Abstract
Anaplastic lymphoma kinase-positive anaplastic large T-cell lymphoma is characterized by morphological variability. Morphological variants (non-common subtype) are associated with a poor outcome. They display abundant reactive bystander cells admixed with the lymphoma cells. So far, the difficulty in distinguishing lymphoma cells from bystander cells by visual inspection has prevented detailed and reliable immunophenotypic analysis using conventional immunohistochemistry. To overcome these limitations, we analyzed 124 cases of pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma treated within clinical trials using immunofluorescence multi-staining and digital image analysis combining antibodies against anaplastic lymphoma kinase to specifically identify lymphoma cells with antibodies against CD30, CD3, CD5, CD8, Ki67 and phosphorylated STAT3. Non-common type anaplastic lymphoma kinase-positive anaplastic large cell lymphomas express CD8 more frequently than common type anaplastic lymphoma kinase-positive anaplastic large cell lymphomas (35.4% and 5.6%, respectively; P=0.0002). CD8 expression was associated with a poorer outcome. Importantly, in a multivariate analysis including clinical risk factors, histological subtype and CD8 expression, CD8-positivity proved to be an independent prognostic predictor of worse outcome (hazard ratio for survival 3.38, P=0.042).
Introduction
Anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) is a CD30-positive T-cell lymphoma predominantly diagnosed in children and young adults.1 The genetic hallmark of ALK-positive ALCL is chromosomal translocations involving the anaplastic lymphoma kinase gene (ALK) at chromosome 2p23. These translocations lead to aberrant expression of fusion proteins between ALK and its translocation partners in lymphoma cells, which can be detected by immunohistochemistry using anti-ALK antibodies.2,3 Post-partum, ALK is expressed only in scattered neurons of the central nervous system. Immunohistochemical detection of ALK expression in lymphatic cells, therefore, serves as a specific tumor cell marker.2 In addition to ALK, the lymphoma cells display strong expression of CD30 and down-regulation of pan-T-cell markers such as CD3 and CD5 along with the presence of cytotoxic proteins, such as granzyme B, perforin and TIA, in the cytoplasm.1
The relapse rate of ALCL in children and adolescents is 25% to 35% following a first-line treatment strategy based on short-pulse chemotherapy over a period of 3 to 6 months.4,5 According to retrospective analyses, patients with ALCL relapse have a 30% to 60% chance of reaching a second continuous remission with relapse therapies as variable as maintenance treatment with vinblastine, autologous or allogeneic hematopoietic stem cell transplantation.6,7
Clinical risk factors have been defined and used for therapy stratification.8,9 Minimal disseminated disease, as detected by polymerase chain reaction analysis for NPM-ALK in blood or bone marrow, serum antibody titers against ALK and the morphological subtype are strong predictors of clinical outcome.10–14 The main morphological subtypes are: (i) the common type, which is composed of large tumor cells with pleomorphic nuclei; (ii) the small cell variant with ALK protein-expressing tumor cells that do not differ in size from reactive T-cells; (iii) the lymphohistiocytic variant with abundant histiocytes admixed with the lymphoma cells which are as small as in the small cell variant; and (iv) other rare subtypes. Especially in the non-common type of ALCL (small cell and lymphohistiocytic variant) the tumor cells are admixed with a large number of reactive, non-neoplastic bystander cells, predominantly T cells, which can account for more than 90% of the neoplastic infiltrate. This histological feature complicates the assessment of the immunophenotype of the lymphoma cells almost prohibiting a detailed determination of the immunophenotype of non-common type ALCL by conventional immunohistochemistry. The difficulties in assessing the immunophenotype might be one important reason why immunophenotypic markers predicting the outcome of ALCL could not be detected in large series.11,15–18 Flow-cytometric immunophenotyping has only been performed on a very limited number of samples not taking the morphological subtype into account.19,20 To overcome these limitations we performed immunofluorescence multi-staining combining antibodies for ALK to specifically identify lymphoma cells with antibodies directed against CD30, CD3, CD5, CD8, Ki67, CD56 and phosphorylated STAT3 (pSTAT3). Using digital image analysis we were able to determine the exact lymphoma phenotype of a large number of pediatric ALK-positive ALCL cases treated in consecutive prospective trials and to correlate our findings with the morphological subtype and patients’ outcome.
