Abstract
Effects of Epimedium extract and its constituent icariin on peripheral nerve repair were investigated in a crush injury rat model. Animals were divided into four groups: sham, control, Epimedium extract, and icariin groups. At postoperative weeks 1, 2, 4, and 8, nerve regeneration and functional recovery were evaluated by sciatic functional index (SFI), nerve electrophysiology, nerve pinch test, and muscle wet weight. Results showed that at 2 and 4 weeks after surgery rats in the Epimedium group displayed a better recovery of nerve function than that in the icariin and control groups, with better recovery in the icariin group than in the control group. The nerve pinch test showed that nerve regeneration was greater in the Epimedium group and the icariin group as compared to the control group. In addition, the muscle wet weight in the Epimedium group was significantly improved when compared with the icariin group, and the improvement in the icariin group was better than that in the control group at 8 weeks after operation. Our findings suggest that Epimedium extract effectively promotes peripheral nerve regeneration and improves the function of damaged nerves.
1. Introduction
Treatment of peripheral nerve injury is a major challenge in clinical practice. With advances in molecular biology and development of microsurgical techniques and tissue engineering, peripheral nerve repair procedures have been greatly improved [1]. In the last 10 decades, most treatments for peripheral nerve injury in animal models have achieved histological and functional recovery. Approaches in humans, however, produce insufficient recovery, especially for proximal nerve injury [2–4]. The discrepancy in results from experiments and clinical trials mainly results from the longer distance between organ and points of damage in humans [5]. Moreover, the speed of nerve regeneration is relatively slow, and the regenerated axons often need 3 or sometimes up to 10 months to eventually grow into target organs and tissues. Therefore, long term treatment is essential when inducing nerve regeneration with neurotrophic factors [6]. Most neurotrophic factors are mainly the neuropoietic cytokines [7] such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin (NF). However, only a few of these factors are used in clinical treatment because they often cause side effects, and the treatment with these factors is usually costly. Therefore, it is imperative to find other factors to promote peripheral nerve regeneration. Increasing attention has been paid to the traditional Chinese medicine (TCM) for promoting peripheral nerve regeneration [8–10] since these remedies often display effective clinical outcome, minor side effects, and effectiveness for multiple targets. Although TCM has complex ingredients and the specific pharmacological mechanisms for their effectiveness are still unclear, an effective clinical outcome is welcomed by many clinicians.
In our previous studies, results showed that systemic administration of a traditional formula, which mainly contains the Radix Hedysari, Epimedium, and so forth, could enhance the peripheral nerve regeneration in rats [11]. Thus, we hypothesized that Epimedium may be a major component promoting the peripheral nerve regeneration in the formula. Epimedium has been used in China to treat erectile dysfunction, postmenopausal syndrome, and osteoporosis for thousands of years [12]. Icariin is a major component of Epimedium [13, 14]. Based on findings in clinical pharmacodynamic studies on Epimedium, Shindel et al. have demonstrated that icariin can promote bone formation and has neurotrophic effects in both in vivo and in vitro experiments [15]. Tohda and Nagata also found that an extract of Epimedium koreanum could promote recovery of muscle function after spinal cord injury in rats [16]. In the present study, the effects of Epimedium extract and its main ingredient, icariin, on peripheral nerve regeneration were investigated.
2. Materials and Methods
2.1. Animals and Animal Model
A total of 42 healthy adult male SD rats weighing 200–220 g (SPF grade) were purchased from the Beijing Unilever Animal Co., Ltd., and housed in the Animal Center of People's Hospital of Peking University. Animals were maintained in a specific pathogen-free (SPF) environment with controlled humidity and 12:12 h light-dark cycle and were given ad libitum access to water and food. All efforts were made to minimize animal suffering and to reduce the number of animals used. All of the surgical procedures, experimental manipulations, and perioperative care were performed in strict accordance with the Chinese Guidelines for the Care and Use of Laboratory Animals and were approved by the Hospital Medical Ethics of Peking Union Medical Collage.
