Abstract
Immunoglobulin light chain amyloidosis remains incurable despite recent therapeutic advances, and is particularly difficult to treat in patients with amyloid cardiomyopathy. Based on evidence of activity in multiple myeloma, we designed a pilot study of an oral regimen of lenalidomide in combination with dexamethasone and low-dose melphalan in order to evaluate its safety and efficacy in patients with amyloidosis, including those with advanced cardiac involvement. Twenty-five patients were enrolled. Ninety-two percent of patients had cardiac involvement by amyloidosis, and 36% of patients met the criteria for Mayo Clinic cardiac stage III disease. Patients received up to nine cycles of treatment, consisting of lenalidomide 10 mg/day orally on days 1 – 21 (28-day cycle); melphalan 0.18 mg/kg orally on days 1–4; and dexamethasone 40 mg orally on days 1, 8, 15, and 22. High rates (33%) of cardiac arrhythmias and low rates of treatment completion (12.5%) were observed. Ten patients died during the study, all within the first several months of treatment due to acute cardiac events. The overall hematologic response rate was 58%, however organ responses were seen in only 8% of patients. The overall survival rate at 1 year was 58%. While we confirmed the hematologic response rates observed with similar regimens, front-line treatment with melphalan, lenalidomide and dexamethasone was toxic, ineffective, and did not alter survival outcomes for patients with high-risk cardiac disease. Our data highlight the importance of developing novel treatment approaches for amyloid cardiomyopathy. This trial was registered at www.clinicaltrials.gov (NCT00890552).
Introduction
Immunoglobulin light chain amyloidosis (AL) is a plasma cell malignancy characterized by light chain tissue deposition, resulting in progressive damage and organ failure.1 Long-term survival outcomes are poor, particularly in patients with extensive cardiac amyloidosis.2 The goal of current AL therapy is to control the malignant plasma cell clone and thereby reduce the production of amyloidogenic light chains.3
Because multiple myeloma and AL result from clonal plasma cell dyscrasias, similar treatment strategies are employed in both diseases. Historically, AL treatment has been based on alkylating agents, such as melphalan, in combination with corticosteroids.2,4,5 While high-dose melphalan with autologous stem cell infusion is considered the standard of care, the majority of AL patients are not eligible for this treatment because of severe organ dysfunction.6,7
Newer agents such as the immunomodulators, thalidomide and lenalidomide, and the proteasome inhibitor bortezomib demonstrate significant activity in the treatment of AL.8–15 Lenalidomide with dexamethasone showed efficacy in AL in two phase 2 studies, although patients required dose reductions.11,12 The addition of alkylators to steroids and immunomodulators has demonstrated activity in both multiple myeloma and AL, and allowed agents to be effectively combined at lower doses with reduced toxicity.9,16–19 Two studies recently reported hematologic response rates of 50 and 58% with lenalidomide, melphalan, and dexamethasone (MDR) in previously treated and newly diagnosed AL, respectively.20,21 Three additional studies demonstrated similar hematologic response rates of 55–60% with lenalidomide in combination with cyclophosphamide and dexamethasone in both treatment-naïve and pretreated patients.22–24 The trial described here was a pilot study of MDR in AL, including patients with advanced AL cardiac involvement and poor performance status in order to evaluate the feasibility of this regimen in such cases.
Methods
Patients
Eligible patients had biopsy-proven AL and evidence of an underlying plasma cell dyscrasia, including clonal bone marrow plasma cells, detection of a monoclonal gammopathy by immunofixation electrophoresis of serum or urine, and/or an abnormal serum free light chain ratio. Patients were required to have measurable hematologic and organ disease.25 Adequate organ function for inclusion required: absolute neutrophil count ≥1.0×109/L, platelet count ≥75×109/L, creatinine clearance ≥15 mL/minute, total bilirubin ≤2 times upper limit of normal, and ECOG performance status of ≤3. After the first 15 patients had been enrolled, the protocol was amended to include only patients with ≤class II symptoms according to the New York Heart Association (NYHA) classification. All study participants were registered into the mandatory RevAssist® program. Females of childbearing potential were required to have a negative pregnancy test within 10 – 14 days prior to and within 24 hours of prescribing lenalidomide. Patients had to take aspirin, warfarin, or low molecular weight heparin as prophylactic anticoagulation. Patients were excluded if they had a history of hypersensitivity to thalidomide or previous lenalidomide treatment. All patients provided written, informed consent to participation in the study.