Methods
A detailed description of the methods is given in the Online Supplementary Material.
Patients’ specimens
Formalin-fixed, paraffin-embedded specimens from 128 patients younger than 18 years of age were analyzed. The cases were selected from the archives of the Department of Pathology, Hematopathology Section and Lymph Node Registry, University of Kiel. The final cohort was composed of 119 patients, all treated in the successive NHL-BFM 90, NHL-BFM V95, NHL-BFM 95 and ALCL99 trials. These trials had a comparable BFM-type chemotherapeutic backbone. Data from a subset of cases (n=36) were included in a previous publication.21 The cohort was composed of 85 (71%) males and 34 (29%) females. All clinical data of the cohort are shown in Table 1. ALK-antibody titer and minimal disseminated disease were measured as described previously.10,13
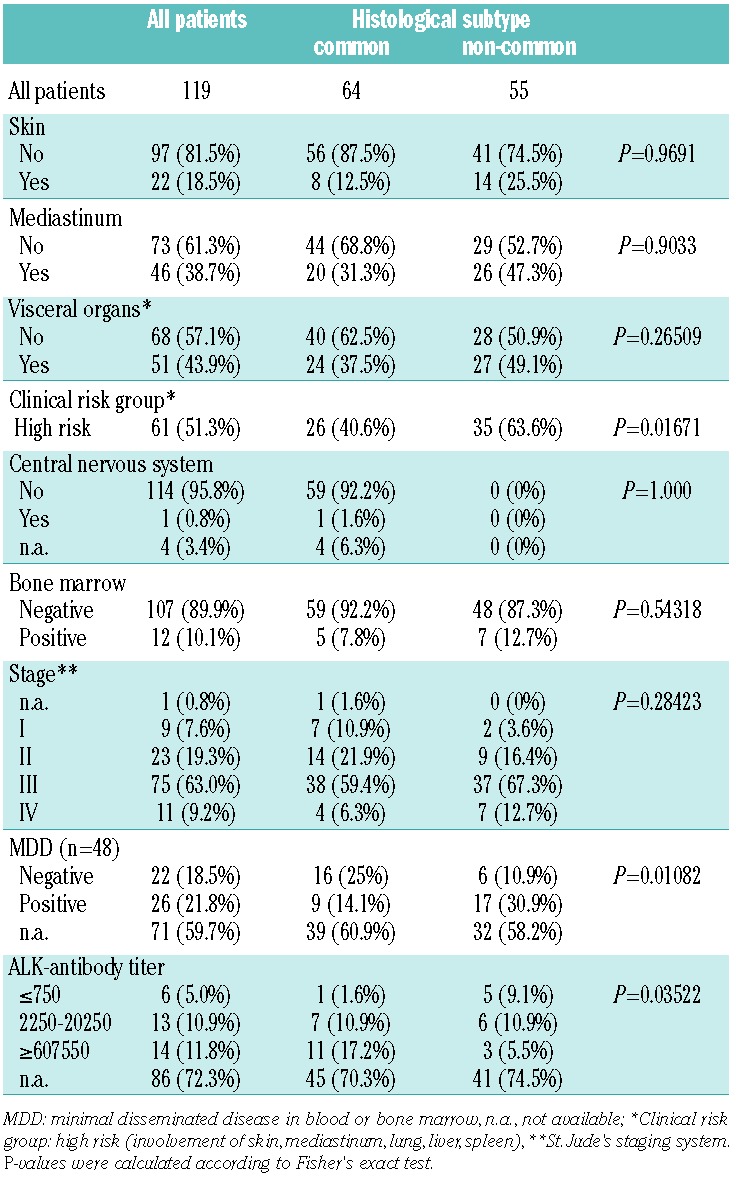
Table 1.
Association of histological subtype (common versus not common) with patients’ characteristics, clinical risk factors and biological risk factors of ALK-positive ALCL.
