disinhibits acetylcholine release from cholinergic interneurons which results in increased motor activity. There is a correlation between the number of these receptors and age which may predispose younger patients to antipsychotic-associated EPS.8,9 While all antipsychotics antagonize D2 receptors, FGAs bind tightly and generally dissociate more slowly than their second generation counterparts. This is hypothesized to be one mechanism underlying increased EPS liability from older agents.10 In addition to this theory, atypical antipsychotics, unlike conventional agents, antagonize serotonin-2A (5-HT2A) receptors. Antagonism of these receptors results in an increased release of dopamine in the nigrostriatal pathway, which helps counteract the negative effects of D2 blockade in this area of the brain.9
Clinical observations suggest that children and adolescents may be more sensitive to EPS, even from some SGAs, and often require adjunctive therapy with anticholinergic agents. Anticholinergic medications that are centrally acting are used to treat EPS. Commonly used agents include benztropine, diphenhydramine and trihexyphenidyl. While these agents may alleviate EPS in many patients, both pediatric and adult, these medications are also associated with cognitive impairment, which is an issue of great concern in this patient population.11 Anticholinergic agents have a dose-dependent negative effect on cognition.12 These agents impair verbal learning of new material and declarative memory, although in the clinical setting the potential adverse consequences of anticholinergic treatments often go unchecked.
The rationale for this study is that clinical observations suggest that anticholinergic medications are commonly utilized in pediatric patients taking both FGAs and SGAs, despite the theoretical decrease in incidence of EPS of these second generation agents. However, the scope of anticholinergic use and differences across antipsychotic classes and individual agents has not been extensively studied. In this study, we characterized the proportion of pediatric patients at a major Midwest academic medical center taking an antipsychotic who received an anticholinergic agent for the management of EPS, and compared the usage of anticholinergics across children and adolescents receiving aripiprazole, risperidone and quetiapine, known to be the three most commonly prescribed SGAs for younger patients at this center.
Methods
This study was a retrospective, medical record evaluation of patients seen at a major Midwest academic medical center, approved by the local Institutional Review Board (IRB). The primary objective of this study was to determine the proportion of patients five to 18 years of age who received anticholinergic therapy during the initial stages of antipsychotic treatment. The secondary objective was to compare anticholinergic use across patients receiving aripiprazole, risperidone, and quetiapine. These three agents are known from previous drug utilization analyses (data not presented here) to be the most commonly prescribed SGAs at the present institution. Additionally, characteristics such as demographic information, psychiatric diagnoses, antipsychotic doses and use of other medications (psychotropic and non-psychotropic) were evaluated with regard to anticholinergic use. Children and adolescents five to 18 years of age were included for study if they filled a prescription for any antipsychotic agent between January 1, 2005 and September 1, 2008 at one of the institutional pharmacies. The database for five outpatient pharmacies affiliated with the hospital was accessed and a search performed to identify prescription fill history of patients meeting these criteria. Patients who received at least two consecutive months of an antipsychotic were selected for inclusion. Use of an antipsychotic for a minimum of two consecutive refills per the institution's pharmacy database was corroborated with documentation of utilization for a period spanning a minimum of 60 days in the electronic medical record during the same time period. For patients who received more than one therapeutic trial of an antipsychotic during 2005-2008, only data from the first medication trial was included in these analyses. Exclusion criteria consisted of age greater than 18 years, as well as presence of a non-antipsychotic medication (such as metoclopramide or prochlorperazine) or neurologic disorder known to elicit EPS. In addition we excluded patients who had documented utilization of an antipsychotic agent in the previous 60 days as noted by either prescription records or electronic medical records. Patients for whom there was not enough information available in the medical records were also excluded from this study.
Data collected included demographic characteristics (age at time of antipsychotic initiation, gender, race, height and weight), medical and psychiatric diagnoses, antipsychotic therapy, anticholinergic therapy, medication doses and schedules, length of antipsychotic and anticholinergic therapies, and documented incidence of EPS. Antipsychotic class was defined as first generation, second generation, or polypharmacy (the simultaneous use of two or more scheduled antipsychotics). Of note, while patients and antipsychotics agents used were identified through the pharmacy prescription database, additional medication information (i.e. usage of medications other than antipsychotic agents) was derived from the electronic medical record. Extrapyramidal symptoms were defined as documented by the treating physician. For the purposes of determining whether extrapyramidal side effects were documented in patients receiving anticholinergic agents, symptoms consistent with akathisia (e.g. inner restlessness, or restlessness qualified as akathisia by the treating clinician), pseudoparkinsonism (e.g. tremor, cogwheel rigidity, akinesia), or dystonia defined the presence of EPS. One instance of “EPS-associated agitation” and another instance describing “EPS” but without further details were both considered as “documented EPS” for the purposes of the data reported herein. Patient diagnoses were defined as documented in the medical record by the treating physician, as well. Those diagnoses consistent with categories outlined in the Diagnostic and Statistics Manual for Mental Disorders fourth edition text revision (DSM-IV-TR 2000) were categorized as “Axis I Psychiatric Diagnoses” for the purposes of comparisons outlined in Tables 3 and 4 and Figure 1.
