Summary
Iron is essential for cell survival and function; yet excess iron is toxic to cells. Therefore, the cellular and whole-body levels of iron are regulated exquisitely. At least a dozen proteins participate in the regulation of iron homeostasis. Hemochromatosis, a genetic disorder of iron overload, is caused by mutations in at least five genes, namely HFE, hemojuvelin, Transferrin receptor 2, ferroportin, and hepcidin. Retina is separated from systemic circulation by inner and outer blood-retinal barriers; therefore it is widely believed that this tissue is immune to changes in systemic circulation. Even though hemochromatosis is associated with iron overload and dysfunction of a variety of systemic organs, little is known on the effects of this disease on the retina. Recent studies have shown that all five genes that are associated with hemochromatosis are expressed in the retina in a cell type-specific manner. The retinal pigment epithelium, which forms the outer blood-retinal barrier, expresses all of these five genes. It is therefore clearly evident that iron homeostasis in the retina is maintained locally by active participation of various iron-regulatory proteins. Excess iron is detrimental to the retina as evidenced from human studies and from mouse models of iron overload. Retinal iron homeostasis is disrupted in various clinical conditions such as hemochromatosis, aceruloplasminemia, age-related macular degeneration, and bacterial and viral infections.
Keywords: Hemochromatosis, age-related macular degeneration, iron toxicity, oxidative damage, retina
Introduction
Iron is an essential micronutrient necessary for the function of many proteins/enzymes that are indispensible for normal cellular activity. Diet is the only source of iron for the body. There are two forms of dietary iron: heme (∼15% of iron in average diet) and nonheme (∼85% of iron in average diet) (1). Heme iron is obtained from hemoglobin and other hemoproteins, mostly from animal products. Iron in plant foods is in the form of nonheme iron, the form of iron added to iron-fortified foods. Heme iron is absorbed in the intestine more efficiently than nonheme iron (1). Intestinal absorption of nonheme iron is facilitated by vitamin C but inhibited by tannins, calcium, polyphenols, and phytates. Iron is the most abundant mineral on earth; yet iron deficiency is the most prevalent nutritional disorder in humans (2). Iron deficiency may result from low dietary intake, inadequate intestinal absorption, excessive blood loss, or due to a variety of genetic mutations (2). Although iron is essential for the survival of cells, excess iron is toxic. Iron is a prooxidant; excess iron can undergo Fenton reaction, catalyzing the conversion of H2O2 to hydroxyl radical, arguably the most reactive oxygen species. Hydroxyl radicals cause lipid peroxidation, DNA strand breaks, and degradation of cellular components resulting in tissue damage (3). Since only small amounts of iron are lost daily from the body by processes such as sloughing of intestinal mucosal and skin cells, and excretion in urine and bile, with women having additional losses associated with menstruation, the whole body iron status is regulated primarily at the level of intestinal absorption (4).
In the retina, there are many iron-containing proteins that are involved in the phototransduction cascade. RPE65, an iron-containing protein expressed in retinal pigment epithelium (RPE), is an isomerohydrolase responsible for the conversion of all-trans-retinyl ester to 11-cis-retinol in the visual cycle (5). Photoreceptor cells constantly shed and synthesize their outer segment discs and thus depend highly on fatty acid desaturase, an iron-containing enzyme necessary for the synthesis of membrane lipids. RPE plays an important role in the maintenance of photoreceptor excitability by phagocytosis of shed photoreceptor outer segments (6). The photoreceptor outer segments are digested, and essential substances such as retinal are recycled and returned to photoreceptors to rebuild light-sensitive outer segments from the base of the photoreceptors. However, like other cells, retinal cells are also susceptible to oxidative stress caused by excess iron. Excessive iron impairs phagocytosis in RPE (6). Iron is also necessary for guanylate cyclase to synthesize cGMP, the second messenger in the phototransduction pathway. Due to prolonged exposure to light, photooxidation generates more than normal levels of reactive oxygen species in the retina. Docosahexaenoic acid, a polyunsaturated fatty acid present abundantly in this tissue, is subject to lipid peroxidation in the presence of reactive oxygen species, thus compromising retinal function. Because of these reasons, regulation of iron homeostasis is particularly important in the retina; disruption of this regulation is likely to have significant biological and clinical consequences.
Proteins Involved In Iron Import In The Retina
Non-heme iron binds to transferrin in the blood in the form of Fe3+; upon binding to the transferrin receptor (TfR), the transferrin-bound iron is taken up into cells via receptor-mediated endocytosis through clathrin-coated pits. The endocytosis of the TfR was recently found to be a regulated process that requires activated src kinase (7). Recent studies also show that if there is a defect in clathrin-mediated endocytosis, TfR is internalized by a caveolae-dependent pathway (8). Once inside the cell, iron is released from transferrin in acidic endosomal compartment, and is exported to the cytosol. Transferrin receptor 1 (TfR1) is the primary protein involved in cellular iron uptake. TfR2 is a homolog of TfR1; it also binds and internalizes transferrin-Fe3+ but with a 30-fold lower affinity than TfR1 (9). However, cellular iron uptake might not be the function of TfR2 in vivo, because loss-of-function mutations in TfR2 lead to hepatic iron accumulation rather than iron deficiency (10, 11). TfR2 is one of the determinants of whole-body iron homeostasis. Divalent metal transporter-1 (DMT1) is a proton/Fe2+ symporter located on the apical surface of intestinal epithelial cells, which mediates the uptake of dietary Fe2+(12). In most cell types, this transporter is also present on endosomal membrane, facilitating the export of Fe2+ into cytosol following receptor-mediated endocytosis of transferrin-iron.