Histology and tissue microarrays
The tissue microarray was constructed using two cores, 1 mm in diameter, from each case containing tumor-rich areas, which were selected using light microscopy on whole hematoxylin and eosin-stained slides. One-hundred three cases (87%) showed nuclear and cytoplasmic ALK staining suggestive of an underlying NPM-ALK fusion and 16 cases (13%) showed staining restricted to the cytoplasm.3 The histological subtype was assessed according to previous publications including the guidelines of the World Health Organization (WHO) classification and the European Pathology Panel for Pediatric Lymphomas.11 We distinguished the pure common type of ALCL (n=64, 54%) from all other non-common morphological variants: lymphohistiocytic, small cell variants, and mixed variants (n=55, 46%).
Informed consent was obtained in all cases from the patients and/or parents at registration in the respective study of BFM study group. In addition the study was conducted in concordance with the recommendations of the local ethics committee.
Fluorescence double staining and immunohistochemistry
Tumor cells were detected using an ALK1 mouse monoclonal antibody (Dako, 1:10 dilution) or ALK/p80 rabbit monoclonal antibody (Epitomics, 1:50 dilution). To determine the tumor cell immunophenotype, the ALK1 stains were combined with a second marker using the following antibodies: CD30 mouse mono-clonal antibody (self-produced clone BerH2,1:5 dilution), CD3 mouse monoclonal antibody (1:100 dilution), CD5 mouse mono-clonal antibody (1:25, Novocastra, Newcastle, United Kingdom), CD8 rabbit monoclonal antibody (1:25, NeoMarkers, Freemont, CA, USA), anti-pSTAT3 rabbit mAb (1:25, Epitomics, 1:100, Burlingame, CA, USA) and Ki67 mouse monoclonal antibody (self-produced clone Ki-S5 22). CD56 was stained with a mouse monoclonal antibody (1:20 dilution, Novocastra, Newcastle, United Kingdom) using an automated staining system (Bond, Leica, Germany).
Digital image analysis of tumor cell immunophenotypes
Individual photographs from a representative area were obtained for ALK to identify tumor cells, DAPI to identify cell nuclei and the second immuno-marker, respectively (see above). The photographs were subsequently transformed into black and white images and further analyzed with TissueQuest 2.2 software (TissueGnostics, Vienna, Austria). The conventional staining for CD56 was scored as positive and negative by one observer (DA) using a light microscope.
Results
Correlation of the histological subtype with clinical and biological characteristics
All clinical data regarding the patients with common and non-common type ALCL are summarized in Table 1. Patients with common type ALCL less frequently belonged to the clinical high-risk group than did patients with non-common type ALCL (26/64 and 35/55, respectively; P=0.01671). Furthermore, cases of common type ALCL less frequently presented with minimal disseminated disease (9/25 and 17/23, respectively; P=0.01082) and showed higher anti-ALK antibody titers than did non-common type ALCL (P=0.03522, Table 1). The non-common type morphology was associated with poorer event-free and overall survival, confirming our previous results10,23 (Online Supplementary Figure S1).
Immunophenotype of common and non-common type anaplastic large cell lymphomas
Using multiple immunofluorescence stains and digital image analysis we determined the exact immunophenotype of ALCL, without bias from the reactive bystander cells, by assessing the phenotype quantitatively only in ALK-positive lymphoma cells. Figure 1 shows a representative example of double staining of ALK and CD8.
Figure 1.
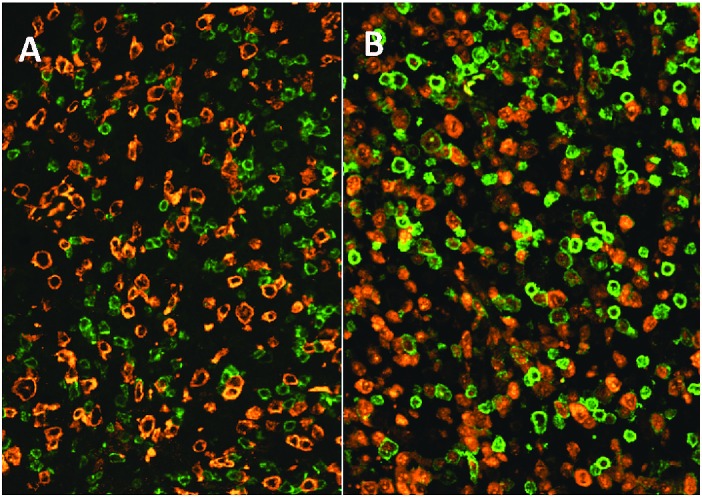
Representative examples of a (A) CD8-negative and a (B) CD8-positive ALCL. Green = CD8, red = ALK. Original magnification ×400.