Table 3. Anticholinergic use at 30 days from AP initiation.
| Variable | Mean ± standard deviation | P valuee | |
|---|---|---|---|
|
| |||
| On AC (n=28) | Not on AC (n=124) | ||
| Age | 14.5 ± 2.3 | 14.5 ± 2.4 | 0.99 |
| Sex (% Male) | 68% | 45% | 0.03 |
| Starting antipsychotic dosea | 551 ± 436 | 384 ± 378 | 0.045 |
| Total number of medications at antipsychotic initiationb | 4.5 ± 2.3 | 3.5 ± 1.8 | 0.02 |
| Total number of Axis I diagnosesc | 2.2 ± 0.7 | 1.8 ± 0.8 | 0.02 |
| Total number of diagnosesd | 3.4 ± 1.3 | 3 ± 1.4 | 0.16 |
AC: Anticholinergic
Dose in chlorpromazine equivalents
Total number of scheduled medications documented in the medical record
Chart documented psychiatric diagnoses categorized in the Diagnostic and Statistics Manual for Psychiatric Disorders Text Revision
Total number of medical and psychiatric diagnoses documented in the most recent (relative to antipsychotic initiation) primary care and psychiatry notes of treating physicians
Mann-Whitney U Test
Table 4. Univariate analysis of anticholinergic use at 30 days.
| Analysis of Maximum Likelihood Estimates | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter | Estimate | Standard Error | Pr > χ2 | OR | 95% CI |
| Age | 0.00903 | 0.0876 | 0.9179 | 1.009 | 0.850 – 1.198 |
| Sex (male vs. female) | 0.9414 | 0.4431 | 0.0336 | 2.563 | 1.076 – 6.109 |
| Total number of diagnosesa | 0.1827 | 0.1518 | 0.2285 | 1.201 | 0.892 – 1.616 |
| Total number of Axis I diagnosesb | 0.4980 | 0.2434 | 0.0408 | 1.646 | 1.021 – 2.652 |
| Antipsychotic classc | 2.6731 | 0.5431 | <.0001 | 14.485 | 4.996 – 42.000 |
| Daily antipsychotic dose at initiationd | 0.00110 | 0.000527 | 0.0367 | 1.001 | 1.000 – 1.002 |
| Daily antipsychotic dose at one monthd | 0.00100 | 0.000489 | 0.0407 | 1.001 | 1.000 – 1.002 |
Total number of medical and psychiatric diagnoses recorded in the medical record at the most recent primary care and psychiatry notes of treating physicians
Chart documented psychiatric diagnoses categorized in the Diagnostic and Statistics Manual for Psychiatric Disorders Text Revision
FGA or polypharmacy vs. SGA
Dose in chlorpromazine equivalents
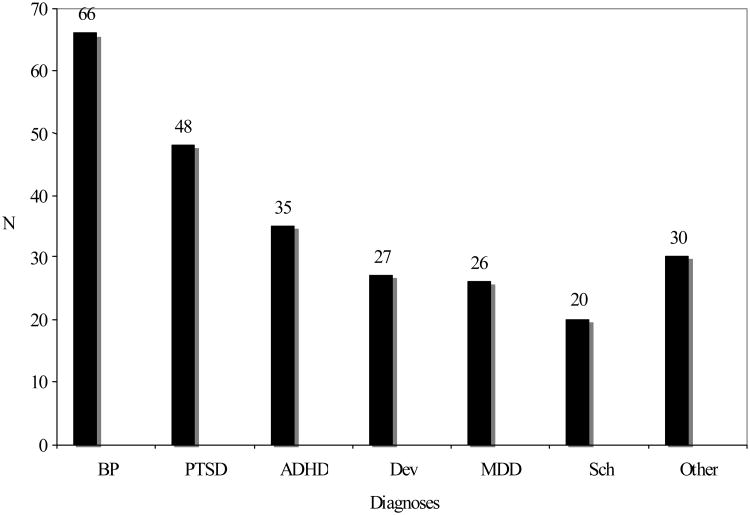
Figure 1. Documented psychiatric diagnoses for patients included in analysesa.
aBP – bipolar disorder; PTSD – post-traumatic stress disorder; ADHD – attention deficit hyperactivity disorder; Dev – developmental disorder (e.g. autism, Asperger's disorder, or mental retardation); MDD – major depressive disorder; Sch – schizophrenia
The number of patients carrying a chart diagnosis of the following psychiatric diagnoses is summarized. The “Other” category included psychosis not otherwise specified (NOS), impulse control disorder, intermittent explosive disorder, oppositional defiant disorder, reactive attachment disorder, mood disorder NOS, or social anxiety disorder.
