Abstract
Primary myelofibrosis is a member of the myeloproliferative neoplasms, a diverse group of bone marrow malignancies. Symptoms of myelofibrosis, particularly those associated with splenomegaly (abdominal distention and pain, early satiety, dyspnea, and diarrhea) and constitutional symptoms, represent a substantial burden to patients. Most patients eventually die from the disease, with a median survival ranging from approximately 5–7 years. Mutations in Janus kinase 2 (JAK2), a kinase that is essential for the normal development of erythrocytes, granulocytes, and platelets, notably the V617F mutation, have been identified in approximately 50% of patients with myelofibrosis. The approval of a JAK2 inhibitor in 2011 has improved the outlook of many patients with myelofibrosis and has changed the treatment landscape. This article focuses on some of the important issues in current myelofibrosis treatment management, including differentiation of myelofibrosis from essential thrombocythemia and polycythemia vera, up-dated data on the results of JAK2 inhibitor therapy, the role of epigenetic mechanisms in myelofibrosis pathogenesis, investigational therapies for myelofibrosis, and advances in hematopoietic stem cell transplant. Three myelofibrosis cases are included to underscore the issues in diagnosing and treating this complex disease.
Introduction
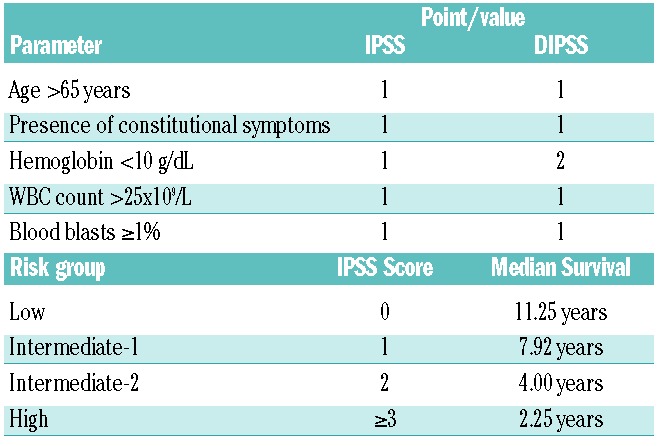
Primary myelofibrosis (PMF) is a member of the myeloproliferative neoplasms (MPN), a diverse group of bone marrow malignancies that includes chronic myelogenous leukemia (CML), essential thrombocythemia (ET), polycythemia vera (PV), chronic neutrophilic leukemia (CNL), chronic eosinophilic leukemia, NOS (CEL-NOS), and systemic mastocytosis (SM). ET and PV are capable of evolving into a myelofibrotic state (post ET-related MF and post PV-related MF) that resemble PMF, and these three entities are collectively termed MF. MF is characterized by a hyperproliferative bone marrow with dysmyelopoiesis and hypolobulated megakaryocytes, bone marrow fibrosis, cytopenias or cytosis, and progressive splenomegaly.1 Symptoms of myelofibrosis, particularly those associated with splenomegaly (abdominal distention and pain, early satiety, dyspnea, and diarrhea) and constitutional symptoms, represent a substantial burden to patients. Most patients eventually die from the disease with a median survival ranging from approximately 5–7 years.2,3 As shown in Table 1, the prognosis of PMF depends on several factors, including age, presence of constitutional symptoms, anemia, white blood cell (WBC) count, and percentage of peripheral blood blasts.4
Table 1.
The International Prognostic Scoring System (IPSS) for myelofibrosis 2 and the dynamic IPSS (DIPSS).4
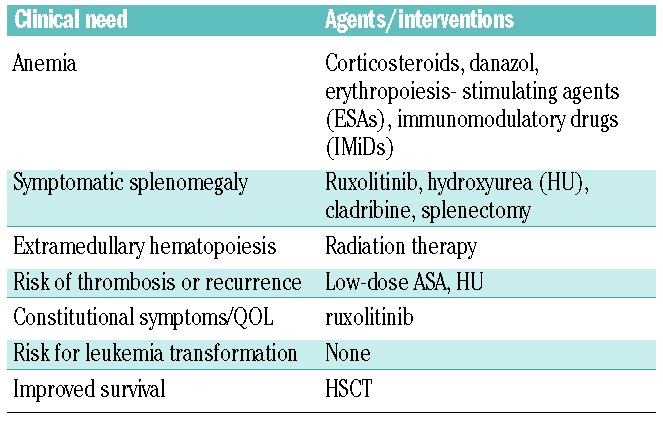
Treatment of MF is generally based on the severity of the patient’s disease and often directed to address an individual’s clinical features (Table 2).5 Hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment; however, it is associated with significant morbidity and mortality, and may not be a viable option for many MF patients who are advanced in age and who suffer from other significant competing comorbidities. In recent years, the molecular abnormalities underlying the natural history of MF have been extensively studied. Mutations in Janus kinase 2 (JAK2), a kinase that is essential for the normal development of erythrocytes, granulocytes, and platelets, notably the V617F mutation, have been identified in approximately 50% of patients with MF.6 The FDA approval of JAK2 inhibitor therapy in 2011 has improved the outlook of many patients with MF. However, JAK2 tyrosine kinase inhibitor therapy does not cure MF.
Table 2.
Therapeutic options for myelofibrosis patients based on clinical need.
This article focuses on some of the important issues in current MF treatment management, including differentiation of MF from ET and PV, up-dated data on the results of JAK2 inhibitor therapy, the role of epigenetic mechanisms in MF pathogenesis, investigational therapies for MF, and improvements in the approach of HSCT. Three MF cases are also presented to underscore the issues in diagnosing and treating this disease.
Diagnosis of primary myelofibrosis and its relationship to essential thrombocythemia
Among the MPNs, PMF shares similarities with ET and PV, both with respect to its molecular basis and its clinical presentation.7 For example, JAK2 mutations are estimated to be present in approximately 95%, 50%–60%, and 50% of PV, ET, and PMF patients, respectively.8 Furthermore, isolated thrombocytosis can be the presenting sign for all three conditions.7 However, PV, ET, and PMF are distinct clinical entities with unique pathologies and varying prognoses. Thus, the use of precise diagnostic criteria that can distinguish among these MPNs is essential for proper patient management. Case 1 illustrates the complexities of making a diagnosis of PMF.