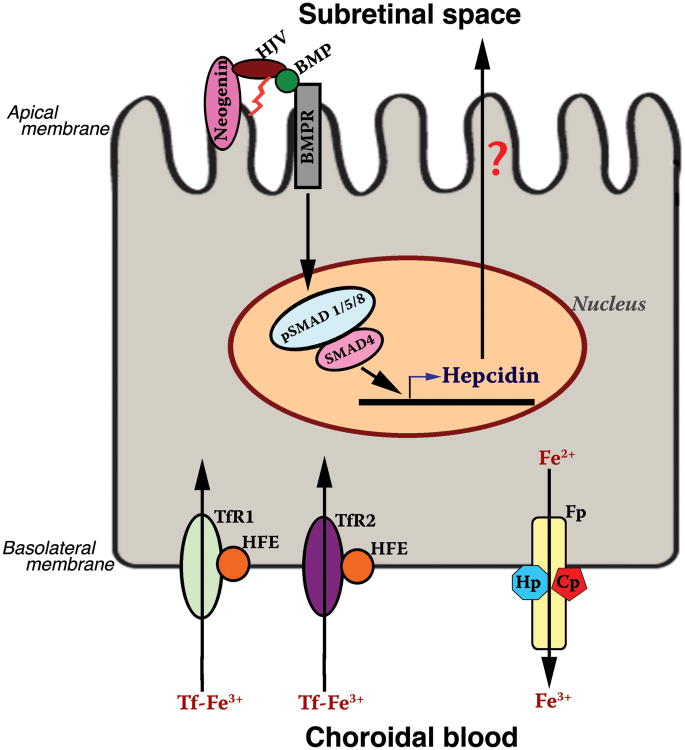
Immunohistochemical studies have revealed the expression of transferrin in RPE and in the inner and outer segments of photoreceptors, as well as in the inner retina (13). TfR1 is detected in the ganglion cell layer, inner nuclear layer, outer plexiform layer, photoreceptor inner segment, RPE, and choroid (13). TfR2 expression is found in RPE (14). Both receptors are located in the basolateral membrane of RPE (Fig. 1), indicating that these receptors play a role in the entry and regulation of transferrin-iron from choroidal circulation into RPE. DMT1 is expressed in rod bipolar cell bodies, rod bipolar cell axon termini, horizontal cell bodies, and photoreceptor inner segments (15).
Fig. 1.
Expression pattern of hemochromatosis genes in RPE. TfR, transferrin receptor; HFE, HLA-like protein involved in iron homeostasis; Fp, ferroportin; Hp, hephaestin; Cp, ceruloplasmin; HJV, hemojuvelin; BMP, bone morphogenic proteins; BMPR, BMP receptors. It is speculated that secretion of hepcidin from RPE may be directional, preferentially into the subretinal space across the apical membrane.
Proteins Involved In Intracellular Iron Storage
Ferritin is a cytoplasmic protein involved in iron storage. It consists of heavy (H) and light (L) chains. Ferritin can bind up to 4,500 iron atoms. H-ferritin is a ferroxidase, and oxidation of iron facilitates its incorporation into ferritin. L-Ferritin does not have ferroxidase activity but promotes iron storage. Recently, a novel receptor specific for L-ferritin, scavenger receptor, member 5 (Scara5), has been identified (16). This receptor mediates the delivery of non–transferrin-bound iron (i.e., iron bound to ferritin) into cells. Ferritin has other biologic functions in addition to its role in iron storage. By binding iron tightly, it prevents iron-induced oxidative stress. Ferritin also interacts with p53 upon oxidative stress, indicating that this iron-storage protein and p53 cooperate to protect cells from oxidative stress (17). Ferritin also plays a role as a regulator of angiogenesis (18). HKa (cleaved product of high molecular weight kininogen) is an endogenous inhibitor of angiogenesis, and ferritin binds to HKa with high affinity and prevents the anti-angiogenic effects of HKa. Thus ferritin functions both as an antioxidant and a pro-angiogenesis factor. Also, hereditary hyperferritinaemia/cataract syndrome (HHCS) arises as a result of various point mutations or deletions within a protein binding sequence in the 5′-UTR of the L-ferritin mRNA, which results in increased efficiency of L-ferritin translation. Each unique mutation confers a characteristic degree of hyperferritinaemia and severity of cataract in affected individuals (19, 20).