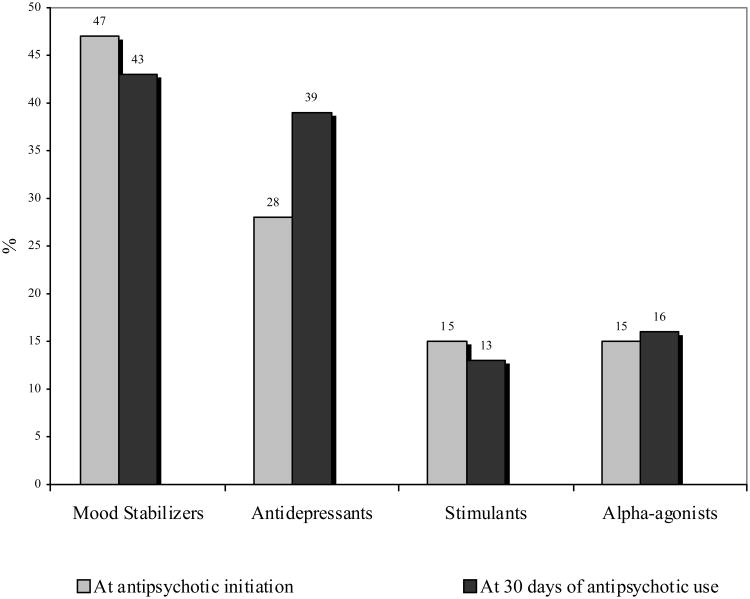
In addition to antipsychotic agents, use of other psychotropic medications as documented in the electronic medical record was identified. These agents were classified into four categories: “mood stabilizers”, antidepressants, “stimulants”, and “α agonists”. Categories of these additional psychotropic medications were compared between those patients prescribed an anticholinergic agent 30 days after antipsychotic initiation and those who were not prescribed an anticholinergic agent. Mood stabilizer medications included lithium, carbamazepine, divalproex sodium, lamotrigine, or oxcarbazepine. Antidepressants used by this patient population included selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, selective serotonin and norepinephrine reuptake inhibitors (SNRIs), mirtazepine, and atomoxetine. Atomoxetine, indicated for the treatment of attention deficit hyperactivity disorder, was categorized as an antidepressant because of mechanistic similarities (norepinephrine reuptake inhibition) and to separate it from the “stimulant” category. Stimulants included amphetamine derivatives such as dextroamphetamine and amphetamine salts, as well as methylphenidate products. Alpha agonists used included clonidine and guanfacine. Clinical data were reliably available at the time of antipsychotic initiation and at one month follow-up appointments. Subsequent clinical visits were inconsistent. Therefore, the analyses presented here represent utilization over the first 30 days of therapy.
Statistical Analysis
Summary statistics for demographic characteristics, antipsychotic use, anticholinergic use, indication, diagnoses and relative doses were tabulated for the study sample as a whole. Chlorpromazine equivalents were calculated using previously described methods by Kane (1996) and Woods (2003) in an effort to standardize antipsychotic exposure.13,14 Chlorpromazine equivalents are doses of FGAs that result in the same D2 receptor occupancy as 100 mg of chlorpromazine. For SGAs, the minimum effective doses of various SGAs versus haloperidol were used to estimate chlorpromazine equivalents using the convention that 2 mg of haloperidol is equivalent to 100 mg of chlorpromazine.14 Recognizing the limitations of these conversions, in our study, the use of chlorpromazine equivalents was reserved for secondary analyses to gain insight on dose as a potential predictor of anticholinergic use in patients receiving any antipsychotic.