Diagnosis of primary myelofibrosis versus essential thrombocythemia
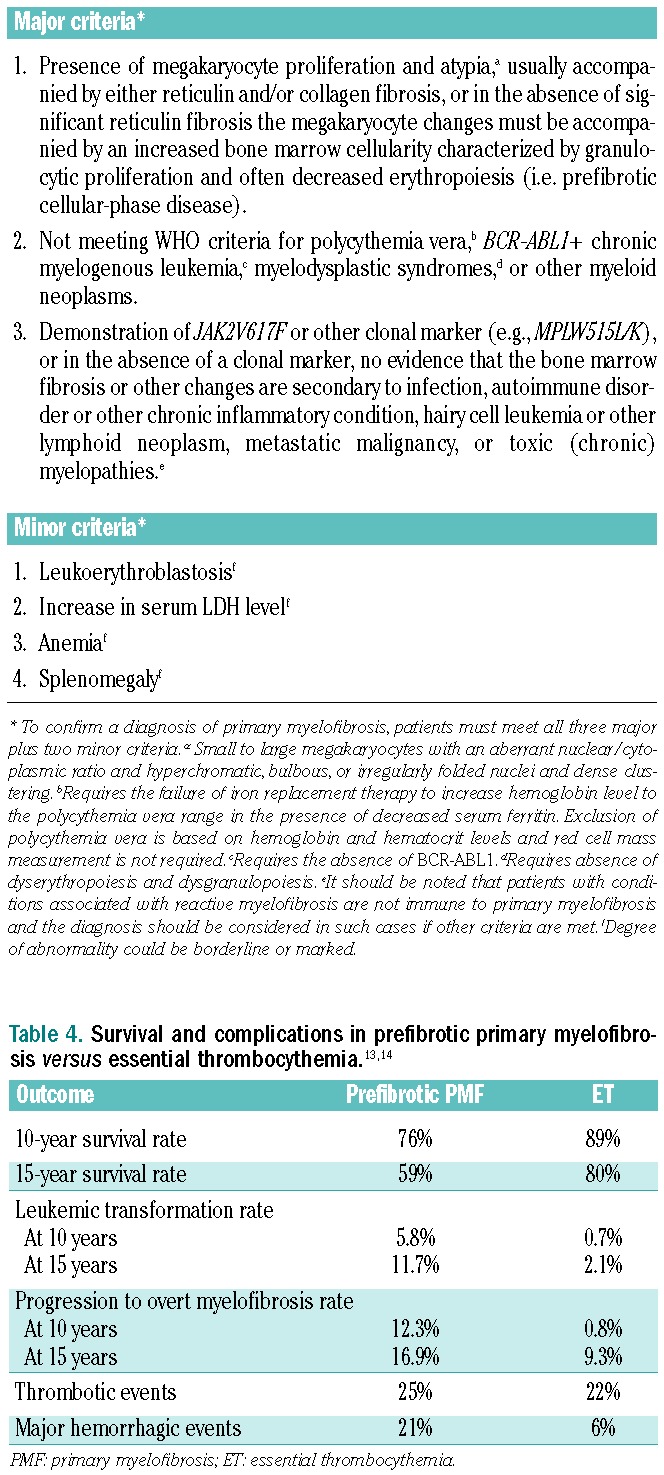
The WHO 2008 diagnostic criteria for PMF have been widely accepted (see Table 3 for a summary).9 To confirm a diagnosis of PMF, patients must meet all three major criteria plus two minor criteria. The major criteria are largely histopathology-based. For example, the presence of increased megakaryopoiesis with a preponderance of atypical megakaryocytes is an important characteristic of PMF. Such findings are usually associated with increased bone marrow cellularity. The latter, although less frequently, can also occur in cases of ET. The integration of major and minor criteria makes the diagnosis specific. More commonly fulfilled minor criteria include the presence of a high lactate dehydrogenase (LDH) level, splenomegaly, and/or anemia. Although reproducibility of the histological parameters of the WHO classification has been questioned in some studies, there is some published evidence to support its validity.10 A gap in the literature is evidence that a broad spectrum of pathologists find the criteria applicable and reproducible.
Table 3.
WHO 2008 Diagnostic Criteria for Primary Myelofibrosis.9
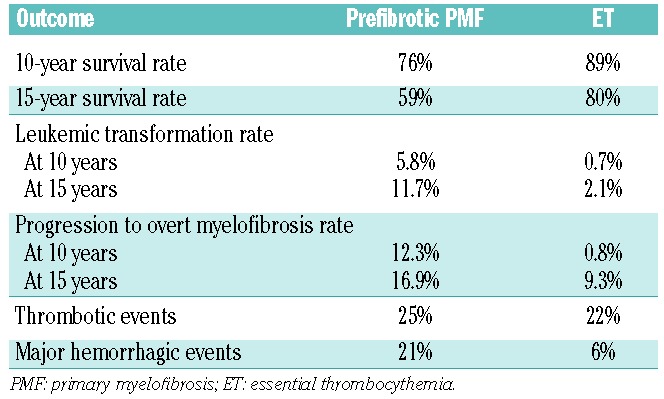
The long-term survival of patients with ET is significantly better than that for patients with prefibrotic PMF.11 Patients with ET also have higher event-free survival (EFS) rates.12 The differences in survival, progression, and complications between prefibrotic PMFs and ET patients are summarized in Table 4.13,14 Although some of the same complications, especially evolution to overt MF, occur in ET patients, their incidence and severity are generally much lower than in PMF patients.
Table 4.
Survival and complications in prefibrotic primary myelofibrosis versus essential thrombocythemia.13,14
Prefibrotic PMF and ET are reported to have distinct morphological characteristics.11 As illustrated in Figure 1, prefibrotic PMF is associated with a hypercellular marrow characterized by the presence of increased numbers of hypolobulated megakaryocytes with atypical forms often present in clusters, increased granulopoiesis, and decreased erythropoiesis.11 By contrast, ET bone marrow is notably less cellular with largely unremarkable erythropoiesis and granulopoiesis. Megakaryopoiesis is increased with large hyperlobulated (staghorn-like) megakaryocytes. Problems may arise when there is a mixture of megakaryocyte morphology.
Figure 1.
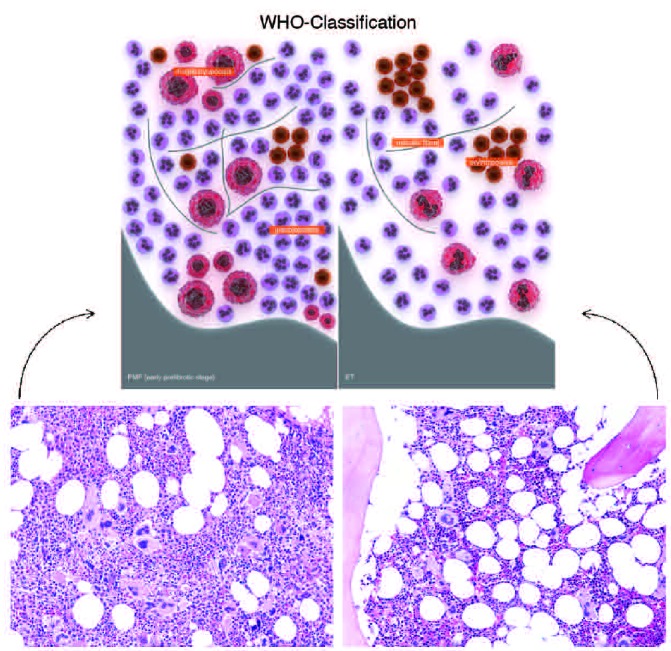
Prefibrotic PMF is associated with a hypercellular marrow characterized by the presence of increased numbers of hypolobulated megakaryocytes with atypical forms often present in clusters, increased granulopoiesis and decreased erythropoiesis (left). ET bone marrow is less cellular with largely unremarkable erythropoiesis and granulopoiesis (right). Megakaryopoiesis is increased with large hyperlobulated (staghorn-like) megakaryocytes.
(Top): republished with permission of American Society of Hematology. Permission conveyed through Copyright Clearance Center, Inc.11
Polycythemia vera versus essential thrombocythemia and primary myelofibrosis
In contrast to ET, which has no specific distinguishing clinical or laboratory features and, therefore, remains a diagnosis of exclusion,7 a unique set of PV diagnostic criteria has been developed.15 A diagnosis of PV requires the presence of both major criteria and one minor criterion or the presence of the first major together with two minor criteria. Importantly, 50% of ET patients harbor JAK2V617F. Although the diagnostic criteria for PV are useful, they may not capture early phases of the disease. The so-called “pre-polycythemic” PV must be excluded in thrombocytotic patients, particularly those with microcytic anemia, low ferritin, and/or who lack stainable bone marrow iron. The first major WHO criterion for diagnosing PV is often not fulfilled in such patients and they may be erroneously considered to have ET.