Ferritin is expressed in retina, primarily in the inner segments of photoreceptors, RPE, choroid, inner nuclear layer, and the ganglion cell layer (15). The regions with the highest iron levels coincide with the regions of ferritin expression. L- and H-ferritin antibodies label the photoreceptor inner segments, outer plexiform layer, inner nuclear layer cell bodies, and the innermost inner plexiform layer near the ganglion cell layer (21). In the inner nuclear layer, much of the label is found in the axon terminals of the rod bipolar cells, suggesting that ferritin may play a role in the transport or storage of iron in the pre-synaptic terminal.
Proteins Involved In Iron Export In The Retina
Iron that is not utilized or stored by the cell is exported by the transporter ferroportin coded by SLC40A1(22). All cell types that export iron express ferroportin, including duodenal enterocytes, phagocytes, Kupffer cells, astrocytes, retinal cells, and placental cells. Iron is exported by ferroportin in Fe2+ form, but must be oxidized to Fe3+ before it can bind to transferrin in circulation. Oxidation of Fe2+ is accomplished by two ferroxidases, ceruloplasmin and hephaestin, in a cell type-specific manner.
In mouse retina, ferroportin is localized to RPE, photoreceptor inner segments, the inner and outer plexiform layers, and the ganglion cell layer (21). In RPE, the localization is predominantly basolateral, suggesting that ferroportin functions to facilitate iron export from RPE into choroidal blood (Fig. 1). Ceruloplasmin and hephaestin are also co-expressed with ferroportin in RPE and Muller cells (23). These ferroxidases work with ferroportin to oxidize Fe2+ during its export from cells.
Iron-Regulatory Proteins In Retina
Hepcidin
Hepcidin is an iron-regulatory hormone coded by HAMP. It was originally identified as an antimicrobial peptide secreted by the liver (HAMP, hepatic antimicrobial peptide), but later found to be a critical iron-regulatory hormone (24). Ferroportin is the target for hepcidin. This hormone is produced by liver as an 84-amino-acid pre-pro-hormone that is processed to generate the bioactive 25-amino-acid peptide (24). Pro-hepcidin lacks biological activity (25). Hepcidin binding to ferroportin leads to the recruitment and activation of Janus Kinase2 (Jak2), which is required for phosphorylation of ferroportin (26). α2-Macroglobulin (α2M) binds hepcidin in circulation (27). The binding of hepcidin to α2M probably enables efficient sequestration of hepcidin, a process that may be important to regulate the functions of hepcidin. α2M-hepcidin complex is more effective in inducing ferroportin degradation than hepcidin alone (27). Hepcidin expression is influenced by systemic stimuli such as iron stores, the rate of erythropoiesis, inflammation, hypoxia and oxidative stress (24). These stimuli control hepcidin levels by acting through hepatocyte cell surface proteins including HFE, TfR2, hemojuvelin, TMPRSS6 and the interleukin 6 receptor (28). These cell surface proteins activate various cell signal transduction pathways, including the BMP-SMAD, JAK-STAT and HIF1 pathways, to alter expression of hepcidin (28). Various stimuli can signal through multiple pathways to regulate hepcidin expression, and the interplay between positive and negative stimuli is critical in determining the net hepcidin level. The BMP-SMAD pathway is particularly important; disruption of this pathway abrogates the response of hepcidin to many stimuli. CREBH (cyclic AMP response element-binding protein H), an endoplasmic reticulum (ER) stress-activated transcription factor, binds and transactivates the hepcidin promoter, thus linking the intracellular response involved in protein quality control to innate immunity and iron homeostasis (29). ER stress induces a complex network of pathways collectively termed the unfolded protein response. This process is associated with induction of CHOP expression, which in turn regulates the levels of C/EBPα, an important transcription factor regulating hepcidin expression (30). The expression levels of ferroportin and ferritin H are also increased by ER stress (30).
Even though it is widely believed that hepcidin is secreted only by the liver, our recent studies have shown that this hormone is expressed abundantly in the retina, detectable in all retinal cell types (31). In RPE, ferroportin is expressed in the basolateral membrane (21); thus ferroportin in RPE may be subject to regulation by hepatic hepcidin present in systemic blood. In contrast, ferroportin expressed in Müller cells and photoreceptor cells does not have access to circulating hepcidin. Therefore, the recent findings showing abundant expression of hepcidin in Müller cells, photoreceptor cells, and RPE have biological significance. The retina-derived hepcidin may play a critical role in the regulation of ferroportin in the inner retina independent of the liver-derived hepcidin (31). We do not know yet the directionality of hepcidin secretion from RPE cells, but we speculate that the hormone is released preferentially into the subretinal space across the apical membrane (Fig. 1).