All the animals were randomly divided into 4 groups: sham group (n = 6), control group (n = 12), Epimedium extract group (n = 12), and icariin group (n = 12). Rats were anesthetized with a single intraperitoneal injection of 2% pentobarbital solution (30 mg/kg). In the control, Epimedium extract, and icariin groups, the right sciatic nerve was exposed, clamped at 5 mm above the first branch of the nerve for 1 min, and marked with a 10-0 nylon microscopic suture in the epineurium under aseptic conditions. In the sham group, the right tibial nerve was only exposed, without nerve crush. Animals in the sham and the control group were intragastrically treated daily with 1 mL of distilled water. Rats in the Epimedium extract group and the icariin group were intragastrically fed daily with 1 mL of Epimedium extract and icariin, respectively.
2.2. Preparation of Epimedium Extract and Icariin
Epimedium was purchased from Beijing Tong Ren Tang Pharmacy and icariin from China Pharmaceutical and Biological Products. Dried Epimedium leaves (1000 g) were immersed in pure water at a volume ratio of 1 : 10 and boiled for 1 h. This was repeated once and the supernatant was collected by using a 200-mesh gauze filter. The collected supernatant was kept at room temperature overnight to remove sediments. It was then concentrated to 1000 mL by boiling and kept at 4°C for use. Five grams of icariin was dissolved in 1000 mL of pure water and kept at 4°C for use.
2.3. Rat Sciatic Functional Index (SFI)
The sciatic functional index (SFI) was measured at weeks 1, 2, 4, and 8 after surgery. A walking track box (50 cm in length) was made and a white paper was cut to the appropriate dimensions and placed on the bottom. The hindlimbs were dipped into carbon ink and each rat was permitted to walk down the box in order to record the bilateral footprints 4-5 times. The following parameters were determined: (1) footprint length (PL), defined as the distance from the heel to toe; (2) toe spread (TS), defined as the distance from the first to fifth toes; and (3) intermediary toe spread (IT), defined as the distance between the second and fourth toes. The right footprints (E) were recorded and measured, and the left footprints served as controls (N). Three parameters were calculated: print length factor (PLF) = (EPL-NPL)/NPL, toe spread factor (TSF) = (ETS-NTS)/NTS, and intermediary toe spread factor (ITF) = (EIT-NIT)/NIT. These parameters were utilized to calculate the Bain-Mackinnon-Hunter (BMH) sciatic function index (SFI) as follows: SFI = −38.3 (PLF) ± 109.5 (TSF) ± 13.3 (ITF) − 8.8. The recovery rate of SFI was calculated as a score of 0–100. Zero represents normal and 100 as no function.
2.4. Nerve Pinch Test
Six rats were randomly selected from the control, Epimedium extract, and icariin groups for nerve pinch test at postoperative week 1. Rats were anesthetized with a single intraperitoneal injection of 2% pentobarbital solution (20 mg/kg). The right sciatic nerve was exposed again, and a clamp was gradually applied from the distal tibial nerve to the proximal tibial nerve. Then, the pain reflex was observed, and the distance between the farthest reflective site and the crushed site was recorded. Experiments in this study were double blind.
2.5. Immunofluorescence Staining of Growth Associated Protein-43 (GAP-43)
After nerve pinch test, animals were killed under anesthesia and sciatic nerves were collected from the 5 mm proximal crush point to the 20 mm distal point. Sciatic nerves were fixed in 4% paraformaldehyde for 6 h and then dehydrated in 15% sucrose for 8–12 h and 30% sucrose overnight. The nerves were embedded in OCT compound. At −20°C, 10 μm sections were obtained. The slices were dried at room temperature for 2 h, kept at 4°C, fixed in acetone at −20°C for 20 min, and washed thrice with 0.3% triton-PBS (5 min for each). These sections were blocked with 10% normal goat serum for 1 h and incubated overnight with mouse anti-rat GAP-43 monoclonal antibody (1 : 500, Sigma) at 4°C. The slides were incubated with rabbit anti-mouse IgG-FITC antibody (1 : 100, Sigma) at room temperature for 1 h after being washed thrice with 0.3% triton-PBS (5 min for each). Sections were observed under a fluorescence microscope.