Early stage PV can be distinguished from ET by bone marrow histology. PV can be suspected in cases with a hypercellular panmyelotic marrow with pleomorphic megakaryocytes. In addition, a marrow fibrosis grade 1–2 on a scale of 4 is much more commonly seen in PV than in ET.
A diagnosis of prefibrotic PMF raises several questions that still have to be answered. 1) Can IFN or JAK2 inhibitor therapy be used to prevent disease progression? 2) Should low-dose aspirin or hydroxyurea (HU) be used for thrombosis prophylaxis? 3) Is observation and blood count monitoring sufficient? 4) Should bone marrow biopsies be periodically repeated? Unfortunately, the therapeutic implications of distinguishing between ET and early PMF remain uncertain, and a therapeutic strategy that prevents or delays disease progression to overt MF has yet to be identified. Cases 2 and 3 (see below) illustrate how some of these questions are answered.
JAK2 inhibitors
Based on the observation that approximately 50% of patients with MF harbor the JAK2V617F mutation,6 development of JAK2 inhibitor therapies has been actively pursued. The first such agent to be developed and given to patients is ruxolitinib (Jakafi, Incyte; JAKAVI, Novartis), a small-molecule inhibitor of JAK1/JAK2 that was approved in 2011 for the treatment of patients with intermediate- or high-risk MF, including PMF, post-PV MF, and post-ET MF.16 Ruxolitinib was evaluated in a phase I/II study of patients with MF that established the safety and efficacy of this agent in terms of symptom improvement and spleen reduction, and provided the rationale for phase III studies.17 This study also established what has subsequently become a standard for determining response to JAK inhibitors in clinical studies, which is the use of magnetic resonance imaging (MRI) to determine spleen volume based on the correlation between a 35% reduction in volume and a 50% reduction in palpable spleen length. The COMFORT-I and COMFORT-II studies were among the first randomized phase III studies to be conducted in MF patients.18,19 These studies had similar designs; COMFORT-I was conducted in the United States, Canada, and Australia and compared ruxolitinib to placebo, while COMFORT-II was conducted in Europe and compared ruxolitinib to best available therapy.
Up-dated safety and efficacy results for the COMFORT-I and COMFORT-II studies were presented at the American Society of Hematology (ASH) meeting in December 2012 (Table 5).20,21 Ruxolitinib treatment resulted in a significant decrease in spleen reduction, with 41.9% of patients in COMFORT-I achieving a 35% reduction or more in spleen volume at Week 24 and 28.5% of patients in COMFORT-II achieving a 35% or more reduction in spleen volume at Week 48. The degree of spleen reduction appeared to correlate with improved outcome. A subgroup analysis of COMFORT-II revealed that the vast majority of both JAK2V617F+ and JAK2V617F–patients experienced a reduction in spleen volume.22 Overall, responses were observed for ruxolitinib-treated patients in all subgroups and were higher than those in patients receiving best available therapy (BAT). Statistically significant differences in overall survival (OS) were observed between study arms in COMFORT-I (Figure 2), and a survival benefit was also observed in COMFORT-II. The dose and duration of ruxolitinib therapy, as well as the degree of spleen reduction, appear to be critical to survival outcomes.
Table 5.
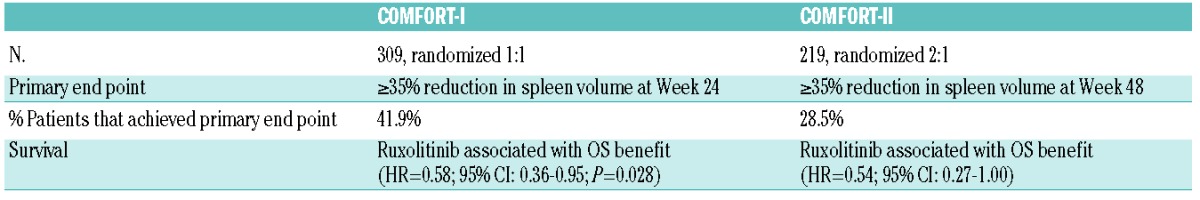
Figure 2.
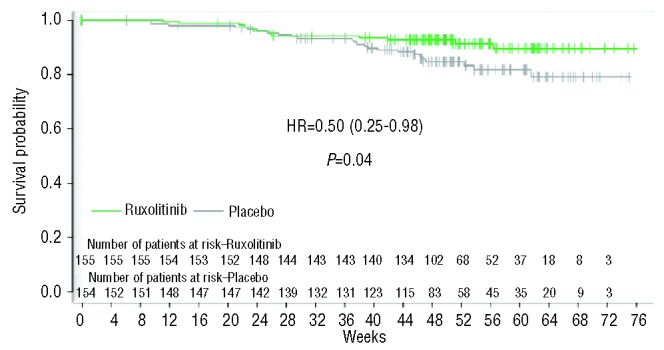
Ruxolitinib-treated patients in COMFORT-I experienced a statistically significant increase in overall survival (OS) compared to placebo.
Reprinted with permission from Massachusetts Medical Society.18
The most common adverse events in both studies were anemia and thrombocytopenia, although it should be noted that the majority of patients had grade 1–2 anemia and/or thrombocytopenia at baseline. To address the anemia, a small number of patients (n=13) in the COMFORT-II study received recombinant erythropoiesis-stimulating agents (ESAs), which appeared to be well tolerated and, interestingly, did not result in an increase in spleen size. The management of thrombocytopenic patients treated with ruxolitinib is currently being evaluated in the EXPAND phase Ib study and a study sponsored by Incyte. Non-hematologic adverse events associated with ruxolitinib therapy were not serious in nature and the most frequently reported were headache, dizziness, and easy bruising unrelated to the platelet count. Both of these studies were also up-dated at the ASH 2012 meeting and appear to show equivalent efficacy and tolerability in this subgroup of patients.23,24
Although no specific pattern of return of symptoms after withdrawal of ruxolitinib was reported in either COMFORT study, an investigator at a single institution has reported the occurrence of life-threatening hemodynamic instability after abrupt cessation of ruxolitinib.25 Attempts to taper ruxolitinib whenever possible, combined with the use of prednisone taper to blunt the acute return of symptoms, are generally recommended.