Study design
Patients were enrolled in this single center, single arm, open-label pilot study of lenalidomide, melphalan, and dexamethasone at the Stanford Amyloid Center in Stanford (CA, USA) between 2009 and 2012. The primary objective was to evaluate the safety of MDR in patients with AL, including those with advanced organ dysfunction. The secondary objectives were to evaluate the hematologic response rate, organ response rate, time to progression, event-free survival, and overall survival. The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and was approved by Stanford University’s Institutional Review Board. The trial was registered at www.clinicaltrials.gov (NCT00890552).
Patients received lenalidomide 10 mg/day orally on days 1–21, melphalan 0.18 mg/kg orally on days 1–4, and dexamethasone 40 mg orally once weekly of a 28-day cycle. Patients received up to nine cycles of treatment, with the option to continue on lenalidomide as a single agent if they responded to treatment.
Assessment
Safety was assessed according to version 3.0 of the NCI Common Terminology Criteria for Adverse Events (CTCAE) prior to each cycle.26 Hematologic responses were defined by the updated guidelines from the 12th International Symposium on Amyloidosis.27 Kidney and liver organ responses to treatment were defined by the 10th International Symposium on Amyloidosis.25 Cardiac organ response and progression was defined by changes in N-terminal pro-brain natriuretic protein (NT-proBNP) concentration such that the values both increased by >30% and were >300 ng/L.27 Hematologic and organ responses were assessed at the end of every cycle, at study completion, and every 3 months after treatment until progression or death.
Statistical analysis
For the primary objective, we assessed the safety of MDR. The percentage of patients experiencing toxicities was determined utilizing NCI CTCAE 3.0 guidelines.26 Survival curves were plotted with the Kaplan-Meier method and compared by using the log-rank test.
Results
Patients’ characteristics
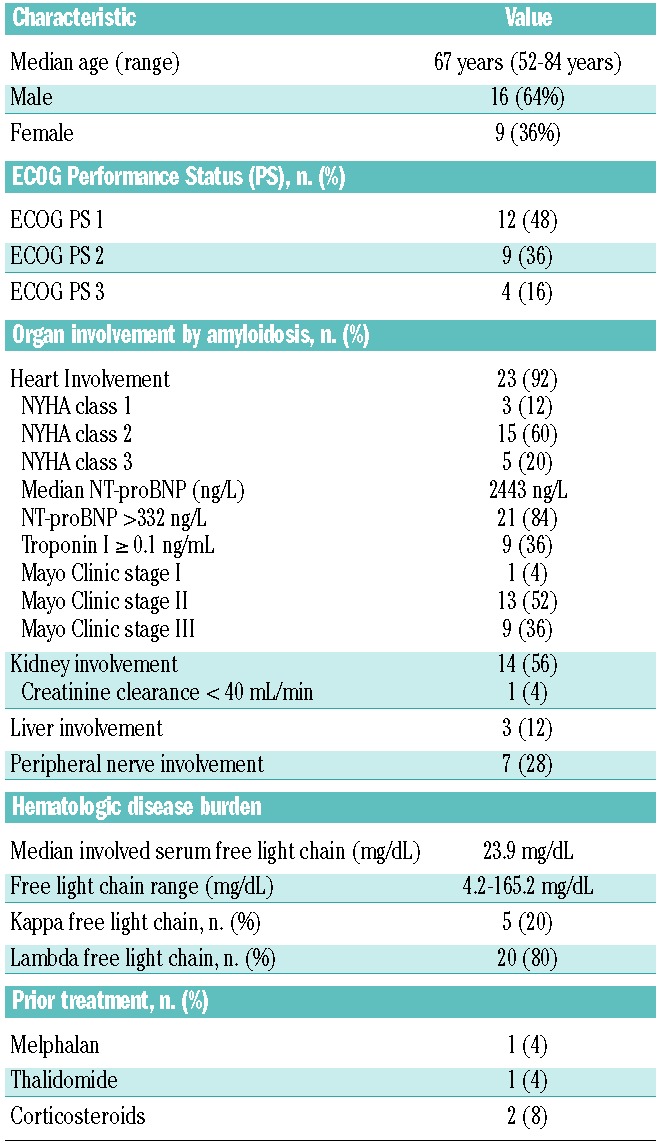
Twenty-five patients were enrolled into the study between April 2009 and September 2012. Twenty-three patients were newly diagnosed, and two had relapsed after one or two prior therapies. The median time from diagnosis to treatment was 1 month (range, 0–41 months). One patient was withdrawn before starting treatment because of progressive disease and death, leaving 24 evaluable patients. The patients’ baseline characteristics are shown in Table 1. The median age was 67 years (range, 52–84 years). Thirteen (52%) patients had an ECOG