2.6. Electrophysiological Examination
At week 8, rats were anesthetized and sciatic nerves were exposed. Electrophysiological recordings were performed in a quiet room with an ambient temperature at 22-23°C. For the detection of compound muscle action potential (CMAP), the recording electrodes were located on the central portion of the triceps and reference electrodes on the ipsilateral thigh muscle. Paraffin was applied around the neural stem to reduce fluid pathway conduction. CMAP was recorded after a stimulus with the intensity of 0.9 mA was given, using a pulse width of 0.1 ms and a frequency of 1 Hz. The latency of CMAP was recorded after stimulation of the nerve at crushed site and 30 mm proximal from the sciatic nerve, and the nerve conduction velocity (NCV, m/s) was obtained semiautomatically by dividing the distance between two stimulating sites by the difference in the onset latency.
2.7. Measurement of Muscle Wet Weight and Histological Staining
Experimental rats were sacrificed by overdose anesthesia after electrophysiological test. Bilateral triceps were carefully separated, collected, and weighted as the muscle wet weight. The ratio of right weight to left weight was calculated. A 5 mm muscle was harvested with a scalpel blade from the middle of the muscles, formalin-fixed, and paraffin-embedded for HE staining. The morphology of muscles was observed under a microscope.
2.8. Statistical Analysis
Data were expressed as mean ± standard deviation. Comparisons of means among different groups were done using one-way analysis of variance (ANOVA). A value of P less than 0.05 was considered statistically significant.
3. Results
3.1. SFI
The SFI of the sham, control, Epimedium extract, and icariin groups was −7.54 ± 1.75, −86.95 ± 4.54, −85.98 ± 5.30, and −88.39 ± 7.64, respectively, at week 1; −5.88 ± 2.03, −70.08 ± 9.71, −56.65 ± 8.36, and −63.58 ± 9.23, respectively, at week 2; −6.32 ± 2.90 −27.50 ± 7.22, −18.61 ± 4.91, and −23.79 ± 3.03, respectively, at week 4; −6.15 ± 2.24, −12.71 ± 3.79, −10.20 ± 3.98, and −13.12 ± 2.94, respectively, at week 8. In the control, Epimedium extract, and icariin groups, SFI showed a gradual recovery of sciatic function, but the sciatic function was worse in the control group. At weeks 2 and 4, the neurological function was significantly improved in the Epimedium group and the icariin group as compared to the control group. At week 8, the SFI was comparable among these groups (Figure 1).
Figure 1.
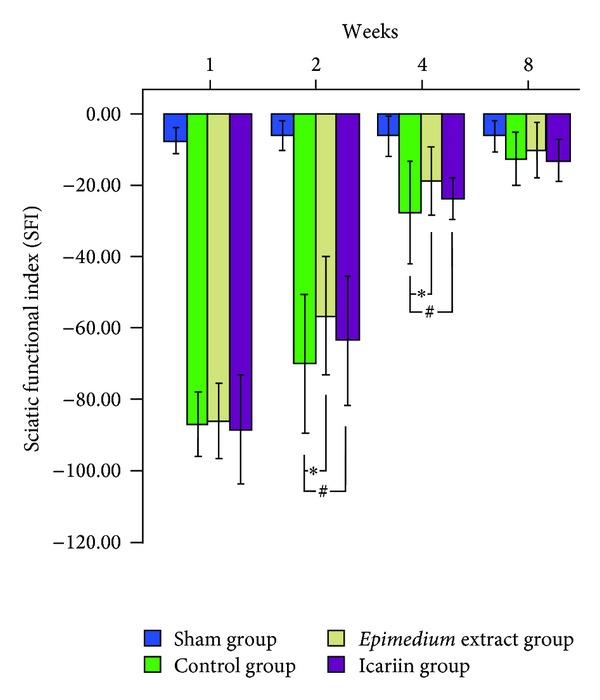
Effect of Epimedium extract and icariin on rat sciatic functional index (SFI). At week 2 and week 4, the SFI values are significantly better in rats of the Epimedium group than those of the control group. Moreover, the icariin group is better than the control group. At week 8, the sciatic functional index was not significantly different among the control, Epimedium extract and icariin groups. *P < 0.05, the Epimedium group versus the control group; # P < 0.05, the icariin group versus the control group (n = 6).