In addition to ruxolitinib, there are a significant number of other JAK inhibitors at different phases of development. Most of them show the same profile of efficacy as ruxolitinib, i.e. spleen and symptom reduction. However, as data from early phase studies indicate, some of these agents have different tolerability and efficacy. A phase I/II study26 with SAR302503 reported that SAR302503 was generally well tolerated with frequent grade 1 adverse events, and significantly improved symptoms, such as anorexia and pruritus. However, in contrast to the findings with ruxolitinib, these effects occurred in the absence of a marked reduction in serum pro-inflammatory cytokine levels (e.g. IL-2, IL-6, IL-8, TNF-α) and in the absence of significant JAK1 inhibition. Two unique, potentially significant aspects of response to this agent include a decrease in the JAK2V617F allele burden during therapy in the mutation-positive subjects and the recently reported reduction in marrow fibrosis scores.27 Following six cycles, 16 of 20 (80%) patients with a base-line JAK2V617F allele burden of more than 20% experienced a median reduction of 61%. These preliminary results reported with SAR302503 are promising and of great interest if confirmed in phase III studies, although their clinical significance remains to be assessed, as the benefit is unlikely to equate to the reduction in the level of BCR/ABL1 burden in CML. The phase III JAKARTA study has completed recruitment and results are expected this year; in addition, JAKARTA 2, evaluating this agent in patients resistant or refractory to ruxolitinib, is also open. In the phase II study with SB1518 (pacritinib), rapid and sustained responses in spleen have been seen for MF at the 400 mg/die dose.28 In an update presented at the ASH 2011 meeting, after a median time on-study of 8.2 months (range 0.5–12.1), 50% of patients had discontinued treatment and response rates were 44% on physical examination and 32% by MRI (35% reduction in volume). Two patients met criteria for anemia response,28 and a phase III study has been launched. Pardanani recently presented data from a phase I/II multicenter study with CYT387 demonstrating the anticipated improvements in splenomegaly and constitutional symptoms, as well as in transfusion requirements.29 Subjects have now reached a minimum of nine months on study, and up-dated safety and efficacy results were presented; 166 subjects were enrolled and the median duration (range) of follow up is 16.1 months (0.7–31.0 months). Particular novel data of interest with this compound are transfusion independence responses that were observed in more than half of the RBC transfusion-dependent subjects with a maximal transfusion-free period exceeding two years and still ongoing. In addition, the percentage of all subjects requiring RBC transfusions substantially decreased over the treatment period. As has been previously reported, treatment with CYT387 resulted in rapid and sustained reductions in splenomegaly, with maximal response duration now approaching two years. Concerning safety, the most common treatment-related AEs were thrombocytopenia, peripheral neuropathy, dizziness, diarrhea, nausea, and headache. Treatment-related peripheral neuropathy is a characteristic of this agent. It was reported as sensory, and mainly grade 1. There were no treatment-related deaths. A phase III study with this agent is also due to start shortly.
Although JAK inhibition clearly reduces the symptomatic burden of MF, it is neither curative nor effective in reducing the risk of leukemic transformation. Strategies to improve upon JAK inhibition therapy include exploration of other investigational small molecule JAK inhibitors, combining JAK inhibitors with other agents, and combining JAK inhibitors with HSCT. The key to optimal JAK inhibitor therapy is likely to lie between patient selection and proper dosing strategy.
The evolving role of histone deacetylase inhibitor therapy for myeloproliferative neoplasms
Epigenetic changes are increasingly being recognized as playing an important role in the progression of malignancies, including MPNs. Such changes encompass biochemical modifications to DNA or histones that are somatically heritable from mother cell to daughter cell. Epigenetic modifications lead to changes in the expression of downstream genes, and also contribute to genomic instability and drug resistance. Thus far, two of the most well-studied epigenetic processes are DNA methylation and histone deacetylation. DNA methyltransferases (DNMTs) catalyze the methylation of CpG islands within promoter sites of DNA, thereby down-regulating transcription by blocking access to transcription factor complexes. Observations that support deregulation of DNA methylation in MPNs include hypermethylation of key genes important for cell cycling, differentiation, and homing of hematopoietic cells to the bone marrow,30–32 and hypermethylation of genes that negatively regulate the hyperactive JAK/STAT signaling pathway.33
Histone deacetylases (HDACs) remove acetyl groups from lysine residues on histone tails, thereby inducing an inactive or closed conformation that restricts access of transcription factors to DNA and thus downregulation of transcription. HDACs fall into four classes that differ in their subcellular localization, tissue distribution, substrates, and binding partners. Two HDAC inhibitors (HDACis) have been FDA-approved for the treatment of cutaneous T-cell lymphoma, and several others are in clinical development for a wide array of cancers. HDACis modulate the acetylation status of histones as well as other non-histone proteins, resulting in a variety of different biological effects, including growth and cell cycle arrest, cellular differentiation, inhibition of angiogenesis, apoptosis, and immune surveillance (Figure 3). Enhanced HDAC expression has been observed in patients with PMF.35 Pre-clinical data support the use of HDACis in MPN.36–38 Characteristics and preliminary results for the use of several HDAC inhibitors in MPNs are described below.
Figure 3.
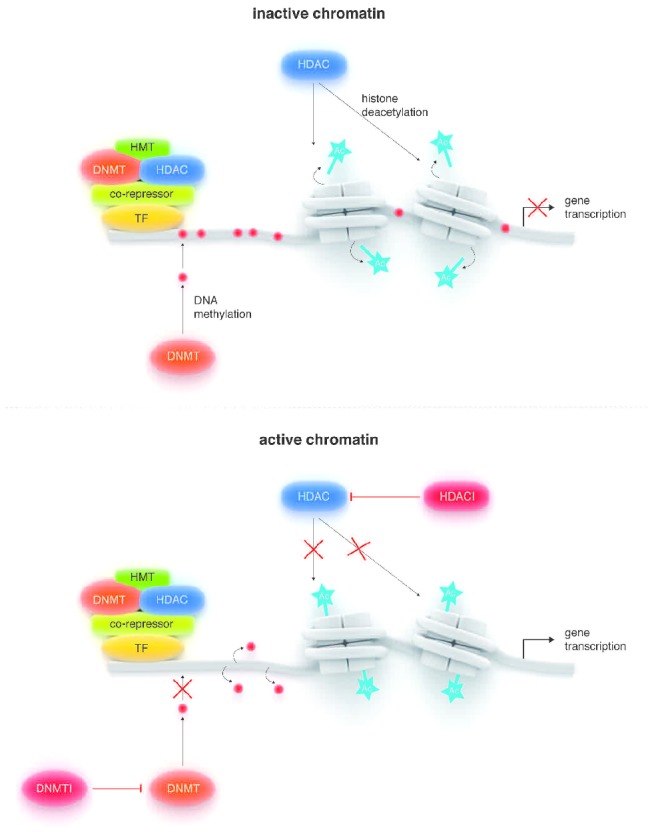
Cartoon depicting the regulation of transcription by two types of chromatin modifying agents. HDAC inhibitors (HDACI) inhibit the enzymatic activity of HDACs which result in hyperacetylation of histones. DNA methyltransferase inhibitors (DNMTI) inhibit the methylation of DNA sequences by inhibiting the catalytic activity of DNMT. Increased acetylation of histones and reduction in DNA promoter site methylation allow for transcription of genes that result in a variety of different biological effects, including growth and cell cycle arrest, cellular differentiation, inhibition of angiogenesis, apoptosis, and immune surveillance.
Reproduced by kind permission of Springer Science+Business Media.34
Pracinostat is an orally bioavailable pan-HDACi that has 1,000-fold selectivity for class 1 and 2 HDACs.39,40 In a phase II study of 22 patients with intermediate- and high-risk MF, single-agent pracinostat resulted in reductions in splenomegaly in 27% of patients. The most common adverse event was fatigue, and grade 3–4 toxicities included neutropenia in 13% of patients and thrombocytopenia in 21% of patients; no grade 3–4 anemia was observed.41
Givinostat is an orally bioavailable inhibitor of class 1 and 2 HDACs.42 The safety and activity of single-agent givinostat were demonstrated in a phase II study in patients with JAK2V617F+ MPNs (MF, n=16; ET, n=1; PV, n=12).43 Overall, 38% of patients with MF and 75% of patients with ET/PV experienced a reduction in splenomegaly, prompting phase II evaluation in patients with ET/PV. In a separate study, combination givinostat/HU in patients with JAK2V617F+ PV who had suboptimal responses with single-agent HU resulted in a response rate of 45–50% and a notable reduction in pruritus in a majority of patients; no grade 3–4 toxicities were observed.44
Panobinostat is a pan-HDACi with superior nanomolar potency for inhibition of class 1, 2, and 4 HDACs compared to other HDAC inhibitors.45–47 A phase II study of single-agent panobinostat was conducted in 35 patients with intermediate/high risk MF.48 Molecular assays showed decreased JAK2 and PRV1 mRNA expression, increased histone acetylation, and decreased JAK2 and p-STAT3/5 protein levels in peripheral blood mononuclear cells from patients treated with panobinostat. While 69% of patients who received at least one dose of panobinostat experienced a 25% or more reduction in spleen size, the dose of 40 mg thrice weekly was found to be too high according to its toxicity profile (e.g. 53% grade 3–4 thrombocytopenia, 28% grade 3–4 anemia, and 22% grade 3–4 fatigue) that led to an early discontinuation rate. Two patients achieved a 2 g/dL increase in hemoglobin level (maintained less than 8 weeks) and a single patient achieved a clinical improvement (CI) in spleen reduction by international working group (IWG) criteria. A phase I dose-escalation study of single-agent panobinostat administered at lower doses was conducted in 18 patients with MF and proved to be clinically well tolerated (no non-hematologic grade 3–4 adverse events).49 Thrombocytopenia was determined to be the dose-limiting toxicity and 25 mg thrice weekly the recommended phase II dose. Prolonged administration of panobinostat in 5 patients resulted in elimination of leukoerythroblastic blood features, improvement in anemia, resolution of splenomegaly, reduction in MF symptoms, and in 2 cases improvement in bone marrow histopathological features and regression of marrow fibrosis. Thus far, low-dose, single agent, oral panobinostat appears to be both clinically and biologically active as well as well tolerated.