The Sapiro-Wilk test was used to determine that age, number of total diagnoses, number of psychiatric diagnoses, antipsychotic doses (measured in chlorpromazine equivalents), total number of scheduled medications at antipsychotic initiation, and total number of psychotropic medications at antipsychotic initiation all significantly deviated from a normal distribution (p<0.05). The Mann-Whitney U test was therefore used to compare age, number of total diagnoses, number of psychiatric diagnoses, antipsychotic doses (measured in chlorpromazine equivalents), total number of scheduled medications at antipsychotic initiation, and total number of psychotropic medications at antipsychotic initiation and those who were not (Table 3). Pearson's chi-square test was used to compare the proportion of males and females between patients who were prescribed an anticholinergic agent 30 days after antipsychotic initiation and those who were not (Table 3). Pearson's chi-square test was also used to compare anticholinergic use 30 days after antipsychotic initiation between patients using FGAs, SGAs, and polypharmacy groups. We also determined a priori to compare anticholinergic use across second generation agents only. Based on our clinical observations and prior drug utilization analyses of antipsychotic utilization at the institution (data not shown), the three most commonly used SGAs at this institution were risperidone, quetiapine, and aripiprazole. Due to the potential low utilization of other second generation agents (e.g. clozapine, olanzapine, ziprasidone), we determined a priori that our primary comparison of anticholinergic use across SGAs would be across these three drugs. Pearson's chi-square test was used to determine whether anticholinergic use significantly differed across these three agents.
Univariate and multiple logistic regression analyses were performed to assess odds ratios of potential predictors of anticholinergic use after 30 days. The dependent variable was defined as anticholinergic use after 30 days as opposed to non-use. Independent variables included sex (male vs. female), age, total number of diagnoses, number of psychiatric diagnoses, antipsychotic class (second generation agent vs. first generation agent or polypharmacy), starting antipsychotic dose, and dose of antipsychotic after 30 days. A multivariate analysis followed, which included variables with potentially significant evidence for association at the p<0.10 level identified in univariate analyses. The final model was chosen according to a backward elimination model selection technique, which resulted in the inclusion of three variables. Model fit assessment was conducted with the Hosmer and Lemeshow goodness of fit test.
All statistical analyses were conducted using SAS JMP version 7 and SPSS version 15.0.
Results
A total of 235 antipsychotic treatment courses were identified, representing 195 patients. Of these, 179 treatment courses or 152 patients were included in the following analyses. For patients with multiple antipsychotic courses, only the first course was included for assessment (n=152). The most common reason for exclusion of treatment courses was lack of information in the medical records (n=19), followed by absence of records indicating usage of antipsychotic for at least two months (n=9) and turning age greater than 18 years during the follow-up time frame (n=7). Other reasons for exclusion included prescription fill dates prior to or beyond the dates of interest (n=4), as well as EPS associated with use of a non-antipsychotic medication, as documented in the electronic medical record (n=3), and misidentification of one patient resulting in records of prescriptions under two different names (n=1).
Demographic characteristics are summarized in Table 1. Patients assessed in this study ranged from early adolescence to late teens. Psychiatric diagnoses included bipolar disorder (BP), major depressive disorder (MDD), schizophrenia, post-traumatic stress disorder (PTSD), attention-deficit hyperactivity disorder (ADHD), developmental disorders (e.g. autism and mental retardation) and “other” disorders (e.g. intermittent explosive disorder and oppositional defiant disorder). The most common psychiatric diagnosis was BP (n=66). The frequencies of documented psychiatric diagnoses in this study may be seen in Figure 1. Patients had a median of two (range 1-3) psychiatric diagnoses documented in the medical record.
Table 1. Demographic characteristics of patients included for analysisa.
| Characteristic | Value |
|---|---|
|
| |
| Age | 14.5 ± 2.4 years |
| Gender | 77 female, 75 male |
| Race | 110 – African American |
| 28 – Caucasian | |
| 9 – Hispanic | |
| 3 – Other | |
| 2 – Asian | |
| BMIb | 27.9 ± 6.4 kg/m2 |
Reported for 152 patients
BMI – body mass index
The most commonly prescribed antipsychotics in this study were risperidone (27%, n=41), quetiapine (27%, n=41), and aripiprazole (16%, n=24). Other SGAs used were ziprasidone (10%, n=15), olanzapine (5%, n=8) and clozapine (2%, n=3). Use of the FGAs (fluphenazine, chlorpromazine, and haloperidol) was documented in six (4%) patients. Additionally, 14 patients were prescribed multiple antipsychotics together at the initiation of therapy (9%). Mean daily doses at the first identified time point for use in the clinical record averaged 2 ± 1.8 mg/d for risperidone, 425 ± 293 mg/d for quetiapine, and 14 ± 8.5 mg/d for aripiprazole. Mean daily doses of the other antipsychotics at baseline were: ziprasidone 110 ± 43.8mg/d, olanzapine 15 ±12.5 mg/d, clozapine 150 ± 60.5 mg/d, fluphenazine 25 mg/d, chlorpromazine 300 mg/d and haloperidol 17.5 ± 10.4 mg/d.