HFE
Hemochromatosis, the most prevalent genetic disease associated with iron overload, is caused by disruption of HFE function. HFE, a ∼49 kDa protein, was originally identified as a Human Leukocyte Antigen (HLA) class I-like protein and was subsequently found to function as a regulator of iron homeostasis [HFE, a HLA-like protein involved in iron (FE) homeostasis]. This protein was considered to be involved in the sensing of body's iron status by the crypt cells of the duodenum, the part of the small intestine where the absorption of dietary iron occurs (32). However, later studies have shown that HFE has a central role not in the small intestine but in the liver (33). HFE interacts with β2-microglobulin (β2M) for the presentation of HFE to the cell surface. β2M is essential for the correct subcellular distribution of both HFE and hepcidin, the two proteins that are critical for iron homeostasis (34). HFE interacts with TfR1 and TfR2, thus sensing the saturation of transferrin with iron in blood. TfR2/HFE complex is important for transcriptional regulation of hepcidin (35). Studies with Hfe-/-/TfR2-/- mice suggest that Hfe and Tfr2 regulate hepcidin through parallel pathways involving Erk1/2 and Smad1/5/8 (36). Also, Hfe deficiency triggers iron overload by impairing hepatic BMP6/Smad signaling (37, 38).
Hfe is expressed in the retina; its expression is restricted exclusively to RPE as evident from the detection of mRNA and protein solely in this single retinal cell layer (14). Equally important and interesting is the polarized expression of Hfe protein at the basolateral membrane of RPE (Fig. 1) (14). The basal membrane of the RPE, which is in contact with choroidal blood, is the site of the first step in the cellular uptake of iron from blood. Therefore, the specific location of Hfe at this membrane strongly suggests that this protein can interact with TfR1 as well as TfR2, and thus could play a critical role in the regulation of iron homeostasis in RPE at the level of cellular uptake of iron from choroidal blood.
Hemojuvelin
Hemojuvelin (HJV), another important iron-regulatory protein (molecular size, ∼50 kDa), is either associated with cell membrane through a glycosylphosphatidylinositol anchor or released as a soluble form. Membrane-anchored HJV acts as a coreceptor for BMPs and induces the expression of hepcidin (39). Soluble HJV antagonizes BMP signaling and suppresses hepcidin expression. Release of HJV in soluble form requires the transmembrane receptor neogenin. Neogenin does not, however, play a role in HJV trafficking to the cell surface (40). The findings that HJV release is coupled to lysosomal degradation of neogenin and that cholesterol depletion by filipin blocks both HJV endocytosis and HJV release suggest that neogenin-mediated HJV release occurs after the HJV-neogenin complex is internalized from the cell surface (40). It has been recently found that the serine protease matriptase-2 functions to suppress BMP stimulation of hepcidin transcription through cell surface proteolytic processing of HJV (41). Even though BMP-2, BMP-4, and BMP-6 regulate hepcidin expression in vitro in an HJV-dependent manner (39), a recent study has shown BMP6 to be solely responsible for this process in vivo (42). Two highly conserved BMP-responsive elements located at positions -84/-79 (BMP-RE1) and -2,255/-2,250 (BMP-RE2) of the human hepcidin promoter are critical for both the basal hepcidin mRNA expression and the hepcidin response to BMP-2 and BMP-6 (43). HJV does not seem to be capable of initiating BMP signaling on its own. Recent studies have shown that interaction of HJV with neogenin is necessary for this process (44). ERK activation by holotransferrin provokes increased levels of phospho-Smad complex, highlighting crosstalk between the HJV/neogenin/BMP/Smad 1/5/8 and HFE/TfR2/ERK1/2 pathways (45).
Hjv is expressed widely in the retina, with evidence of expression seen in RPE, Muller cells, photoreceptor cells, and ganglion cells (45). In RPE, the expression is restricted to the apical membrane that faces the neural retina (Fig. 1). The differential polarization of Hfe (basolateral membrane) and Hjv (apical membrane) in RPE is interesting and intriguing. These recent findings suggest that the expression of hepcidin in RPE may be controlled by HFE and HJV; in contrast, the expression of hepcidin in the neural retina may be regulated only by HJV.
Table 1 summarizes the expression pattern of various iron-regulatory proteins in the retina. As mentioned in the previous sections, all five genes (HFE, HJV, TfR2, ferroportin, hepcidin) that are associated with hemochromatosis are expressed in RPE. The differential expression of these gene products in RPE in the retina-facing apical membrane and choroid-facing basolateral membrane is illustrated in Fig. 1.
Table 1.