Several ongoing and planned studies have been designed to evaluate combination HDACi/JAK2 inhibitor therapy, including a phase Ib European study and a phase I/II US study (PRIME) that are investigating combination panobinostat/ruxolitinib therapy in patients with MF, as well as a pracinostat/pacritinib study. Pre-clinical rationale for the combination of these two agents is supported by compelling laboratory and murine models of MF.37,50 In addition, combination HDACi/DNMT inhibitor therapy trials are being planned.
Development of novel drug combinations for treatment of myelofibrosis
A key therapeutic challenge in MF in the post-JAK2 TKI era is to improve the efficacy against JAK2V617F, which can theoretically be accomplished by two strategies: reducing JAK2V617F allelic burden and inactivating downstream components of the JAK/STAT signal transduction pathway. Currently, four classes of investigational agents are being evaluated that target JAK2V617F or downstream signaling molecules, as shown in Figure 4: HDACis/Hsp90 inhibitors, PI3K/mTOR inhibitors, PIM kinase inhibitors, and MEK inhibitors.51
Figure 4.
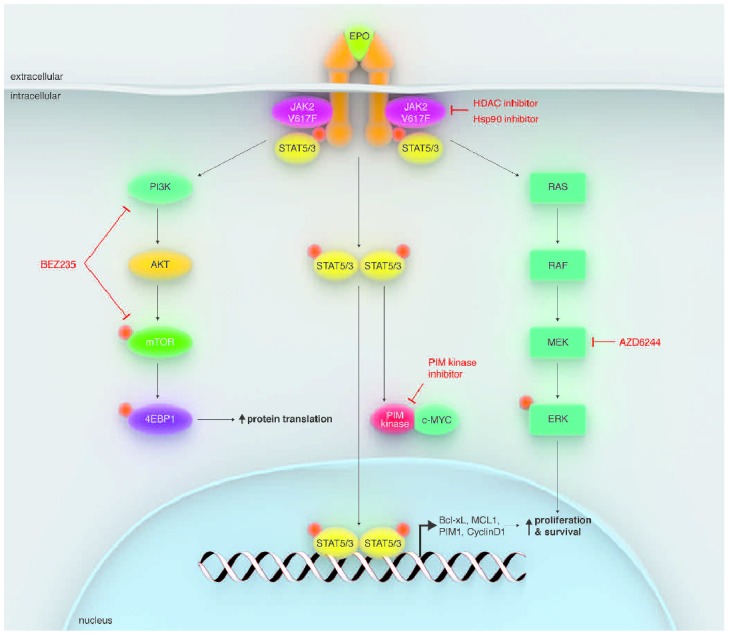
HDACis/Hsp90 inhibitors, PI3K/mTOR inhibitors, PIM kinase inhibitors, and MEK inhibitors target JAK2V617F or down-stream signaling molecules.
Reproduced by kind permission of Elsevier 2012.51
Heat-shock protein 90 (Hsp90) inhibitors
Hsp90 is an ATP-dependent, dimeric molecular chaperone that folds and stabilizes its client proteins, including JAK2 and STAT5, into their active conformations.52 Hsp90 inhibitors bind to the N-terminal ATP-binding domain of Hsp90 and inhibit its chaperone function, which has been shown to induce proteasome-mediated degradation of client proteins in MPN cells. Interestingly and importantly, pan-HDACis, such as vorinostat and panobinostat, induce Hsp90 acetylation, inhibit Hsp90 chaperone function, and promote proteasomal degradation of Hsp90 client proteins in MPN cells.
Hsp90 inhibitors have shown promise in both reducing JAK2V617F allelic burden and overcoming resistance to JAK2 inhibitor therapy. Panobinostat is an HDACi that also mediates acetylation of Hsp90. This agent has been shown to induce apoptosis in JAK2V617F+ MPN cells, likely via the inhibition of chaperone association between JAK2 and Hsp90, thereby resulting in proteasomal degradation of JAK2.53 Co-treatment of MPN stem/progenitor cells with panobinostat and the JAK2 inhibitor TG101209 results in increased cell death compared to treatment with either compound alone, providing the rationale for combination JAK2/Hsp90 inhibitor therapy as noted above.53
AUY922 is an Hsp90 inhibitor that has been shown to deplete JAK2 and to induce apoptosis in MPN cells. This compound is also known to disrupt chaperone association of JAK2 with Hsp90 and induces degradation of JAK2. Similar to the results observed with panobinostat described above, co-treatment of primary MPN cells with AUY922 and the JAK2 inhibitor TG101209 enhances apoptosis.54
Two lines of evidence suggest that Hsp90 inhibitors also appear to have a potential role in overcoming resistance to JAK2 inhibition. First, JAK2 TKI-resistant MPN cells have been shown to exhibit greater sensitivity to AUY922 than non-resistant cells.54 Second, the Hsp90 inhibitor PU-H71 was demonstrated to abrogate heterodimeric JAK/STAT activation in JAK2 inhibitor therapy-resistant cell lines.55
PI3K/mTOR inhibitors
The PI3K/mTOR signaling pathway plays an important role in cell growth and proliferation in many malignancies, including MF.56–58 BEZ235 is a dual PI3K/mTOR inhibitor that has been shown to induce apoptosis in MPN cells. Co-treatment of PMF cells with BEZ235 and the JAK2 inhibitor SAR302503 enhances JAK2 inhibitor-mediated loss of survival, and BEZ235 also induces apoptosis in JAK2-TKI–resistant MPN cells.59
Additional investigational agents
In addition to agents that abrogate Hsp90 activity, several other targeted therapies are currently being evaluated for their activity in MF. Agents that show activity alone or in combination with a JAK2 inhibitor include the MEK inhibitor AZD6244, the PIM kinase inhibitor SGI1776, the β-catenin antagonist BC2059, and DNMT1 inhibitors.51
Hematopoietic stem cell transplantation for myelofibrosis
Allogeneic HSCT remains the only potentially curative therapy for MF. HSCT is an established treatment capable of eradication of the disease process, normalization of bone marrow findings (including reversal of bone marrow reticulin and collagen fibrosis), and produces durable disease-free survival (DFS). However, this “high-reward” treatment is also associated with high risk, and represents a major commitment for the patient.