Marker expression of common and non-common type ALCL was compared using the percentage of positive cells as a continuous parameter (Figure 2). Common type ALCL more frequently displayed >80% CD30 positive tumor cells, and expression of CD5 compared to the non-common type (P=0.0104, P=0.0003, respectively; Figure 2) whereas common type ALCL cells were less frequently CD8 positive (P=0.0019; Figure 2). There were no statistically significant differences between common and non-common type ALCL with regards to CD56 expression (4 of 63 positive in common type and 4 of 51 positive lymphomas in non-common type ALCL; Online Supplementary Table S1) and CD3 expression (P=0.2240; Figure 2). As shown in Figure 2, the proliferation rate, as assessed by the percentage of Ki67-positive cells, was higher in common type ALCL (P=0.0005). Expression of pSTAT3 was not statistically different between common and non-common type ALCL (P=0.7303; Figure 2).
Figure 2.
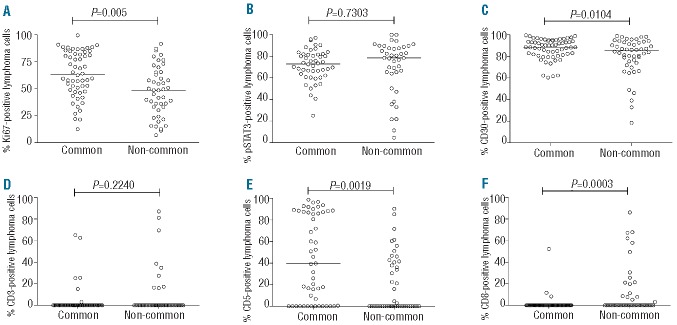
Percentage of (A) Ki67, (B) pSTAT3, (C) CD30, (D) CD3, (E) CD5 and (F) CD8 positive lymphoma cells. The median is indicated by a horizonal bar. The P values were generated by a t-test.
Correlation of immunophenotypic features with outcome
To analyze whether immunophenotypic features of the lymphoma cells correlate with event-free survival and overall survival, independently of histological subtype, lymphoma cell expression of CD30, CD3, CD5, CD8 and CD56 were correlated with clinical outcome in univariate analyses. All cases with any level of expression were counted as positive and compared to those without expression (Online Supplementary Table S1). CD56 expression was observed only rarely in ALCL (altogether 8 of 114 cases, 7%) and was associated with a poorer event-free survival but not overall survival (P=0.03 and P=0.56, respectively; data not shown). No significant correlation between expression of CD30, CD3 or CD5 with event-free survival or overall survival was detected (data not shown). Analyzing Ki67 and pSTAT3 expression, divided into quartiles, we did not observe statistically significant associations with event-free survival (data not shown). However, expression of CD8 was significantly associated with lower event-free and overall survival rates (CD8-negative versus CD8-positive: 5-year event-free survival 68 ± 5% versus 25 ± 10%; P<0.001, 5-year overall survival 84%, SE 4% versus 55%, SE 11%; P=0.003; Figure 3). Patients with lymphomas expressing CD8 were more frequently part of the clinical high-risk group than were patients with CD8-negative lymphomas (18/20 and 36/82, respectively; P<0.001). The percentage of CD8-positive lymphoma cells varied between 2% and 86% (median of all CD8-positive lymphomas 21%; Figure 2). CD8-positive ALCL differed from CD8-negative ALCL by showing less CD30 expression (>80% CD30-positive lymphoma cells in 9/19 and 63/77 cases, respectively; P=0.007) and more CD3-positivity (7/17 and 7/71, respectively; P=0.004). No statistically significant differences between CD8-positive and CD8-positive ALCL were detected for expression of CD5, CD56, pSTAT3 and Ki67 (data not shown). The cytotoxic proteins perforin and granzyme B were expressed in 17/17 and 13/14 of CD8-positive lymphomas, respectively. All morphological and immunophenotypic details of the CD8-positive lymphomas in this cohort are shown in Online Supplementary Table S3.
Figure 3.