Expression pattern of iron regulatory proteins in retina
| Iron-regulatory proteins | Expression pattern in the retina |
|---|---|
|
| |
| DMT1 | Photoreceptor inner segments, rod bipolar cell bodies and axon termini, horizontal cell bodies |
| TfR1 | RPE, photoreceptor inner segment, outer plexiform layer, inner nuclear layer, ganglion cell layer |
| TfR2 | RPE |
| H- and L- Ferritin | RPE, photoreceptor inner segments, outer plexiform layer, inner nuclear layer cell bodies, inner plexiform layer, ganglion cell layer |
| Ferroportin | RPE, photoreceptor inner segments, the inner and outer plexiform layers, and the ganglion cell layer |
| Ceruloplasmin | RPE and Muller cells |
| Hephaestin | RPE and Muller cells |
| HFE | RPE |
| Hemojuvelin | RPE, photoreceptor cells, Muller cells, ganglion cells |
| Hepcidin | RPE, photoreceptor cells, Müller cells |
Role of Iron-Regulatory Proteins In Genetic Retinal Disorders
Aceruloplasminemia
Aceruloplasminemia is a rare genetic disorder in which iron gradually accumulates in the brain, retina and pancreas (47). Patients with aceruloplasminemia develop a variety of movement problems such as rhythmic shaking, jerking movements, eyelid twitching, and ataxia. In addition, iron accumulation in tissues results in iron deficiency in blood, leading to anemia. Affected individuals also have opaque spots and areas of tissue degeneration around the edges of the retina. Aceruloplasminemia is caused by mutations in ceruloplasmin (48). Patients with aceruloplasminemia have impaired iron export from certain tissues as ceruloplasmin dysfunction prevents the conversion of Fe2+ to Fe3+, the form of iron that is exported out of the cells. Mice deficient in ceruloplasmin and hephaestin show age-dependent retinal degeneration (23). By 6–9 months, these mice exhibit iron overload in retina, RPE hypertrophy with increased phagosomes and lysosomes, sub-RPE deposits of collagen, focal areas of RPE hyperplasia, and necrosis along with photoreceptor loss and subretinal neovascularization.
Hemochromatosis
Hemochromatosis is a genetic disease associated with iron overload. The tissues commonly affected in this disease are the liver, pancreas, kidney, pituitary, and heart (49-51). The symptoms associated with the disease include liver cirrhosis, hepatocarcinoma, diabetes, cardiomyopathy, nephropathy, and endocrine dysfunction. Most patients with hemochromatosis have mutations in HFE; a single mutation (C282Y) is responsible for most cases. Wild type HFE binds to TfR1, lowering the affinity of the receptor for transferrin, but the mutant HFE cannot complex with TfR1 and thus is unable to prevent cellular iron accumulation. Other forms of hemochromatosis involve mutations in ferroportin, HJV, TfR2 and hepcidin (52). Mutations in ferroportin reduce iron export from cells. Mutations in HFE, HJV, TfR2 and hepcidin are associated with hepcidin deficiency. Since hepcidin is a hormone that prevents iron export via ferroportin in certain cell types, deficiency of hepcidin leads to increased release of iron from these cells, leading to increased iron in circulation. The resultant increase in transferrin-loaded iron facilitates iron uptake in most cells, leading to iron overload in tissues. Patients with hemochromatosis have excess iron in the peripapillary RPE, ciliary epithelium, and sclera, accompanied with drusen (53, 54). Hfe is expressed in RPE and the protein is specifically associated with the basolateral membrane of the cell, suggesting a potential role for this protein in the maintenance of iron status in RPE/retina. (14). Hfe-/- mice have retinal degeneration at ≥18-months of age but such changes are not evident in younger mice of the same genotype (55). This recapitulates what is known in patients with hemochromatosis in terms of iron accumulation in systemic organs. Iron overload and consequent functional disruption in systemic organs are not evident in hemochromatosis patients until ∼50 years of age. We also found evidence of hypertrophy and hyperplasia in the RPE cell layer (55). Hepcidin knockout mouse was also recently found to exhibit age-related retinal iron accumulation with RPE hyperplasia/hypertrophy (56). Available evidence thus provides strong indication that hemochromatosis-related genes play an important role in the control of retinal iron homeostasis. There have been no studies correlating the incidence of age-related macular degeneration like retinal disorders in hemochromatosis patients. Therefore, the possibility that hemochromatosis may lead to iron overload in the retina with consequent retinal dysfunction merits further investigation.
Age-related macular degeneration (AMD)
AMD is a major cause of gradual, painless, and bilateral loss of central vision in elderly people. At early stages of the disease, there are drusen deposits; as the disease progresses, large areas of atrophy develop in the dry or non-exudative form of AMD whereas choroidal neovascularization occurs in the wet or exudative form. There is overwhelming evidence in support of a genetic component in the etiology of AMD (57, 58). Free radical damage and oxidative stress play a role in the pathogenesis of the disease (59). Patients with dry AMD, when given dietary supplements of antioxidants and zinc, show reduced progression of the disease, suggesting that oxidative stress is involved in its pathogenesis (60). Though a role of oxidative stress in the etiology of AMD is apparent, the underlying factors that lead to oxidative stress are far from clear. Irrespective of the etiology, patients with AMD show evidence of excessive iron accumulation in retina (61, 62). Iron accumulation in the retina observed in the ceruloplasmin/hephaestin double-knockout mouse is associated with hypertrophy and hyperplasia of RPE (63). We observed a similar phenomenon in Hfe-/- mouse (55). These findings indicate that excessive iron accumulation might underlie the cellular and growth pattern changes seen in RPE in AMD. Interestingly, the hyperplasia phenotype of RPE observed in intact retina of Hfe-/- mouse is also seen with primary RPE cell cultures (55). These findings suggest that the iron overload associated with hemochromatosis may have a role in the etiology and/or progression of AMD in humans. The emerging evidence for the involvement of excessive iron in AMD strongly suggests that systematic investigation of the potential association between hemochromatosis and AMD is warranted.