The total number of medications used by patients in this study ranged from 1-11, with a median of approximately 3.5 medications per subject. These patients received a median of two (range 1-4) psychotropic medications at the initiation of antipsychotic therapy. In addition to antipsychotic medications, patients were co-prescribed other psychotropic medications such as stimulants, mood stabilizers, antidepressants and alpha-agonists as summarized in Figure 2. At the documented time of antipsychotic initiation, the use of mood stabilizers was most commonly observed, followed by the use of antidepressants, stimulants, and alpha agonists.
Figure 2. Psychotropic medication use at antipsychotic initiations and after 30 days.
The proportion of patients receiving commonly prescribed psychotropic medications at antipsychotic initiation and after 30 days.
Concomitant use of centrally-acting anticholinergic medications with an antipsychotic at any time was identified in 32 patients (21%). Use of non centrally-acting anticholinergic agents such as oxybutynin was observed but not included for this analysis due to their indications. Initiation of the anticholinergic occurred concurrently with antipsychotic initiation in 20 (62.5%) patients. At 30 days from antipsychotic initiation, a total of 28 of these 32 patients (87.5%) were prescribed anticholinergic medications. Patients were initiated on an anticholinergic agent within 30 days of antipsychotic initiation usually after the occurrence of EPS as documented in the medical record, although time to occurrence of EPS after initiation of an antipsychotic and/or anticholinergic use was not collected. Benztropine was the most frequently prescribed anticholinergic (n=32); one subject also received diphenhydramine that was additionally documented as being used for EPS. Extrapyramidal symptoms were documented in the medical record of 8% of all patients and 12/32 patients using an anticholinergic agent, with specific symptoms and occurrences listed in Table 2.
Table 2. Extrapyramidal symptoms.
| Symptoms | N (Total=12) |
|---|---|
|
| |
| Akathisia | 2 |
| Agitation | 1 |
| Cogwheel rigidity | 2 |
| Hand tremor | 2 |
| Involuntary hand movement | 2 |
| Left sided tremor | 1 |
| Neck tics | 1 |
| Not specified | 1 |
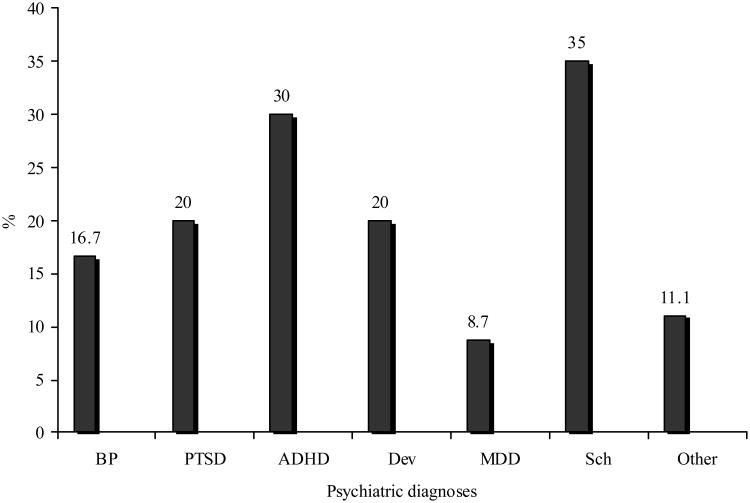
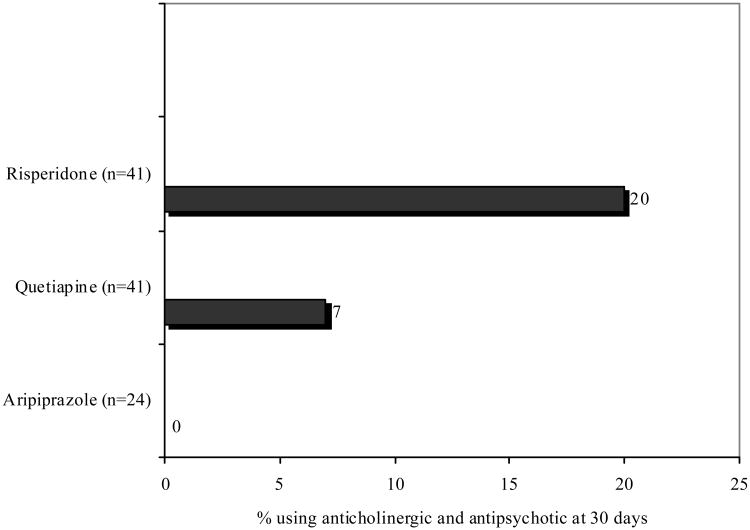
Anticholinergic use was most commonly observed in patients carrying diagnoses of schizophrenia and/or ADHD (see Figure 3). Anticholinergic medication use was also analyzed according to specific agents and antipsychotic class (FGA vs. SGA vs. polypharmacy). Overall, an anticholinergic agent prescribed at 30 days from antipsychotic initiation was most common in patients who were prescribed a conventional antipsychotic (4 of 6 patients, 67%). Of the 14 patients using multiple antipsychotics, nine (64%) were taking an anticholinergic at 30 days after antipsychotic initiation. Of the remaining 132 patients taking a SGA, 17 patients were taking an anticholinergic at 30 days (12.8%; p<0.0001 for comparison across FGA, SGA, and polypharmacy) Among the three most commonly prescribed SGAs in this study, anticholinergic use after one month differed significantly (p=0.03) and was most commonly documented in patients using risperidone (Figure 4). Anticholinergic use was documented in two patients taking aripiprazole at the start of therapy, but these were discontinued prior to the 30 day time point.