performance score ≥2. The median number of organs involved by amyloidosis was two (range, 1–5 organs). The heart was the most commonly involved organ (92%). Twenty (80%) patients had ≥NYHA class II disease, and nine (36%) met criteria for high-risk disease according to Mayo Clinic criteria. Fourteen (56%) patients had kidney involvement by amyloidosis, including one patient with a creatinine clearance <40 mL/min who required a 20% reduction of the dose of melphalan at baseline. The amyloidosis was lambda light chain restricted in 20 (80%) patients. The median level of involved serum free light chains was 23.9 mg/dL. The median percentage of bone marrow plasma cells was 8% (range, 2–37%) by morphological analysis and 15% (range, 5–55%) by immunohistochemical CD138 staining.
Table 1.
Patients’ baseline characteristics (n=25).
Safety and toxicity
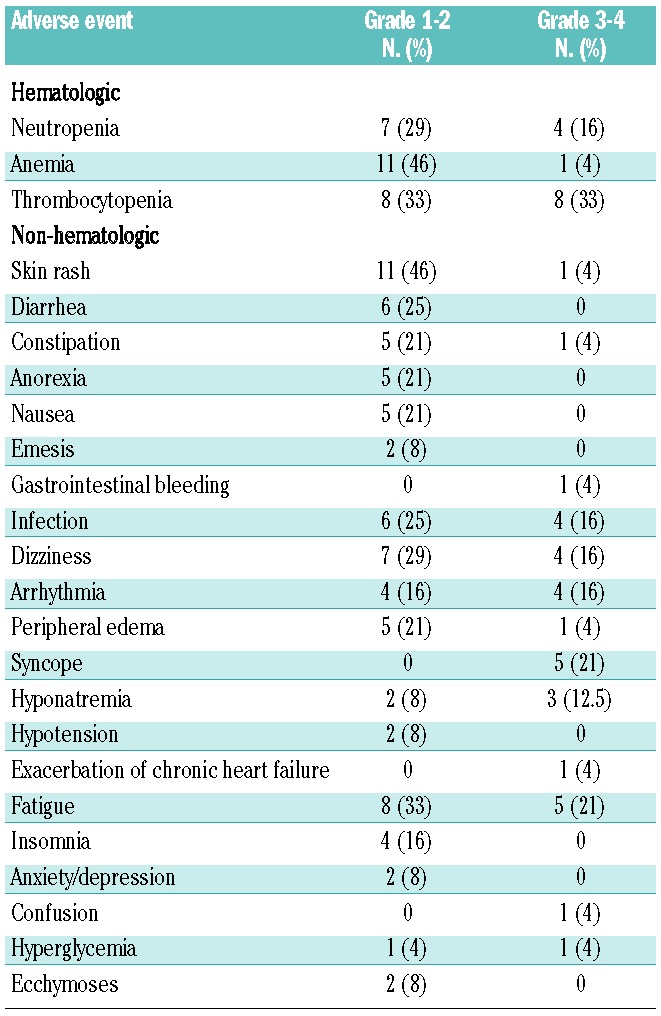
The most common toxicities attributed to the study medications were cytopenias (67%). Nine patients (37.5%) experienced at least one hematologic toxicity ≥ grade 3. Other common treatment-related events were fatigue (54%) and skin rash (50%), both likely attributable to lenalidomide. There were several adverse events in patients with amyloid cardiomyopathy that could be attributed to their organ dysfunction, supportive cardiac medications such as diuretics, and/or study treatment toxicity: eight (33%) patients had a documented cardiac arrhythmia; five (21%) reported syncopal events; ten (42%) reported dizziness; five (21%) patients developed hyponatremia (12.5% ≥grade 3); two (8%) patients experienced grade 2 hypotension; and one (4%) patient had an exacerbation of congestive heart failure. One additional patient who experienced dizziness reported that the symptom was specifically associated with treatment doses and resolved with discontinuing study drugs. The patients’ median pretreatment blood pressure was 111/66 mmHg (range, 86/49–163/100 mmHg). There was no consistent trend in changes in blood pressure after initiation of treatment. The median blood pressure after one cycle of treatment was 107/68 mmHg (range, 78/49–142/86 mmHg) and the median change in systolic blood pressure was −5 mmHg (range, −21 to +18 mm Hg) while that of diastolic blood pressure was −3 mmHg (range, −14 to +14 mmHg). Gastrointestinal toxicity that was most likely treatment-related included diarrhea (25%), constipation (25%), nausea (21%) and anorexia (21%). Ten patients (42%) had infections, and six of these events were possibly treatment related. Adverse events likely attributable to dexamethasone included edema (25%), insomnia (16%), anxiety/depression (8%), and hyperglycemia (8%). Neuropathy was present at baseline in six patients, but did not increase with MDR. No thrombotic events occurred (Table 2).