An important issue regarding the use of HSCT in MF patients is the optimal timing for a particular individual. As shown in Figure 5, patients with early stage disease, as defined by Dynamic International Prognostic Scoring System (DIPSS) risk category, have better survival after transplant; such patients also tend to have lower non-relapse mortality (NRM) rates.60 Unfortunately, the majority of patients with MF are beyond the 6th decade of life and are not ideal candidates for this definitive therapeutic approach.
Figure 5.
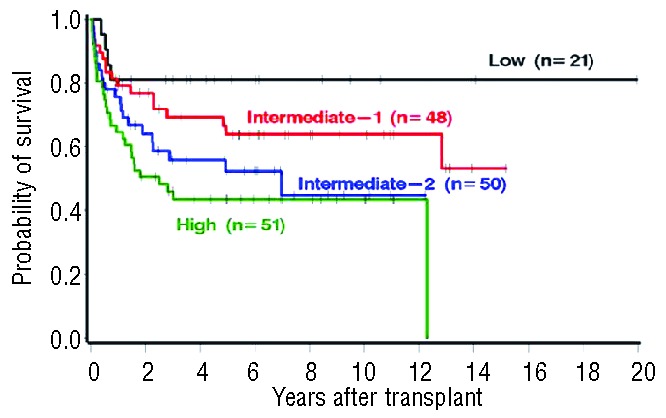
Patients with early stage disease, as defined by Dynamic International Prognostic Scoring System (DIPSS) risk category, have better survival after transplant.
Republished by kind permission of the American Society of Hematology, 2012.60Permission conveyed through Copyright Clearance Center, Inc.
In MF patients, HSCT has conventionally been performed with busulfan/cyclophosphamide-based myeloablative chemotherapy regimens. However, MF patients tend to be older and intolerant to such aggressive regimens. Recently developed reduced intensity conditioning (RIC) regimens have enabled treatment of older MF patients with less transplant-related morbidity and mortality. For example, busulfan/fludarabine (Bu-Flu) conditioning has demonstrated both tolerability and the potential to reverse clinical and pathological evidence of MF.61 Unpublished data from the MD Anderson Cancer Center indicate that pharmacokinetic-based dosing (by area under the curve, AUC) of busulfan rather than body surface area (BSA)-based dosing results in superior survival. HSCT with Bu-Flu conditioning results in a dramatic decrease in JAK2 activity and splenomegaly at one month after transplant, and bone marrow fibrosis decreases to 81%, 72%, 37%, and 19% at 1, 3, 6, and 12 months after transplant. (U Popat, personal communication, 2013). Such low-intensity regimens are increasingly being recognized as an appropriate option for MF patients, and the number of transplants performed in this patient population (including patients aged 60 years and over) has progressively increased in the past two decades.62
Case 1. ET and early phases of PMF: are they different and does it matter? (courtesy of A. Orazi)
A 43-year old man with a history of thrombocytosis of a few years duration was referred for evaluation of a possible MPN. His spleen was palpable 1 cm below the costal margin, and his November 2007 complete blood chemistry (CBC) results were: hemoglobin (Hb) 13.1 g/dL; hematocrit (Hct) 39.5%; mean corpuscular volume (MCV) 80.5 fL; WBC count 6.5×109/L; platelets 483×109/L. His peripheral blood smear showed mild anisopoikilocytosis and thrombocytosis, and the bone marrow aspirate was inadequate. The following test results were also obtained: lactate dehydrogenase (LDH) 248 U/L (normal range 96–200 U/L); JAK2V617 was positive by polymerase chain reaction (PCR); red cell mass (RCM) within normal range; normal erythropoietin; normal bone marrow flow cytometry; normal karyotype; BCR-ABL1− (by fluorescent in situ hybridization, FISH); and TCRγ and IgH chain gene negative (by PCR).
Bone marrow histopathology revealed hypercellularity, increased granulopoiesis and megakaryopoiesis, highly pleomorphic megakaryocytes displaying atypia, tight clusters of megakaryoctyes, grade 1 fibrosis by Bauermeister scale, and lymphoid aggregates most consistent with lymphoid follicle formation. A diagnosis of early stage PMF was made based on abnormal bone marrow histopathology and the presence of two minor criteria, splenomegaly and elevated LDH.
Four years later, repeat biopsy indicated that the patient had progressed to overt PMF based on marrow histopathology. He also had splenomegaly and radiographic (documented splenic vein thrombosis). Portal hypertension resulted in esophageal variceal bleeding which was managed with serial banding and intermittent beta-blockers. Pegylated interferon (IFN) therapy was initiated, but did not effectively reduce the splenomegaly. He is currently receiving low molecular weight heparin for secondary thromboprophylaxis.
Case 2. Symptomatic myelofibrosis (courtesy of C. Harrison)
A 65-year old female who had been previously diagnosed with PV in 1974 was asymptomatic at re-presentation, but was noted to have a ruddy complexion. Initial treatment consisted of phlebotomy and busulfan; however, she was switched to hydroxycarbamide (HC) in 1989 as the risks associated with busulfan became increasingly apparent. The patient developed severe ulcers in January 2009, which resolved four months after HC discontinuation. The ulcers recurred upon HC re-challenge, so she was switched to interferon (IFN). Although she found it difficult to tolerate the IFN-related side effects, she continued to receive this agent. From 2007 to 2008, the patient’s spleen progressively enlarged, she experienced night sweats and fatigue, and she lost a notable amount of weight (15 kg). Treatment with pegylated IFN was initiated in an attempt to reduce IFN-related side effects; however, this did not result in resolution of splenomegaly or symptoms.
In June 2009, the patient was diagnosed with post-PV MF that fulfilled the IPSS high-risk category. Symptoms and signs included massive splenomegaly (32 cm in length), leukocytosis (WBC 30×109/L), anemia, and hepatomegaly. Although HSCT, splenectomy, and low-dose splenic irradiation were also considered, the patient elected to enroll on a JAK2 inhibitor clinical trial.
Case 3. Myelofibrosis in blast phase (courtesy of J. O. Mascarenhas)
A 52-year old woman was referred to a hematologist for evaluation of isolated thrombocytosis, which was documented in November 2001 at 69 ×109/L. Her medical history included hypertension, obesity, osteoarthritis of the bilateral knees, and a Caesarean section 25 years prior. She was currently taking amlodipine and metoprolol. On physical examination she was obese and had no evidence of organomegaly. In addition to the elevated platelets, laboratory results included WBC 8.6×109/L, Hb 12.2 g/dL, and no evidence of leukoerythroblastosis on manual review of peripheral blood smear. Her erythrocyte sedimentation rate (ESR) was 5, her LDH was normal, and she had normal renal and hepatic function. Bone marrow evaluation revealed a hyperproliferative marrow with atypical megakaryocytes seen singly and in clusters with atypical nuclei in the background of dense hematopoietic elements. Molecular studies showed a JAK2V617F allele burden of 13% and BCR-ABL and MPL mutation were negative; her karyotype was normal. Following a diagnosis of ET, she was treated with aspirin 81 mg/die and metoprolol for better blood pressure control; she was also advised to lose weight.