3.2. Nerve Pinch Test
At week 1, nerve pinch test showed that the average distance from reflective site to crushed site in the control, Epimedium extract, and icariin groups was 6.02 ± 0.64 mm, 8.07 ± 0.71 mm, and 6.58 ± 1.03 mm, respectively. The longest distance was found in the Epimedium extract group. The distance in the Epimedium extract group was significantly longer than that in the control group (P = 0.002) and the icariin group (P = 0.026), but no significant difference was observed between the icariin group and the control group (P = 0.303) (Figure 2).
Figure 2.
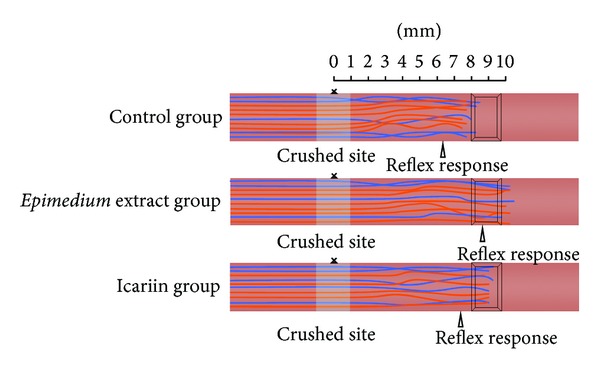
Nerve pinch test was performed to evaluate Epimedium extract and icariin effect on nerve regeneration. The arrow indicates the nerve reflective site. The average distances from the crushed site to the reflective site were 6.02 ± 0.64 mm, 8.07 ± 0.71 mm, and 6.58 ± 1.03 mm in the control, Epimedium, and icariin groups at week 1 after the operation.
3.3. GAP-43 Immunofluorescence Staining
GAP-43 staining was performed to evaluate the regeneration of nerve fibers. GAP-43 expression was obviously observed at the reflective site. At the same site, 9 mm proximate to the crushed site, GAP-43 expression was significantly lower in the control group than in the Epimedium extract and icariin groups at week 1 after operation (Figures 3(a), 3(b), and 3(c)). It is indicated that the nerve fiber regeneration was better in the Epimedium extract group.
Figure 3.