Role of Iron-Regulatory Proteins In Bacterial and Viral Infections
Bacterial infection
Hepatic expression of hepcidin is induced by inflammation. During infection, body tries to sequester iron in tissues to prevent the pathogen survival in circulation. This process is mediated by an increase in the circulating levels of hepcidin that inhibit ferroportin-dependent release of iron into circulation from certain cell types. Recently, we mimicked bacterial infection of the retina by intravitreal injection of bacterial lipopolysaccharide (LPS), and examined the changes in the expression of hepcidin within the retina (31). These studies showed that hepcidin expressed in retinal cells is subject to regulation by LPS with obligatory involvement of the Toll-like receptor-4. The increase in hepcidin expression in response to LPS is associated with a decrease in ferroportin levels in Müller cells, photoreceptor cells, and RPE. We also showed that bacterial LPS is a regulator of hepcidin expression in Müller cells and RPE, both in vitro and in vivo, and that the regulation occurs at the transcriptional level. The upregulation of hepcidin by LPS occurs independent of Hfe and Hjv. Exposure of the retina to LPS causes oxidative stress as monitored by the levels of hydroxynonenol. Several studies have shown that iron overload causes oxidative stress and that hydroxynonenol levels increase under these conditions (64, 65). The LPS-induced oxidative stress in vivo in retina is accompanied with apoptosis in specific cell types. A significant increase in apoptotic nuclei is seen in the outer nuclear layer, which contains the nuclei of photoreceptor cells, and in the inner nuclear layer, which contains, among other cell types, the nuclei of Müller cells (31). Thus retinal iron homeostasis may be regulated in an autonomous manner by hepcidin generated within the retina, and chronic bacterial infection/inflammation of the retina may disrupt iron homeostasis and retinal function.
Viral infection
Infection of mammalian cells with cytomegalovirus (CMV) leads to downregulation of HFE via proteosomal degradation (66, 67). This process is necessary for virus multiplication in host cells because HFE depletion will lead to iron accumulation in cells, which is obligatory for virus proliferation. These findings are relevant to RPE and retina because CMV infection is a major cause of retinitis in immunocompromised individuals that leads to serious functional deficits in vision (68). We found that CMV infection of the retina decreases Hfe but increases Hjv in RPE (46). Though CMV infection leads to decreased levels of Hfe, there was no significant change in hepcidin levels. This is likely due to the concomitant increase in the expression of Hjv. Hfe and Hjv have similar effects on hepcidin, both enhancing its expression. The reciprocal changes that occur in the cellular levels of Hfe and Hjv in response to CMV infection may explain the lack of detectable changes in hepcidin levels. We found that CMV infection in RPE cells decreases TfR1 levels but increases ferritin levels, which are indicative of iron accumulation. These changes are accompanied with evidence of increased lipid peroxidation (46). Thus, while enhanced iron accumulation may initially favor virus multiplication in the host cell, the process eventually leads to disruption of host cell function through oxidative stress. Changes in cellular iron status as a result of CMV infection seem to play an important role in this process. We also found that Hfe-/- mouse RPE cells accumulate iron at significantly higher levels than wild type cells upon CMV infection (46). This may have clinical significance in hemochromatosis patients with regard to CMV multiplication and progression of CMV retinitis.
Conclusions
It was widely believed that retina was protected from changes in systemic iron status by the blood-retinal barriers. There is increasing evidence in recent years however indicating that this may not be the case. Hemochromatosis that cause iron overload in various systemic organs occurs due to mutations in five different genes: HFE, TfR2, HJV, ferroportin, and hepcidin. The expression of all these five genes in the retina has been demonstrated unequivocally in recent studies. Therefore, patients with hemochromatosis are likely to have disruption of iron homeostasis in retina due to dysfunction of these gene products. These recent findings suggest that systematic investigations of retinal involvement in patients with hemochromatosis are warranted. In addition, the potential of retinal iron overload as a causative or modifying factor in AMD also merits investigation. Bacterial and viral infection and inflammation lead to changes in the expression of iron-regulatory proteins in retina, thus disrupting retinal iron homeostasis. Continued investigations of the role of iron and iron-regulatory proteins in the retina in health and disease will provide important clues to understand the pathogenesis of AMD, CMV retinitis and other iron-related retinal disorders, and may thus lead to the design and development of novel preventive or therapeutic strategies for the treatment of a wide variety of retinal diseases.