Seven years later, in November 2008, she re-presented with mild night sweats, progressive fatigue, and global weakness, and complained of left upper quadrant abdominal pain after eating. She had lost 20 pounds intentionally with diet and exercise. Her spleen was now 6 cm below the left costal margin and tender. Her liver tip was barely palpable with deep inspiration. Hematologic values were: WBC 23.5×109/L; Hb 9.5 g/dL; and platelet count 245×109/L, and there was evidence of early myeloid forms on the peripheral blood smear. One percent blasts were noted by manual count and confirmed by flow cytometry, with occasional nucleated red blood cells and large platelet forms. Her LDH was elevated at 330 IU/L, ALT was 80 U/L, creatinine was normal, and she had hyperuricemia. Repeat bone marrow biopsy showed less cellularity with a cluster of atypical tightly clustered megakarytocytes, atypical nuclei, and distortion of the marrow architecture. She also had dense, coarse reticulin fibers and collagen fibrosis. Additionally, her JAK2V617F allele burden was increased to 55% and she had acquired a 20q abnormality as determined by FISH. Her hematologist started her on HU for the symptomatic spleen and she was given the diagnosis of post ET MF. Her DIPSS score was 4 points, placing her in the intermediate-2 risk category with an increased risk of leukemic transformation. Additional treatment choices at that time would be observation, immunomodulatory agents, ruxolitinib, HSCT, or enrollment on to a clinical trial.
The patient initially did well on HU, with improvement in her WBC count and her spleen. She was doing relatively well until November 2012 when she developed worsening night sweats and low-grade fevers, profound fatigue, global weakness, debilitating bone pains, abdominal discomfort, bloating, and diarrhea, and was requiring 2 units of packed red blood cells every 2–3 weeks. Her spleen tip was now 10 cm below the left costal margin, her liver was 3 cm, and she had a WBC count of 44×109/L, Hb 7.5 g/dL, and platelet count 76×109/L. On review of her peripheral blood, she had 45% blasts, which flow cytometry revealed to be myeloblasts. Repeat bone marrow biopsy showed distortion of the marrow architecture, and immunohistochemical staining confirmed the presence of myeloblasts. Thus, her disease has now effectively transformed into acute leukemia, the definition of which is at least 20% blasts either in the peripheral blood or the bone marrow, and the terminology is MF in blast phase. Treatment options include supportive care, induction chemotherapy, hypomethylating agents (azacitidine/decitabine), HSCT, and enrollment onto a clinical trial.
Conclusion
The MPNs are a collection of diseases that have overlapping clinico-pathological features and can present a challenge to the pathologist and hematologist in correctly distinguishing one from the other. This is particularly true in the case of ET and early phase/pre-fibrotic form of MF. MF is a challenging myeloid malignancy to treat effectively, limited by advanced age in the majority of patients, the presence of competing comorbid conditions, and the availability of tolerable disease-modifying agents. The development of oral JAK2 inhibitors and the approval of ruxolitinib for the treatment of MF patients have clearly impacted the treatment paradigm. Recent reports of survival advantage with ruxolitinib therapy are exciting, but are not explained by reversal of bone marrow pathological features or elimination of JAK2V617F in treated patients. Current MPN translational research focuses on developing therapeutic approaches based on pre-clinical rationale and attempts to combine agents that inhibit aberrant oncogenic pathways and target epigenetic alterations. HSCT remains the only treatment approach that offers the potential for cure at a risk of transplant morbidity and mortality that is not insignificant. As conditioning regimens are refined and intensity reduced, and innovative transplant approaches incorporate pre-conditioning JAK2 inhibition, more MF patients of advanced age will become eligible for this treatment modality. In the last six years, advances in the understanding of molecular mechanisms underlying pathological features of MF have resulted in improved therapeutic options and will allow for continued success in developing effective treatments for all MF patients.
Acknowledgments
The authors would like to thank Incyte Corporation for its unrestricted educational grant which allowed this manuscript to move forward, and Hemedicus Inc and Gail Flores, PhD, for their editorial assistance. Incyte did not review nor influence the content of this manuscript.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Thiele J, Kvasnicka HM, Tefferi A, et al. Primary myelofibrosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Lyon: World Health Organization, 2008:44–7 [Google Scholar]
- 2.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901 [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al. Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol. 2012;30(24):2981–7 [DOI] [PubMed] [Google Scholar]
- 4.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWGMRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8 [DOI] [PubMed] [Google Scholar]
- 5.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7(9):673–83 [DOI] [PubMed] [Google Scholar]
- 7.Zhan H, Spivak JL. The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era. Clin Adv Hematol Oncol. 2009;7(5):334–42 [PubMed] [Google Scholar]
- 8.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005; 106(6):2162–8 [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22 [DOI] [PubMed] [Google Scholar]
- 10.Barbui T, Thiele J, Vannucchi AM, Tefferi A. Problems and pitfalls regarding WHO-defined diagnosis of early/prefibrotic primary myelofibrosis versus essential thrombocythemia. Leukemia. 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Thiele J, Kvasnicka HM, Mullauer L, Buxhofer-Ausch V, Gisslinger B, Gisslinger H. Essential thrombocythemia versus early primary myelofibrosis: a multicenter study to validate the WHO classification. Blood. 2011;117(21):5710–8 [DOI] [PubMed] [Google Scholar]
- 12.Barbui T, Thiele J, Carobbio A, Passamonti F, Rumi E, Randi ML, et al. Disease characteristics and clinical outcome in young adults with essential thrombocythemia versus early/prefibrotic primary myelofibrosis. Blood. 2012;120(3):569–71 [DOI] [PubMed] [Google Scholar]
- 13.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29(23):3179–84 [DOI] [PubMed] [Google Scholar]
- 14.Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia. 2012;26 (4):716–9 [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110(4):1092–7 [DOI] [PubMed] [Google Scholar]
- 16.Incyte Corporation Jakafi (R) (ruxolitinib) Full prescribing information. Available from: http://www.incyte.com/products/uspi_jakafi.pdf Accessed 20 February 2013
- 17.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12): 1117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98 [DOI] [PubMed] [Google Scholar]
- 20.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Long-term outcome of ruxolitinib treatment in patients with myelofibrosis: Durable reductions in spleen volume, improvements in quality of life, and overall survival advantage in COMFORT-I. Blood. 2012;120(21):Abstract 800. [Google Scholar]
- 21.Cervantes F, Kiladjian JJ, Niederwieser D, Sirulnik A, Stalbovskaya V, McQuity M, et al. Long-term safety, efficacy, and survival findings from Comfort-II, a phase 3 study comparing ruxolitinib with best available therapy (BAT) for the treatment of myelofibrosis (MF). Blood. 2012;120:(Abstract 801). [Google Scholar]
- 22.Harrison CN, Kiladjian JJ, Gisslinger H, Niederwieser D, Passamonti F, Waltzman RJ, et al. Ruxolitinib provides reductions in splenomegaly across subgroups; An analysis of spleen response in the COMFORT-II study. Blood. 2011;118: Abstract 279. [Google Scholar]