Figure 3. Anticholinergic use by psychiatric diagnosisa.
aBP – bipolar disorder; PTSD – post-traumatic stress disorder; ADHD – attention deficit hyperactivity disorder; Dev – developmental disorder (e.g. autism, Asperger's disorder, or mental retardation); MDD – major depressive disorder; Sch – schizophrenia
Figure 4. Anticholinergic use across the 3 most commonly prescribed antipsychotics.
P=0.03
Comparison of anticholinergic utilization 30 days after antipsychotic start across commonly prescribed second generation antipsychotic agents.
Anticholinergic users and non-users were examined for differences in age, antipsychotic dose, total number of medications, number of psychiatric diagnoses and total number of diagnoses. Statistically significant differences were appreciated between anticholinergic users and non-users in antipsychotic dose, total number of medications, and number of psychiatric diagnoses (see Table 3). The most common Axis I diagnoses in patients using anticholinergic agents were BP (n=5), PTSD (n=5), and developmental disorders (n=5), followed by ADHD (n=3) and MDD (n=3). Other common diagnoses seen in these patients included seizure disorder (n=4), asthma (n=4), and polysubstance abuse (n=2). Conditions such as hypothyroidism, anemia, obesity, enuresis, and insomnia were also documented in patients who were prescribed an anticholinergic medication. The frequency of these diagnoses was not compared between patients who were prescribed an anticholinergic and those who were not.
Univariate logistic regression analyses with anticholinergic use at one month from antipsychotic initiation as the dependent variable were performed, using individual demographic and clinical variables as possible predictors (Table 4). Univariate odds ratios were calculated indicating a potentially significant association between the following variables and anticholinergic use: male gender, total number of Axis I diagnoses, antipsychotic class, starting dose of the antipsychotic and daily dose after one month (p<0.05). A multivariate analysis followed univariate analyses with a predefined inclusion of univariate variables at the p<0.10 significance level. Sex, number of Axis I diagnoses, antipsychotic class, and daily dose at antipsychotic initiation were included in this model. Dose at one month was not included because it was highly correlated to starting dose. Based on the backward model selection technique and goodness of fit test, antipsychotic class, total number of psychiatric diagnoses, and starting antipsychotic dose were selected in the final multivariate model. Of these, antipsychotic class alone was found to be significantly associated with an increased likelihood of receiving an anticholinergic within 30 days (OR=18.98, 95% CI:4.74 –75.95).
Discussion
The primary finding of this study is that the use of anticholinergics was documented in 21% of pediatric and adolescents patients receiving antipsychotics for various psychiatric disorders. Utilization was approximately 13% in patients prescribed only a SGA, with utilization in SGAs most common in patients prescribed risperidone.
Anticholinergic agents were prescribed along with an antipsychotic at some point during the initial 30 days of therapy in 21% of the study patients, which exceeded the documented incidence of EPS of 8% in this study sample. Approximately 39% (12/32) patients receiving an anticholinergic agent had EPS documented in the medical record. Anticholinergic medications were often initiated at the same time as antipsychotic initiation, indicating that they may have been prescribed prophylactically. Risk factors for dystonic reactions, which are most likely to occur early on in therapy, include FGAs, higher antipsychotic doses, and male gender.15 These were characteristics individually identified as being significantly associated with anticholinergic use in this sample, which support our hypothesis that these were prescribed in a prophylactic manner. Of the three most commonly prescribed SGAs, anticholinergic use was greatest in patients prescribed risperidone. However, for risperidone treated patients, 8/9 receiving an anticholinergic had therapy noted after antipsychotic initiation, indicating that this was not prophylactic, rather for antipsychotic-induced EPS. Interestingly, all patients receiving an anticholinergic along with quetiapine had this combination noted at baseline and also at follow-up. While quetiapine does not have appreciable affinity for muscarinic M1 receptors, its metabolite, norquetiapine, has moderate affinity for these receptors, which is thought to account for the extremely low rates of EPS observed clinically with this drug. Thus, the reasons for concomitant use of an anticholinergic with quetiapine at treatment initiation are unclear. Two patients were prescribed an anticholinergic at the initiation of aripiprazole, with no anticholinergic use noted at follow-up. This is consistent with clinical observations that aripiprazole may occasionally be associated with akathisia during the initial stages of therapy, which may be targeted prophylactically.