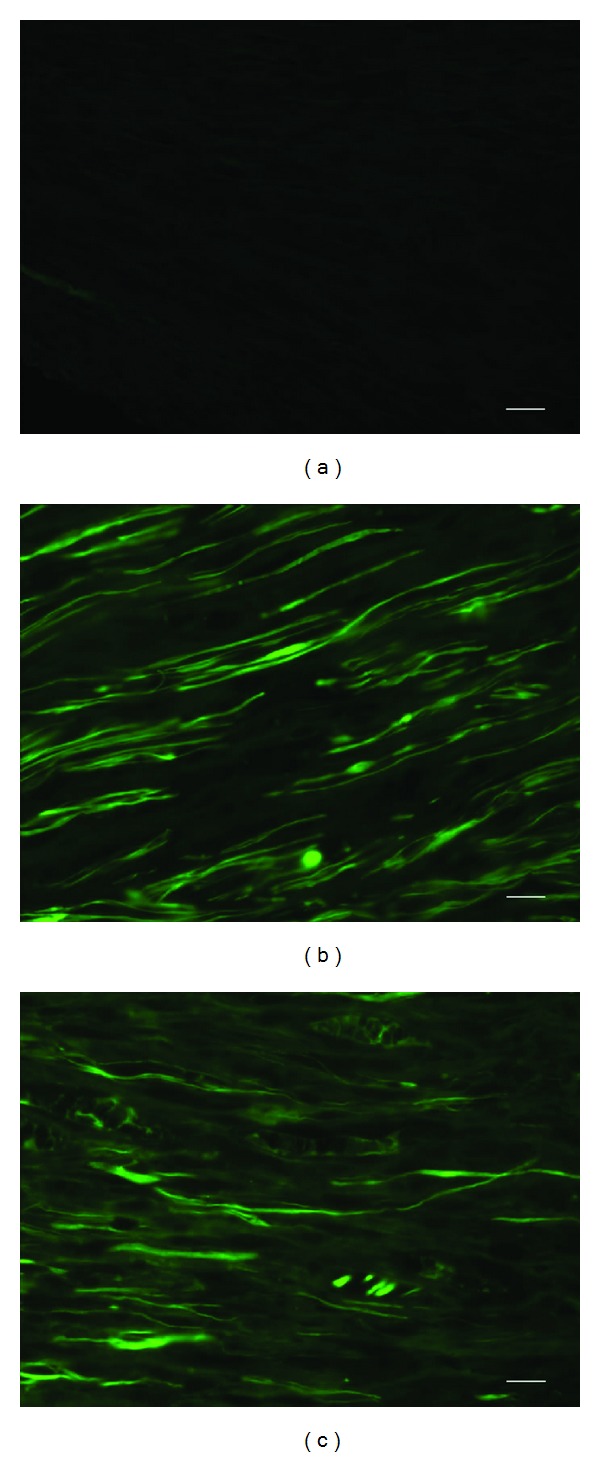
GAP-43 immunofluorescence staining was observed at 9 mm proximate to the crushed site in the control, Epimedium, and icariin groups at week 1 after the operation. (a) GAP-43 staining was hardly seen in the control group. (b) Evident GAP-43 staining was observed in the Epimedium extract group. (c) In the icariin group, GAP-43 staining was detectable but the density was lower than the Epimedium extract group. Scale bar: 5 μm.
3.4. Nerve Electrophysiological Measurements
The nerve conduction velocity of the sham, control, Epimedium extract, and icariin groups was 47.25 ± 2.91 m/s, 28.22 ± 3.55 m/s, 32.02 ± 2.37 m/s, and 29.51 ± 3.29 m/s, respectively, at week 8 after operation. The nerve conduction velocity of the control group was significantly lower than that of the Epimedium extract group (P = 0.044). There was no significant difference between the Epimedium extract and the icariin groups (P = 0.17).
3.5. Muscle Wet Weight and Histological Staining
The bilateral wet muscle weight of the sham group was comparable. Atrophy of the muscle was evident in the control group, however, as compared to the Epimedium extract and the icariin groups. The wet weight of left normal muscle in the sham, control, Epimedium extract, and icariin groups was 1.285 ± 0.098 g, 1.271 ± 0.088 g, 1.303 ± 0.138 g, and 1.307 ± 0.086 g, respectively, showing no significant difference between any two groups. The wet weight of right muscle was 1.283 ± 0.071 g, 0.853 ± 0.042 g, 1.075 ± 0.111 g, and 1.010 ± 0.109 g in the sham, control, Epimedium extract, and icariin groups, respectively. The recovery rate of wet muscle weight was 100.10 ± 5.10%, 67.26 ± 3.67%, 82.56 ± 4.35%, and 77.13 ± 3.79% in the sham, control, Epimedium extract, and icariin groups, respectively. The muscle wet weight and recovery rate of the control, Epimedium, and icariin groups were lower than those of the sham group. The wet muscle weight and recovery of the control group were lower than those in the Epimedium group and the icariin group, and the muscle recovery rate in the Epimedium extract group was better than that in the icariin group.