- 23.Talpaz M, Hamburg SI, Jamieson K, Terebelo HR, Afrin L, Winton EF, et al. Preliminary safety and eficiacy from a phase II study of ruxolitinib in patients with primary and secondary myelofibrosis with platelets counts of 50–100 × 109/L. Haematologica. 2012;97:244 Abstract 597 [Google Scholar]
- 24.Harrison CN, Gisslinger H, Miller CB, Kiladjian JJ, Atienza E, Stalbovskaya V, et al. Expand: a phase 1b, open-label, dose-finding study of ruxolitinib in patients with myelofibrosis and baseline platelet counts between 5×109/L and 99×109/L. Blood. 2012;120 (21):Abstract 177. [Google Scholar]
- 25.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011;365(15):1455–7 [DOI] [PubMed] [Google Scholar]
- 26.Pardanani A, Gotlib J, Jamieson C, Cortes JE, Talpaz M, Stone R, et al. SAR302503: Interim safety, efficacy and long-term impact on JAK2 V617F allele burden in a phase I/II study in patients with myelofibrosis. Blood. 2011;118(21):Abstract 3838. [Google Scholar]
- 27.Talpaz M, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, et al. A phase II randomized dose-ranging study of the JAK2-selective inhibitor SAR302503 in patients with intermediate-2 or high-risk primary myelofibrosis (MF), or post-essential thrombocythemia (ET) MF. Blood. 2012;120(21):Abstract 2837. [Google Scholar]
- 28.Komrokji RS, Wadleigh M, Seymour JF, Roberts AW, To LB, Zhu HJ, et al. Results of a phase 2 study of pacritinib (SB1518), a novel oral JAK2 inhibitor, in patients with primary, post-polycythemia vera, and post-essential thrombocythemia myelofibrosis. Blood. 2011;118(21):Abstract 282. [Google Scholar]
- 29.Pardanani A, Gotlib J, Gupta V, Roberts AW, Wadleigh M, Sirhan S, et al. Phase I/II study of CYT387, a JAK1/JAK2 inhibitor for the treatment of myelofibrosis. Blood. 2012;120(21):Abstract 178. [Google Scholar]
- 30.Wang JC, Chen W, Nallusamy S, Chen C, Novetsky AD. Hypermethylation of the P15INK4b and P16INK4a in agnogenic myeloid metaplasia (AMM) and AMM in leukaemic transformation. Br J Haematol. 2002;116(3):582–6 [DOI] [PubMed] [Google Scholar]
- 31.Jones LC, Tefferi A, Idos GE, Kumagai T, Hofmann WK, Koeffler HP. RARbeta2 is a candidate tumor suppressor gene in myelofibrosis with myeloid metaplasia. Oncogene. 2004;23(47):7846–53 [DOI] [PubMed] [Google Scholar]
- 32.Bogani C, Ponziani V, Guglielmelli P, Desterke C, Rosti V, Bosi A, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells. 2008;26(8):1920–30 [DOI] [PubMed] [Google Scholar]
- 33.Teofili L, Martini M, Cenci T, Guidi F, Torti L, Giona F, et al. Epigenetic alteration of SOCS family members is a possible pathogenetic mechanism in JAK2 wild type myeloproliferative diseases. Int J Cancer. 2008;123(7):1586–92 [DOI] [PubMed] [Google Scholar]
- 34.Mascarenhas J, Roper N, Chaurasia P, Hoffman R. Epigenetic abnormalities in myeloproliferative neoplasms: a target for novel therapeutic strategies. Clin Epigenetics. 2011;2(2):197–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JC, Chen C, Dumlao T, Naik S, Chang T, Xiao YY, et al. Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk Lymphoma. 2008;49(12):2321–7 [DOI] [PubMed] [Google Scholar]
- 36.Shi J, Zhao Y, Ishii T, Hu W, Sozer S, Zhang W, et al. Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res. 2007;67(13):6417–24 [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Zhang W, Ishii T, Sozer S, Wang J, Xu M, et al. Correction of the abnormal trafficking of primary myelofibrosis CD34+ cells by treatment with chromatin-modifying agents. Cancer Res. 2009;69(19):7612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerini V, Barbui V, Spinelli O, Salvi A, Dellacasa C, Carobbio A, et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F). Leukemia. 2008;22(4):740–7 [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Manero G, Chuah C, Wilding G, Chang J, Verstovsek S, Faderl S, et al. Phase I study of the oral histone deacetylase inhibitor SB939 in patients with advanced hematologic malignancies. Blood. 2010;116: Abstract 3292. [Google Scholar]
- 40.Wang H, Yu N, Chen D, Lee KC, Lye PL, Chang JW, et al. Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H-benzim-idazol-5-yl}-N-hydroxyacrylami de (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. J Med Chem. 2011;54(13):4694–720 [DOI] [PubMed] [Google Scholar]
- 41.Quintas-Cardama A, Kantarjian H, Estrov Z, Borthakur G, Cortes J, Verstovsek S. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk Res. 2012;36(9):1124–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golay J, Cuppini L, Leoni F, Mico C, Barbui V, Domenghini M, et al. The histone deacetylase inhibitor ITF2357 has anti-leukemic activity in vitro and in vivo and inhibits IL-6 and VEGF production by stromal cells. Leukemia. 2007;21(9):1892–900 [DOI] [PubMed] [Google Scholar]
- 43.Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150(4): 446–55 [DOI] [PubMed] [Google Scholar]
- 44.Rambaldi A, Finazzi G, Vannucchi AM, Martinelli V, Rodeghiero F, Nobile F, et al. A phase II study of the HDAC inhibitor givinostat in combination with hydroxyurea in patients with polycythemia vera resistant to hydroxyurea monotherapy. Blood. 2011; 118:Abstract 1748. [Google Scholar]
- 45.Prince HM, George D, Patnaik A, Mita M, Dugan M, Butterfoss D, et al. Phase I study of oral LBH589, a novel deacetylase (DAC) inhibitor in advanced solid tumors and nonhodgkin’s lymphoma. J Clin Oncol. 2007; 25(18S):Abstract 3500. [Google Scholar]
- 46.Spencer A, Prince M, De Angelo DJ, Fischer T, Bhalla KN, Giles FJ, et al. Phase IA/II study of oral LBH589, a novel deacetylase inhibitor (DACi), administered on 2 schedules, in patients with advanced hematologic malignancies. Blood. 2007;110: Abstract 907. [Google Scholar]
- 47.Sharma S, Vogelzang NJ, Beck J, Patnaik A, Mita M, Dugan M, et al. Phase I pharmacokinetic and pharmacodynamic study of once-weekly IV LBH589. Eur J Cancer. 2007;5(4):107 : Abstract 702. [Google Scholar]
- 48.DeAngelo DJ, Tefferi A, Fiskus W, Mesa RA, Paley CS, Wadleigh M, et al. A phase II trial of panobinostat, an orally available deacetylase inhibitor (DACi), in patients with primary myelofibrosis (PMF), post essential thrombocythemia (ET), and post polycythemia vera (PV) myelofibrosis. Blood. 2010;116: Abstract 630. [Google Scholar]
- 49.Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161(1):68–75 [DOI] [PubMed] [Google Scholar]
- 50.Baffert F, Evrot E, Ebel N, Roelli C, Andraos R, Qian Z, et al. Improved efficacy upon combined JAK1/2 and pan-deacetylase inhibition using ruxolitinib (INC424) and panobinostat (LBH589) in preclinical mouse models of JAK2V617-F-driven disease. Blood. 2011;118: Abstract 798. [Google Scholar]
- 51.Fiskus W, Ganguly S, Kambhampati S, Bhalla KN. Role of additional novel therapies in myeloproliferative neoplasms. Hematol Oncol Clin North Am. 2012;26(5):959–80 [DOI] [PubMed] [Google Scholar]
- 52.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–72 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114(24): 5024–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiskus W, Verstovsek S, Manshouri T, Rao R, Balusu R, Venkannagari S, et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin Cancer Res. 2011;17(23):7347–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–48 [DOI] [PubMed] [Google Scholar]
- 57.Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Tozzi L, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118(8):2069–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogani C, Bartalucci N, Martinelli S, Tozzi L, Guglielmelli P, Bosi A, et al. mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms. PLoS One. 2013;8(1):e54826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S, et al. Mol Cancer Ther. 2013;12(5):577–88 [DOI] [PubMed] [Google Scholar]
- 60.Scott BL, Gooley TA, Sorror ML, Rezvani AR, Linenberger ML, Grim J, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119(11):2657–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114 (26):5264–70 [DOI] [PubMed] [Google Scholar]
- 62.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120(7):1367–79 [DOI] [PMC free article] [PubMed] [Google Scholar]