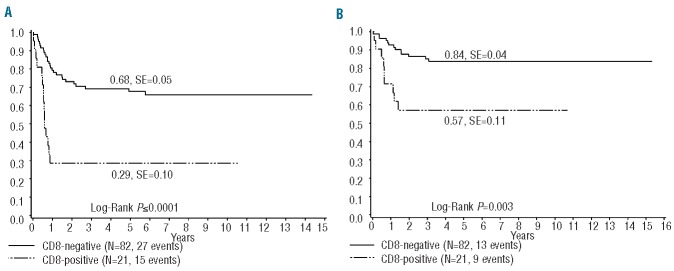
(A) Event free survival and (B) overall survival according to CD8 expression in ALCL.
Using a cut-off of 10% positivity for CD8 indicates that high expression of CD8 was associated with a poor outcome (Online Supplementary Figure S2). Similar data were obtained for a cut-off of 15% (data not shown). Excluding the CD8-positive cases from the analysis, the morphological subtype retained its prognostic significance for event-free survival but not for overall survival (Online Supplementary Figure S3).
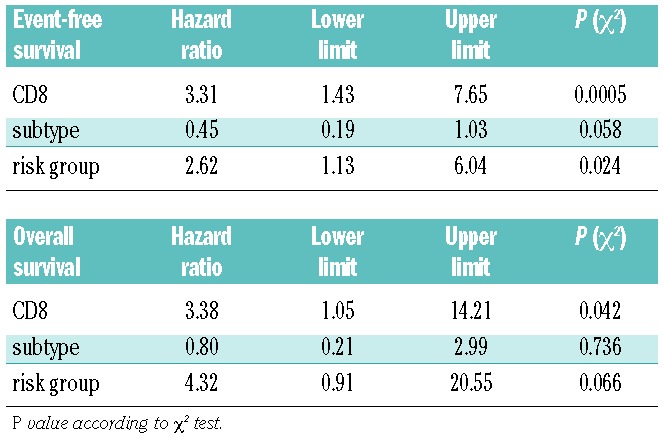
A Cox regression analysis of all prognostic histological factors (any CD8 expression versus no expression, common versus non-common histological subtype) and clinical risk group revealed that clinical risk group and CD8 expression were independent risk factors for event-free survival (hazard ratio 2.61, 3.31 and 2.39, respectively; Table 2). For overall survival only CD8 expression proved to be an independent risk factor (Table 2).
Table 2.
Multivariate Cox analysis of prognostic histological features and clinical risk groups.
Discussion
Several histological features hamper the assessment of the immunophenotype of ALCL by conventional immunohistochemistry: (i) the disease frequently presents as a paucicellular lymphoma with abundant reactive non-malignant bystander cells; (ii) the lymphoma cells in the non-common type ALCL do not differ in size from the bystander cells; (iii) the reactive bystander cells are predominantly T cells and thus share immunophenotypic features with the lymphoma cells; and (iv) the lymphoma cells in ALCL express low levels of surface T-cell markers such as CD3.1,24–26 All these features prevent a reliable assessment of the immunophenotype by conventional immunohistochemistry and ‘eyeballing’. Since ALCL predominantly presents as a tumor, flow cytometry can only rarely be used for immunophenotyping. Given these difficulties, it is not surprising that immunohistochemical markers that were established as prognostic in one cohort18 were not reproducible in other cohorts.17 In our study we used multiple fluorescence staining and digital image analysis to obtain a quantitative immunophenotype of the lymphoma cells. This technique obviously allows assignment of the expression of a T-cell marker to the tumor cell population more precisely than does conventional staining, especially in non-common type ALCL, in which the tumor cells do not differ in size from reactive bystander cells. Comparing the data in our study with those from the few published studies using flow cytometry, we detected expression of CD3 less frequently and CD5 expression more frequently19,20 (Online Supplementary Table S2). However, these studies did not correlate the findings with morphological subtype. A recently published large study that used conventional immunohistochemistry found that more cases expressed CD3 although, as in our study, this marker tended to be more often in non-common type ALCL11 (Online Supplementary Table S2). The levels of expression of CD8 and CD5 is our study were in the ranges of those reported for both markers analyzed by conventional immunohistochemistry (Online Supplementary Table S2). However, the possibility of quantifying expression more exactly in non-common type ALCL by using fluorescence multi-staining might explain the differences regarding the prognostic impact between our study and a previously published study.11
We analyzed a large and clinically very homogeneous group of cases of ALCL, focusing on ALK-positive lymphomas in children treated in prospective trials, allowing a very reliable clinical correlation. Using the technique of fluorescence multi-staining we identified CD8 expression as a powerful prognostic bio-marker in ALCL, being associated with a poorer outcome independently of clinical risk factors. According to our data a cut-off of 10% positive cells might be able to delineate a poor prognostic group. In addition to clinical factors and morphology, minimal disseminated disease with involvement of blood and bone marrow and the antibody response against ALK have been shown to be associated with survival in pediatric ALCL.10,13 Unfortunately, there were too few cases in our cohort with available data on minimal disseminated disease and anti-ALK titer for correlation with the immunophenotypic data.