The HE staining of normal muscle in the sham group showed clear boundaries of muscle fibers with uniform staining and similar diameter. In the control, Epimedium extract, and icariin groups, muscle fiber staining was not uniform, and the muscle diameter was smaller than that in sham group. Muscle diameter of the control group was the smallest (Figure 4).
Figure 4.
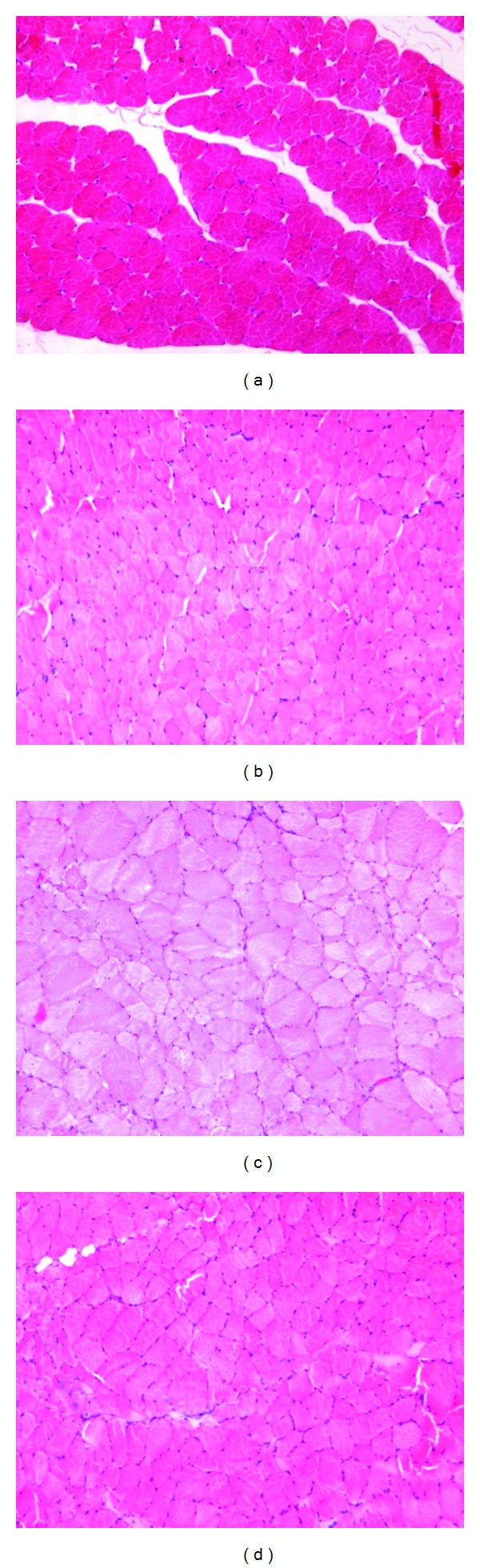
HE staining of muscle histology. (a) The sham group; (b) the control group; (c) the Epimedium extract group; (d) the icariin group.
4. Discussion
The SFI most commonly employed is the BMH SFI formula [17–19]. In this formula, an SFI of 0 is normal, and an SFI of −100 indicates complete impairment. In this study, the SFI was significantly improved in the Epimedium group and the icariin group as compared to the control group at 2 and 4 weeks after operation, suggesting that Epimedium extract and icariin are able to accelerate the functional recovery following injury. The extent of axonal regeneration distal to the injured site was also measured at week 1 by using the nerve pinch test and GAP-43 staining (staining of regenerating axons). Results demonstrated that Epimedium extract accelerated axonal growth, which is helpful to explain why the SFI in the Epimedium group was significantly improved at 2 and 4 weeks after operation as compared to the control group. Findings in nerve electrophysiological measurement, muscle wet weight measurement, and histological staining also supported that both Epimedium extract and icariin can exert protective effects on the motor function recovery and conductivity recovery in rats. It is presumed that icariin alone is effective to promote nerve regeneration, and may be one of the major components of Epimedium extract promoting the regeneration of peripheral nerves. However, the effects of Epimedium extract and icariin on the peripheral nerve regeneration are different, suggesting that components other than icariin in Epimedium extract may also facilitate the regeneration of peripheral nerves or coordinate this growth.