Abbreviations
- HFE
HLA-like protein involved in iron homeostasis
- HJV
hemojuvelin
- TfR
transferrin receptor
- BMP
bone morphogenetic protein
- AMD
age-related macular degeneration
- RPE
retinal pigment epithelium
- LPS
lipopolysaccharide
- CMV
cytomegalovirus
References
- 1.Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr. 2003;23:283–301. doi: 10.1146/annurev.nutr.23.011702.073139. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GJ, Vulpe CD. Regulation of intestinal iron transport. In: Templeton DM, editor. Molecular and Cellular Iron Transport. New York: Marcel Dekker; 2002. pp. 559–596. [Google Scholar]
- 5.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci (USA) 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Lukas TJ, Du N, Suyeoka G, Neufeld AH. Dysfunction of the retinal pigment epithelium with age: increased iron decreases phagocytosis and lysosomal activity. Invest Ophthalmol Vis Sci. 2009;50:1895–1902. doi: 10.1167/iovs.08-2850. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Chen J, Krueger EW, McNiven MA. Src-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2009 doi: 10.1128/MCB.00330-09. Dec 2009 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri C. Loss of myosin VI no insert isoform (NoI) induces a defect in clathrin-mediated endocytosis and leads to caveolar endocytosis of transferrin receptor. J Biol Chem. 2009;284:34998–35014. doi: 10.1074/jbc.M109.012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 10.Camaschella C, Roetto A, Calì A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TfR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–5. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 11.Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci (USA) 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 13.Yefimova MG, Jeanny JC, Guillonneau X, Keller N, Nguyen-Legros J, Sergeant C, Guillou F, Courtois Y. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Invest Ophthalmol Vis Sci. 2000;41:2343–2351. [PubMed] [Google Scholar]
- 14.Martin PM, Gnana-Prakasam JP, Roon P, Smith RG, Smith SB, Ganapathy V. Expression and polarized localization of the hemochromatosis gene product HFE in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2006;47:4238–4244. doi: 10.1167/iovs.06-0026. [DOI] [PubMed] [Google Scholar]
- 15.He X, Hahn P, Iacovelli J, Wong R, King C, Bhisitkul R, Massaro-Giordano M, Dunaief JL. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007;26:649–673. doi: 10.1016/j.preteyeres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Jang H, Cho EJ, Youn HD. Ferritin binds and activates p53 under oxidative stress. Biochem Biophys Res Commun. 2009;389:399–404. doi: 10.1016/j.bbrc.2009.08.125. [DOI] [PubMed] [Google Scholar]
- 18.Coffman LG, Parsonage D, D'Agostino R, Jr, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci (USA) 2009;106:570–575. doi: 10.1073/pnas.0812010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaumont C, Leneuve P, Devaux I. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nature Genetics. 1995;11:444–446. doi: 10.1038/ng1295-444. [DOI] [PubMed] [Google Scholar]
- 20.Girelli D, Corrocher R, Bisceglia L. Molecular basis for the recently described hereditary hyperferritinemia–cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the ‘Verona mutation’) Blood. 1995;86:4050–4053. [PubMed] [Google Scholar]
- 21.Hahn P, Dentchev T, Qian Y, Rouault T, Harris ZL, Dunaief JL. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol Vis. 2004;10:598–607. [PubMed] [Google Scholar]
- 22.Rice AE, Mendez MJ, Hokanson CA, Rees DC, Björkman PJ. Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol. 2009;386:717–732. doi: 10.1016/j.jmb.2008.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci (USA) 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematol Am Soc Hematol Educ Program. 2006;507:29–35. doi: 10.1182/asheducation-2006.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Gagliardo B, Kubat N, Faye A, Jaouen M, Durel B, Deschemin JC, Canonne-Hergaux F, Sari MA, Vaulont S. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J Hepatol. 2009;50:394–401. doi: 10.1016/j.jhep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 26.De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci (USA) 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E, Huang ML, Rahmanto YS, Richardson DR, Vyoral D. Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood. 2009;113:6225–6236. doi: 10.1182/blood-2009-01-201590. [DOI] [PubMed] [Google Scholar]
- 28.Darshan D, Anderson GJ. Interacting signals in the control of hepcidin expression. Biometals. 2009;22:77–87. doi: 10.1007/s10534-008-9187-y. [DOI] [PubMed] [Google Scholar]
- 29.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira SJ, Pinto JP, Picarote G, Costa VM, Carvalho F, Rangel M, de Sousa M, de Almeida SF. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnana-Prakasam JP, Martin PM, Mysona BA, Roon P, Smith SB, Ganapathy V. Hepcidin expression in mouse retina and its regulation via lipopolysaccharide/Toll-like receptor-4 pathway independent of Hfe. Biochem J. 2008;411:79–88. doi: 10.1042/BJ20071377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming RE, Britton RS. Iron imports. VI HFE and regulation of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2006;290:G590–G594. doi: 10.1152/ajpgi.00486.2005. [DOI] [PubMed] [Google Scholar]