CD8 expression was a stronger predictor of outcome than histological subtype. Our study was based on fluorescence double staining and was designed for optimal use of our digital image analysis software. We did not compare this method to alternative methods, such as conventional double staining which might be easier to perform in a diagnostic setting. It will be a challenge to introduce these techniques into routine diagnosis. Nevertheless, independently of their potential diagnostic use, our findings provide important insights into ALCL biology. Previous studies have shown that ALK activates STAT3, a process that can subsequently alter the immunophenotype of the lymphoma cells.27 Gene expression studies have demonstrated that the majority of genes regulated by ALK in ALCL are dependent on STAT3.28 Thus phosphorylated STAT3, analyzed in our cohort, might be a good biomarker for ALK activity within tumor cells. Interestingly, we did not detect a difference in pSTAT3 expression between lymphomas with cytoplasmic or nuclear and cytoplasmic ALK expression (data not shown), although it has recently been suggested that nuclear ALK is inactive.29 Future analysis will need to correlate additional markers for ALK activity within lymphoma cells in vivo using the methodology described here. ALCL is a T-cell lymphoma that shows little or no expression of the T-cell receptor and its associated molecules, such as CD3.26,30 Although the expression of membranous T-cell markers in ALCL is low or undetectable, expression of CD3, CD5 and CD8 was detectable in a subset of ALCL.
Interestingly, CD8, which as a single marker was strongly associated with poor outcome, is more frequently expressed in non-common type ALCL, linking the morphology and the immunophenotype of this apparently more aggressive subgroup of ALCL. Given the fact that the majority of ALCL have been reported to express CD4,1,31 one might speculate that the CD8-positive subgroup of ALCL does not only differ in clinical course and morphology but also in its cell of origin or at least T-cell lineage differentiation from common type ALCL. Interestingly, the morphology, when reported, of the few published cases of CD8-positive ALK-positive ALCL seems to be predominantly of the non-common type.32–34 In the current study, we did not assess CD4 expression because it is unlikely to delineate a poor prognostic group due to its widespread expression. Furthermore, assessment of CD4 expression is difficult by immunohistochemistry on tumor cells because many macrophages are also positive. In our experience, the distinction of CD4 on macrophages and lymphoma cells is difficult even using image analysis. To the best of our knowledge, expression of both CD8 and CD4 in the same ALCL does not occur and has never been reported in the literature. However, this issue might be addressed in future studies using full slides and high-resolution techniques such as confocal microscopy. Although gene expression has revealed several genes differentially expressed between common and non-common type ALCL, indications of a different lineage differentiation have not been reported.15 Given the low level of expression and the abundance of reactive bystander cells in the tumor, fluorescence multi-staining might be a much more promising approach than gene expression analysis to define expression in non-common type ALCL.
The composition of reactive bystander cells differs between non-common and common types of ALCL; for example, the lymphohistiocytic variant of ALCL shows abundant histiocytes admixed with the lymphoma cells.24 Furthermore, an antibody response against the ALK protein can be detected in ALCL patients and predicts clinical outcome.13 Both observations suggest that the interaction between the lymphoma and immune system plays a pivotal role in control of the lymphoma and clinical outcome after chemotherapy. CD8 on T lymphocytes binds with major histocompatibility complex molecules and mediates efficient cell-cell interactions within the immune system. The function of CD8 has been linked to the activity and binding of the T-cell receptor.35 It is worth noting that the group of CD8-positive ALCL was significantly more frequently positive for CD3, which is a part of the T-cell receptor complex, although the T-cell receptor has been shown to be absent in the majority of ALCL.26 Characterizing the function of CD8 on ALCL cells might provide valuable insights into the interaction between the lymphoma and the immune system as a prerequisite to developing new therapeutic strategies for the CD8-positive subsets of ALCL.