Table 2.
Treatment emergent adverse events (n=24).
Nine patients required dose reductions. The dose of melphalan was reduced because of cytopenias, that of lenalidomide because of rash and cytopenias and that of dexamethasone in a patient who reported symptoms of fatigue, dizziness and edema.
The median number of cycles received was three (range, 1–9). Only three patients were able to complete the scheduled nine cycles of treatment, of whom two elected to continue on maintenance therapy. Eight patients went off study because of toxicity, five because of organ progression, one because of lack of hematologic response, one because of an infection unrelated to treatment, and six died while actively receiving treatment on protocol. Toxicities requiring treatment discontinuation included four cases of ≥ grade 3 cytopenias, with one of these cases occurring in a patient who had a baseline creatinine clearance <40 mL/min which required a 20% reduction in the melphalan dose. Two patients discontinued treatment because of ≥ grade 2 fatigue. One patient with AL gastrointestinal involvement developed grade 3 thrombocytopenia and gastrointestinal hemorrhage, leading to study discontinuation. One patient discontinued treatment because of grade 4 hyponatremia, leading to seizures, and subsequently died of cardiac arrest. The five (21%) patients taken off study due to progressive organ disease included four patients who met criteria for cardiac progression and one patient who met the criteria for renal progression by AL as defined by the Consensus Criteria.20
Hematologic response
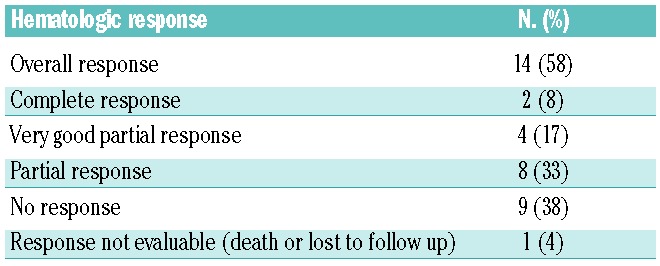
Overall the hematologic response rate was 58%, including complete responses in two patients (8%), very good partial responses in four patients (17%), and partial responses in eight patients (33%). Nine patients (38%) did not respond to treatment, however no patients met criteria for hematologic progression on study. One patient died during the first cycle of treatment, and was therefore not assessable (Table 3). The median time to first hematologic response (partial or better) was 1 month (range, 0.5–2). All responding patients achieved their best hematologic response within this time frame, except for one patient who achieved a partial response within 1.75 months of beginning treatment and then went on to have a very good partial response by 5.25 months of treatment.
Table 3.
Best hematologic response (n=24).