After controlling for other variables associated with anticholinergic use, antipsychotic class was the primary predictor of use. Our findings suggested that anticholinergics were used prophylactically with FGAs. The possibility of characteristics other than antipsychotic class as predictors of anticholinergic use has been investigated, although the literature is lacking. Antipsychotic dose, for example, has been suggested as a predictor of anticholinergic use. In a review by Pierre, the likelihood of EPS was determined to increase when higher doses of SGAs were administered, due to a greater blockade of D2 receptors.16 In adult patients, risperidone has been found to have a dose-response relationship with movement disorders, suggested by a higher incidence of motor disturbances (specifically Parkinsonism) associated with larger doses.17 However, the results presented in a case series by Kumra et al. are conflicting.18 In this study, the rate of antipsychotic-related dyskinesia was assessed in 34 children and adolescents diagnosed with schizophrenia receiving clozapine, olanzapine, risperidone or haloperidol (haloperidol in combination with benztropine). Comparing patients with dyskinesia to patients without dyskinesia, no significant difference was found in gender, age and other variables, including admission doses of antipsychotics. These results suggest that the incidence of EPS and need for anticholinergic medications may not be accurately predicted by antipsychotic dose alone; further evidence is necessary to validate the predictive capability of antipsychotic dose as well as other characteristics such as gender and number of psychiatric diagnoses. In our study, antipsychotic dose was found to differ significantly between patients who were prescribed an anticholinergic and those who were not; however, due to the wide variation in dosing, a set dose (in terms of chlorpromazine equivalents) above which patients were more likely to require an anticholinergic could not be defined.
There are limitations to our study that warrant consideration when interpreting the results. The external validity of this sample is unknown due to the relatively modest sample size, demographic distribution, and the retrospective nature of data collection from a single center. Time points for clinical follow-up past 30 days were inconsistent. Thus, while 60 days of documented antipsychotic use was an inclusion criterion, the clinical follow-up for many patients was highly variable which led us to focus our analyses on the initial 30 days of treatment. Long-term use of anticholinergics was therefore not analyzed in this study.
Several of the patients initially identified had transferred to this institution from outside facilities, from which records were not available. This made the psychiatric history and prior antipsychotic and anticholinergic medication use difficult to discern/ascertain and also resulted in the exclusion of a number of patients from our potential study sample. In our efforts to obtain a best estimate of newly initiated antipsychotics, we excluded patients with documented antipsychotic use in the previous 60 days. This was done through an assessment of information in the prescription database as well as the electronic medical record. Despite these efforts, there were some patients identified with doses at drug initiation that were higher than one would expect. This could be due to initial therapy started during a brief period prior to referral that was not documented for some patients. However, due to the aforementioned efforts to identify those receiving antipsychotics in the previous 60 days, these data are still representative of the initial stages of therapy.
In addition, there were a large number of prescribers in this study (n=75). Due to the number of prescribers, we were not able to adequately assess whether an individual physician's prescribing behavior or preference is a predictor of anticholinergic use. However, the high number of prescribers and the extensive heterogeneity of the diagnoses limit the likelihood that our results were skewed by individual prescribers or diagnoses.
Another important consideration of our sample is the method of identification. By using the institution's pharmacy database, patients receiving their medications through outside pharmacies were not included. We do not know if there are characteristics that differ between those populations that may have influenced the results here. Finally, one of the reasons for conducting this study is that anticholinergic use may be associated with impaired scholastic performance. This hypothesis is based on prior studies investigating the relationship between anticholinergic activity and cognition. It is important to note that we did not assess the relationship between anticholinergic use and scholastic performance in this sample, which should be the topic of future investigations.