Acknowledgments
This work was supported by the José-Carreras Foundation (DJCLS R08/09) and the Kinderkrebsinitiative Buchholz, Holm-Seppensen. We thank K. Dege for editing the manuscript and Olivera Batic and Charlotte Botz von Drathen for excellent technical support.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Reference
- 1.Swerdlow SH, Campo E, Harris N, Jaffe E, Pileri S, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumors of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008 [Google Scholar]
- 2.Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997;89(4):1394–404 [PubMed] [Google Scholar]
- 3.Damm-Welk C, Klapper W, Oschlies I, Gesk S, Rottgers S, Bradtke J, et al. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: a molecular-histological correlation. Br J Haematol. 2009;146 (3):306–9 [DOI] [PubMed] [Google Scholar]
- 4.Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699–706 [DOI] [PubMed] [Google Scholar]
- 5.Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group J Clin Oncol. 2009;27(6):897–903 [DOI] [PubMed] [Google Scholar]
- 6.Brugieres L, Pacquement H, Le Deley MC, Leverger G, Lutz P, Paillard C, et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27(30):5056–61 [DOI] [PubMed] [Google Scholar]
- 7.Woessmann W, Zimmermann M, Lenhard M, Burkhardt B, Rossig C, Kremens B, et al. Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after Berlin-Frankfurt-Muenster (BFM)-type first-line therapy: a BFM-group study. J Clin Oncol. 2011; 29(22):3065–71 [DOI] [PubMed] [Google Scholar]
- 8.Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008; 111(3):1560–6 [DOI] [PubMed] [Google Scholar]
- 9.Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010; 28(25):3987–93 [DOI] [PubMed] [Google Scholar]
- 10.Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK positive anaplastic large cell lymphoma. Blood. 2007; 110(2):670–7 [DOI] [PubMed] [Google Scholar]
- 11.Lamant L, McCarthy K, d’Amore E, Klapper W, Nakagawa A, Fraga M, et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol. 2011; 29(35):4669–76 [DOI] [PubMed] [Google Scholar]
- 12.Mussolin L, Pillon M, d’Amore ES, Santoro N, Lombardi A, Fagioli F, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005;19(9): 1643–7 [DOI] [PubMed] [Google Scholar]
- 13.Ait-Tahar K, Damm-Welk C, Burkhardt B, Zimmermann M, Klapper W, Reiter A, et al. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood. 2010; 115(16):3314–9 [DOI] [PubMed] [Google Scholar]
- 14.Mussolin L, Damm-Welk C, Pillon M, Zimmermann M, Franceschetto G, Pulford K, et al. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia. 2013; 27(2):416–22 [DOI] [PubMed] [Google Scholar]
- 15.Lamant L, De Reynies A, Duplantier MM, Rickman DS, Sabourdy F, Giuriato S, et al. Gene expression profiling of systemic anaplastic large cell lymphoma reveals differences depending on ALK status and two distinct morphological ALK+ subtypes. Blood. 2007;109(5):2156–64 [DOI] [PubMed] [Google Scholar]
- 16.d’Amore ES, Menin A, Bonoldi E, Bevilacqua P, Cazzavillan S, Donofrio V, et al. Anaplastic large cell lymphomas: a study of 75 pediatric patients. Pediatr Dev Pathol. 2007;10(3):181–91 [DOI] [PubMed] [Google Scholar]
- 17.Nasr MR, Laver JH, Chang M, Hutchison RE. Expression of anaplastic lymphoma kinase, tyrosine-phosphorylated STAT3, and associated factors in pediatric anaplastic large cell lymphoma: a report from the children’s oncology group. Am J Clin Pathol. 2007;127(5):770–8 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki R, Kagami Y, Takeuchi K, Kami M, Okamoto M, Ichinohasama R, et al. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood. 2000;96(9):2993–3000 [PubMed] [Google Scholar]