There are three animal nerve injury models frequently used in studies on the physiology and function of peripheral nerves: crush, transection, and graft/conduit [20, 21]. In this study, the sciatic nerve crush model, which has been employed in our previous studies, was used to investigate the influence of Epimedium extract on the peripheral nerve regeneration. This model is easy to establish and can be appropriately standardized, and it successfully reflects the restoration of nerve function. The sciatic nerve crush may produce a Sunderland type II injury, in which the myelin sheath and the axons are disrupted, but the basal lamina Schwann cell tubes remain intact [22]. Thus, the recovery of functional index is excellent and contrasts to the relatively poor recovery after nerve transection and repair. In this study, no difference in SFI was observed among these groups at 8 weeks after operation. This may be explained that the rat nerves achieve full restoration at 8 weeks after operation.
The dose of Epimedium extract used in this study was determined according to our previous studies [23]. The proportion of icariin in Epimedium extract was determined by high-performance liquid chromatography. The average proportion of icariin is about 4.873 mg/mL. Thus, the concentration of icariin prepared in this study was identical in both Epimedium extract and icariin solution.
Clinical and experimental studies have demonstrated that many TCM can promote peripheral nerve regeneration. For example, Wei et al. found that Hedysari extract (a TCM) prompted the regeneration of peripheral nerves in animal experiments [24]. Hsiang et al. also found that puerarin also promoted the regeneration of peripheral nerves [25]. In addition, Chen et al. showed that extract seemed to promote the PC12 differentiation and the regeneration of lateral nerve bud [26], efficiently improving the restoration of peripheral nerve function in experimental animals. Many molecular and pathophysiological changes are found to be involved in the process of nerve regeneration, which is in turn under the control of other factors. Recent studies have demonstrated that the interaction of multiple factors is important for the nerve regeneration and may exert better effects than single factor. For example, the nerve growth factors secreted by Schwann cells are a collection of multiple factors having more potent effects on the axonal growth and maturation of myelin than any individual factor. These studies suggest that the microenvironment involved in the nerve regeneration is really a collection of influences that can more efficiently promote the overall regeneration of peripheral nerves. Since TCM have multiple components in each treatment, they have the potential to effectively promote nerve regeneration. This was a preliminary study on the effect of Epimedium on nerve regeneration and growth, and the detailed mechanisms are still unclear. Further studies are warranted.
Conflict of Interests
The authors certify that they do not have a direct financial relation with the companies mentioned in their paper and have no other conflict of interests in connection with the submitted paper.
Acknowledgments
This study was supported by the National Natural Science Fund (31171150, 31271284, 81171146, 30971526, and 30801169), the New Star Program of Beijing Science and Technology (A-2008-10), the New Century Excellent Talents Support Project of Educational Ministry (BMU 20110270), and the Innovation Team Program of Educational Ministry (IRT1201).