- 33.Vujić Spasić M, Kiss J, Herrmann T, Galy B, Martinache S, Stolte J, Gröne HJ, Stremmel W, Hentze MW, Muckenthaler MU. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab. 2008;7(2):173–8. doi: 10.1016/j.cmet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Bhatt L, Horgan CP, McCaffrey MW. Knockdown of beta2-microglobulin perturbs the subcellular distribution of HFE and hepcidin. Biochem Biophys Res Commun. 2009;378:727–731. doi: 10.1016/j.bbrc.2008.11.118. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50:1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 37.Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al Saati T, Coppin H, Roth MP. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114:2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 38.Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B, Jr, Lin HY, Pietrangelo A, Babitt JL. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137:1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111:5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang AS, Yang F, Meyer K, Hernandez C, Chapman-Arvedson T, Bjorkman PJ, Enns CA. Neogenin-mediated hemojuvelin shedding occurs after hemojuvelin traffics to the plasma membrane. J Biol Chem. 2008;283:17494–17502. doi: 10.1074/jbc.M710527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, López-Otín C. Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica. 2009;94:840–849. doi: 10.3324/haematol.2008.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med. 2009;87:471–480. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang AS, Yang F, Wang J, Tsukamoto H, Enns CA. Hemojuvelin-neogenin interaction is required for bone morphogenic protein-4-induced hepcidin expression. J Biol Chem. 2009;284:22580–22589. doi: 10.1074/jbc.M109.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94:765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gnana-Prakasam JP, Martin PM, Zhang M, Atherton SS, Smith SB, Ganapathy V. Expression of the iron-regulatory protein hemojuvelin in retina and its regulation during cytomegalovirus infection. Biochem J. 2009;419:533–543. doi: 10.1042/BJ20082240. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Takahashi S, Kawanami T, Kato T, Sasaki H. Retinal degeneration in hereditary ceruloplasmin deficiency. Ophthalmologica. 1998;212:11–14. doi: 10.1159/000027251. [DOI] [PubMed] [Google Scholar]
- 48.Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RT, Gitlin JD. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci (USA) 1995;92:2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan A, Andrews NC. The molecular regulation of iron metabolism. Hematol J. 2004;5:373–380. doi: 10.1038/sj.thj.6200540. [DOI] [PubMed] [Google Scholar]
- 50.Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25:411–419. doi: 10.1055/s-2005-923313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beutler E. Hemochromatosis: genetics and pathophysiology. Annu Rev Med. 2006;57:331–347. doi: 10.1146/annurev.med.57.121304.131310. [DOI] [PubMed] [Google Scholar]
- 52.Pietrangelo A. Non-HFE hemochromatosis. Semin Liver Dis. 2005;25:450–460. doi: 10.1055/s-2005-923316. [DOI] [PubMed] [Google Scholar]
- 53.Davies G, Dymock I, Harry J, Williams R. Deposition of melanin and iron in ocular structures in hemochromatosis. Br J Ophthalmol. 1972;56:338–342. doi: 10.1136/bjo.56.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth AM, Foos RY. Ocular pathologic changes in primary hemochromatosis. Arch Ophthalmol. 1972;87:507–514. doi: 10.1001/archopht.1972.01000020509003. [DOI] [PubMed] [Google Scholar]
- 55.Gnana-Prakasam JP, Thangaraju M, Liu K, Ha Y, Martin PM, Smith SB, Ganapathy V. Absence of iron-regulatory protein HFE results in hyper-proliferation of retinal pigment epithelium mediated by induction of cystine/glutamate transporter. Biochem J. 2009;424:243–252. doi: 10.1042/BJ20090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadziahmetovic M, Song Y, Grieco S, Vaulont S, Dunaief JL. Hepcidin deficient mice have an age-related retinal iron accumulation with RPE hypertrophy/autofluorescence. ARVO 2009. 2009:896. Poster # D. [Google Scholar]
- 57.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 58.Montezuma SR, Sobrin L, Seddon JM. Review of genetics of age-related macular degeneration. Semin Ophthalmol. 2007;22:229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- 59.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 60.AREDS. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no 8 Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dentchev T, Hahn P, Dunaief JL. Strong labeling for iron and the iron-handling proteins ferritin and ferroportin in the photoreceptor layer in age-related macular degeneration. Arch Ophthalmol. 2005;123:1745–1746. doi: 10.1001/archopht.123.12.1745. [DOI] [PubMed] [Google Scholar]
- 62.Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121:1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 63.Hadziahmetovic M, Denntchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Invest Ophthalmol Vis Sci. 2008;49:2728–2736. doi: 10.1167/iovs.07-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammer A, Ferro M, Tillian HM, Tatzber F, Zollner H, Schauenstein E, Schaur RJ. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Rad Biol Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- 65.Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest. 1990;86:1991–1998. doi: 10.1172/JCI114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arieh SVB, Laham N, Schechter C, Yewdell JW, Coligan JE, Ehrlich R. A single viral protein HCMV US2 affects antigen presentation and intracellular iron homeostasis by degradation of classical HLA class I and HFE molecules. Blood. 2003;101:2858–2864. doi: 10.1182/blood-2002-07-2158. [DOI] [PubMed] [Google Scholar]
- 67.Chevalier MS, Johnson DC. Human cytomegalovirus US3 chimeras containing US2 cytosolic residues acquire major histocompatibility class I and II protein degradation properties. J Virol. 2003;77:4731–4738. doi: 10.1128/JVI.77.8.4731-4738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scholz M, Doerr HW, Cinatl J. Human cytomegalovirus retinitis: pathogenicity, immune invasion and persistence. Trends Microbiol. 2003;11:171–178. doi: 10.1016/s0966-842x(03)00066-0. [DOI] [PubMed] [Google Scholar]