- 19.Juco J, Holden JT, Mann KP, Kelley LG, Li S. Immunophenotypic analysis of anaplastic large cell lymphoma by flow cytometry. Am J Clin Pathol. 2003;119(2):205–12 [DOI] [PubMed] [Google Scholar]
- 20.Muzzafar T, Wei EX, Lin P, Medeiros LJ, Jorgensen JL. Flow cytometric immunophenotyping of anaplastic large cell lymphoma. Arch Pathol Lab Med. 2009;133(1):49–56 [DOI] [PubMed] [Google Scholar]
- 21.Abramov D, Oschlies I, Konovalov D, Damm-Welk C, Woessmann W, Klapper W. Intratumoral heterogeneity in anaplastic large cell lymphoma of non-common subtype. J Hematop. 2012;5(3):109–16 [Google Scholar]
- 22.Klapper W, Hoster E, Determann O, Oschlies I, van der Laak J, Berger F, et al. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop. 2009;2(2):103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamant L, McCarthy K, d’Amore E, Klapper W, Nakagawa A, Fraga M, et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol. 2011; 29(35):4669–76 [DOI] [PubMed] [Google Scholar]
- 24.Pileri S, Falini B, Delsol G, Stein H, Baglioni P, Poggi S, et al. Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology. 1990; 16(4):383–91 [DOI] [PubMed] [Google Scholar]
- 25.Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681–95 [PubMed] [Google Scholar]
- 26.Bonzheim I, Geissinger E, Roth S, Zettl A, Marx A, Rosenwald A, et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004;104(10):3358–60 [DOI] [PubMed] [Google Scholar]
- 27.Ambrogio C, Martinengo C, Voena C, Tondat F, Riera L, di Celle PF, et al. NPM-ALK oncogenic tyrosine kinase controls T-cell identity by transcriptional regulation and epigenetic silencing in lymphoma cells. Cancer Res. 2009;69(22):8611–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piva R, Agnelli L, Pellegrino E, Todoerti K, Grosso V, Tamagno I, et al. Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms. J Clin Oncol. 2010;28(9):1583–90 [DOI] [PubMed] [Google Scholar]
- 29.Voena C, Panizza E, Di Giacomo F, Ambrogio C, Manazza AD, Chiarle R. Trapping and silencing of NPM-ALK in the nucleus is a fundamental event for the NPM-ALK mediated cell transformation. XVth Meeting of the European Association for Haematopathology, Uppsala, Sweden 2010. Ref Type: Abstract. [Google Scholar]
- 30.Geissinger E, Sadler P, Roth S, Grieb T, Puppe B, Muller N, et al. Disturbed expression of the T-cell receptor/CD3 complex and associated signaling molecules in CD30+ T-cell lymphoproliferations. Haematologica. 2010; 95(10):1697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pileri SA, Agostinelli C, Bacci F, Sabattini E, Sagramoso C, Falini B, et al. Pathobiology of ALK-negative anaplastic large cell lymphoma. Pediatr. Rep. 2011; 3 (Suppl 2):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaiser T, Geissinger E, Schattenberg T, Scharf HP, Durken M, Dinter D, et al. Case report: A unique pediatric case of a primary CD8 expressing ALK-1 positive anaplastic large cell lymphoma of skeletal muscle. Diagn Pathol. 2012;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang E, Papalas J, Siddiqi I, Stoecker M, Rehder C, Sebastain S, et al. A small cell variant of ALK-positive, CD8-positive anaplastic large cell lymphoma with primary subcutaneous presentation mimicking subcutaneous panniculitis-like T-cell lymphoma. Pathol Res Pract. 2011;207(8): 522–6 [DOI] [PubMed] [Google Scholar]
- 34.Merlin E, Chabrier S, Verkarre V, Cramer E, Delabesse E, Stephan JL. Primary leptomeningeal ALK+ lymphoma in a 13-year-old child. J Pediatr Hematol Oncol. 2008; 30(12):963–7 [DOI] [PubMed] [Google Scholar]
- 35.Gao GF, Jakobsen BK. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol Today. 2000;21(12):630–6 [DOI] [PubMed] [Google Scholar]