References
- 1.Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clinics in Plastic Surgery. 2011;38(4):643–660. doi: 10.1016/j.cps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: bridging the gap. Progress in Neurobiology. 2010;92(3):245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Nectow AR, Marra KG, Kaplan DL. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Engineering B. 2012;18(1):40–50. doi: 10.1089/ten.teb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood MD, Kemp SWP, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Annals of Anatomy. 2011;193(4):321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Höke A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nature Clinical Practice Neurology. 2006;2(8):448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 6.Ide C. Peripheral nerve regeneration. Neuroscience Research. 1996;25(2):101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 7.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurgical Focus. 2009;26(2):p. E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 8.Mani L, Roco ML, Barbaro Paparo S, Guaragna M. Electroacupucture and nerve growth factor: potential clinical applications. Archives Italiennes de Biologie. 2011;149(2):247–255. doi: 10.4449/aib.v149i2.1365. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Huang C, Tsai F, Tsai C, Yao C, Chen Y. Growth-promoting effects of quercetin on peripheral nerves in rats. International Journal of Artificial Organs. 2011;34(11):1095–1105. doi: 10.5301/ijao.5000064. [DOI] [PubMed] [Google Scholar]
- 10.Wei S, Yin X, Kou Y, Jiang B. Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. Journal of Ethnopharmacology. 2009;123(1):51–54. doi: 10.1016/j.jep.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Wei S, Zhang P, Dang Y, Zhang H, Jiang B. Primary study on effect of various components of modified formula radix hedysari on peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22(9):1056–1059. [PubMed] [Google Scholar]
- 12.Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus epimedium: an ethnopharmacological and phytochemical review. Journal of Ethnopharmacology. 2011;134(3):519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Wu C, Zhang J, Li Y. A new strategy for the discovery of epimedium metabolites using high-performance liquid chromatography with high resolution mass spectrometry. Analytica Chimica Acta. 2013;768:111–117. doi: 10.1016/j.aca.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Xue L, Wang Y, Jiang Y, et al. Comparative effects of er-xian decoction, epimedium herbs, and icariin with estrogen on bone and reproductive tissue in ovariectomized rats. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11 pages. doi: 10.1155/2012/241416.241416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shindel AW, Xin Z, Lin G, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo . Journal of Sexual Medicine. 2010;7(4):1518–1528. doi: 10.1111/j.1743-6109.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohda C, Nagata A. Epimedium koreanum extract and its constituent Icariin improve motor dysfunction in spinal cord injury. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6 pages. doi: 10.1155/2012/731208.731208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 1982;77(3):634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 18.Bain J, Mackinnon S, Hunter D. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plastic and Reconstructive Surgery. 1989;83(1):129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Hare G, Evans P, Mackinnon S, et al. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plastic and Reconstructive Surgery. 1992;89(2):251–258. [PubMed] [Google Scholar]
- 20.Wood MD, Kemp SWP, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Annals of Anatomy. 2011;193(4):321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Pfister B, Gordon T, Loverde J, Kochar AS, Mackinnon SE, Kacy Cullen D. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Critical Reviews in Biomedical Engineering. 2011;39(2):81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 22.Moradzadeh A, Brenner MJ, Whitloch EL, et al. Bipolar electrocautery: a rodent model of sunderland third-degree nerve injury. Archives of Facial Plastic Surgery. 2010;12(1):40–47. doi: 10.1001/archfacial.2009.104. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Jiang B, Zhang D, Fu Z, Zhang H. Compound injection of radix Hedysari to promote peripheral nerve regeneration in rats. Chinese Journal of Traumatology. 2002;5(2):107–111. [PubMed] [Google Scholar]
- 24.Wei SY, Zhang PX, Han N, et al. Effects of hedysari polysaccharides on regeneration and function recovery following peripheral nerve injury in rats. American Journal of Chinese Medicine. 2009;37(1):57–67. doi: 10.1142/S0192415X09006618. [DOI] [PubMed] [Google Scholar]
- 25.Hsiang SW, Lee HC, Tsai FJ, Tsai C, Yao C, Chen Y. Puerarin accelerates peripheral nerve regeneration. American Journal of Chinese Medicine. 2011;39(6):1207–1217. doi: 10.1142/S0192415X11009500. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Lin J, Lu T, et al. Earthworm extracts facilitate pc12 cell differentiation and promote axonal sprouting in peripheral nerve injury. American Journal of Chinese Medicine. 2010;38(3):547–560. doi: 10.1142/S0192415X10008044. [DOI] [PubMed] [Google Scholar]