Conclusion
In this study sample of children and adolescents taking antipsychotic agents, concomitant use of an anticholinergic agent was documented in 21% of patients at some point over the course of the first 30 days of therapy. To our knowledge, this is the first investigation to specifically study anticholinergic use during the initial stages of antipsychotic treatment in a “real world” clinical population. Antipsychotic use was observed in both indicated and off-label diagnoses, and there was a discordance between the use of anticholinergic agents and documented indications for their use. Antipsychotic class was the variable associated with the greatest likelihood of anticholinergic use, and there were significant differences across the three most commonly prescribed agents in the SGA class (risperidone, aripiprazole, and quetiapine). This collectively indicates that younger patients may be sensitive to EPS, even from SGAs. This pattern of sensitivity may be different from what is commonly observed in adults, although this comparison is beyond the scope of the current investigation. The overall medication burden, particularly psychotropics, was high in our study sample. Furthermore, with the exception of higher antidepressant use after 30 days, total psychotropic utilization did not change after antipsychotic initiation. Additional larger scale studies involving multiple institutions and investigating other characteristics such as gender, psychiatric diagnoses, and daily antipsychotic doses that may affect the use of anticholinergic agents in pediatric patients taking SGAs are needed to determine unequivocally the relationships between these variables and the risk for anticholinergic use. The diagnostic complexities and the heterogeneous use of multiple psychotropic medications in younger patients with psychiatric disorders make this a difficult population to study. The clinical realities and challenges of psychotropic use in younger patients, however, should not deter further study of younger patients. At this time, anticholinergic therapy may be required for treatment of antipsychotic-induced EPS in both adult and pediatric patients but the risk:benefit ratio of treating young patients with these medications on top of antipsychotic agents needs to be carefully considered.
Footnotes
Prior Presentation: Portions of this work were previously presented as a work in progress abstract and poster at the 2008 American Society of Health-System Pharmacists Annual Meeting
Contributor Information
Irene S. Hong, Email: ihong2@uic.edu, University of Illinois at Chicago College of Pharmacy, Department of Pharmacy Practice, 833 South Wood Street M/C 886, Chicago, IL 60612, Phone: (312) 413-3804, Fax: (312) 996-0448.
Jeffrey R. Bishop, Email: jbishop@uic.edu, University of Illinois at Chicago College of Pharmacy, Department of Pharmacy Practice, 833 South Wood Street M/C 886, Phone: (312) 413-3495, Fax: (312) 996-0379.
References
- 1.Wonodi I, Reeves G, Carmichael D, et al. Tardive dyskinesia in children treated with atypical antipsychotic medications. Mov Disord. 2007;22:1777–82. doi: 10.1002/mds.21618. [DOI] [PubMed] [Google Scholar]
- 2.FDA/CDER resources page. [Accessed January 23, 2010];Food and Drug Administration Pediatric Advisory Committee Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM193204.pdf.
- 3.Jensen PS, Buitelaar J, Pandina GJ, Binder C, Haas M. Management of psychiatric disorders in children and adolescents with atypical antipsychotics: a systematic review of published clinical trials. Eur Child Adolesc Psychiatry. 2007;16:104–20. doi: 10.1007/s00787-006-0580-1. [DOI] [PubMed] [Google Scholar]
- 4.Gebhardt S, Hartling F, Hanke M, et al. Relations between movement disorders and psychopathology under predominantly atypical antipsychotic treatment in adolescent patients with schizophrenia. Eur Child Adolesc Psychiatry. 2008;17:44–53. doi: 10.1007/s00787-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 5.Fleischhaker C, Heiser P, Hennighausen K, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol. 2006;16:308–16. doi: 10.1089/cap.2006.16.308. [DOI] [PubMed] [Google Scholar]
- 6.Remington G, Kapur S. Atypical antipsychotics: are some more atypical than others? Psychopharmacology (Berl) 2000;148:3–15. doi: 10.1007/s002130050017. [DOI] [PubMed] [Google Scholar]
- 7.Lewis R. Typical and atypical antipsychotics in adolescent schizophrenia: efficacy, tolerability, and differential sensitivity to extrapyramidal symptoms. Can J Psychiatry. 1998;43:596–604. doi: 10.1177/070674379804300608. [DOI] [PubMed] [Google Scholar]
- 8.Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004;29:133–45. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- 9.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 10.Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 11.Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(4):26–36. [PubMed] [Google Scholar]
- 12.Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–24. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- 13.Kane JM. Schizophrenia. N Engl J Med. 1996;334:34–41. doi: 10.1056/NEJM199601043340109. [DOI] [PubMed] [Google Scholar]
- 14.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 15.Perry PJ, Alexander B, Liskow B, et al. Psychotropic Drug Handbook. 8th. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 106–109. [Google Scholar]
- 16.Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf. 2005;28:191–208. doi: 10.2165/00002018-200528030-00002. [DOI] [PubMed] [Google Scholar]
- 17.Chouinard G. Interrelations between psychiatric symptoms and drug-induced movement disorder. J Psychiatry Neurosci. 2006;31:177–180. [PMC free article] [PubMed] [Google Scholar]
- 18.Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37:221–227. doi: 10.1097/00004583-199802000-00016. [DOI] [PubMed] [Google Scholar]