Organ response
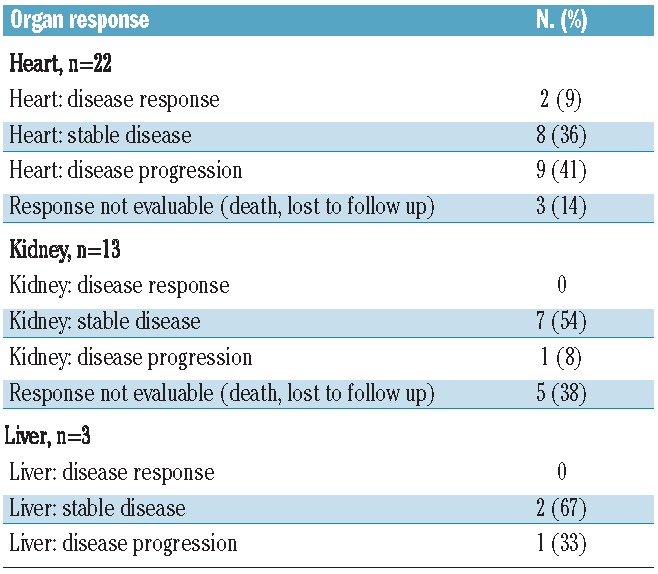
The criteria for an organ response were met in only two patients (8%), who both had a cardiac response. Stable organ disease was seen in eight (36%) patients with cardiac disease, seven (54%) patients with renal disease, and two (67%) patients with liver disease. Patients with cardiac disease had the highest rate of progression, with this occurring in nine (41%) patients. One patient developed decreased cardiac ejection fraction, one patient developed an increased interventricular septal thickness, and seven patients developed increased levels of NT-pro BNP. While four of these patients discontinued treatment early due to cardiac progression, the remaining five patients were found to meet criteria for progression at the end of treatment evaluation (Table 4). In the 12 patients with cardiac involvement who were alive at the time of study closure, one remained on maintenance treatment with lenalidomide, nine had a persistently elevated NT-proBNP off MDR treatment, and two had a normalized NT-proBNP at 1 and 4 months after discontinuing MDR treatment.
Table 4.
Organ response.
Progression and survival
At the cut-off date of October 1, 2012, all patients had completed or discontinued induction therapy and one patient remained on maintenance therapy with lenalidomide as a single agent. The median follow-up time from beginning treatment was 6.4 months (range, 0.25–36 months) for all patients and 16.75 months (range, 1–36 months) for surviving patients. The overall survival rate at 1 year was 58%; the median overall survival was not reached at the time of this assessment (range, 0.25 months to not reached) (Figure 1A). According to Mayo Clinic stage by cardiac biomarkers, the 1-year overall survival was 100% in stage I patients, 75% in stage II patients, and 22% in stage III patients (P<0.005). The median overall survival was 1.75 months in stage III patients (Figure 1B). There was no significant difference in survival outcomes based on depth of hematologic response. Ten (42%) patients died during the study period, including six who died while actively receiving therapy and four who discontinued treatment early for other reasons and died during the follow-up monitoring period. The causes of death were cardiac related due to advanced cardiac amyloidosis in nine patients. All deaths occurred within 3 months of study enrollment; seven (70%) of these patients had high-risk cardiac disease at baseline, while the other three (30%) had intermediate-risk cardiac disease at baseline. The median event-free survival was 3.15 months (range, 0.25 months to not reached). The 1-year event-free survival rate stratified by cardiac stage was 17% in stage II patients and 0% in stage III patients (P<0.04). The one patient with stage I cardiac disease had progressive disease at 1 year (Figure 2). The median event-free survival was 1.75 months in stage III patients. Seven of 14 patients who responded to treatment had not progressed at the time of this report. The median duration of response, defined by the time from first partial response until progression, death, or last follow up, was 9.1 months (range, 0–31.25 months). However, this figure most likely over-estimates the effects of MDR because six patients who achieved a partial response with MDR went on to receive alternative therapy in order to gain a greater depth of response. When we censored for start of next therapy, the median duration of response decreased to 3.1 months (range, 0–31.25 months). Five patients with lack of treatment response received alternative therapy after MDR. Salvage regimens included bortezomib with dexamethasone, lenalidomide with dexamethasone, bortezomib, lenalidomide and dexamethasone, and bortezomib, cyclophosphamide, and dexamethasone. For all surviving patients, the median time to next treatment was 0.9 months (range, 0 months to not reached).
Figure 1.
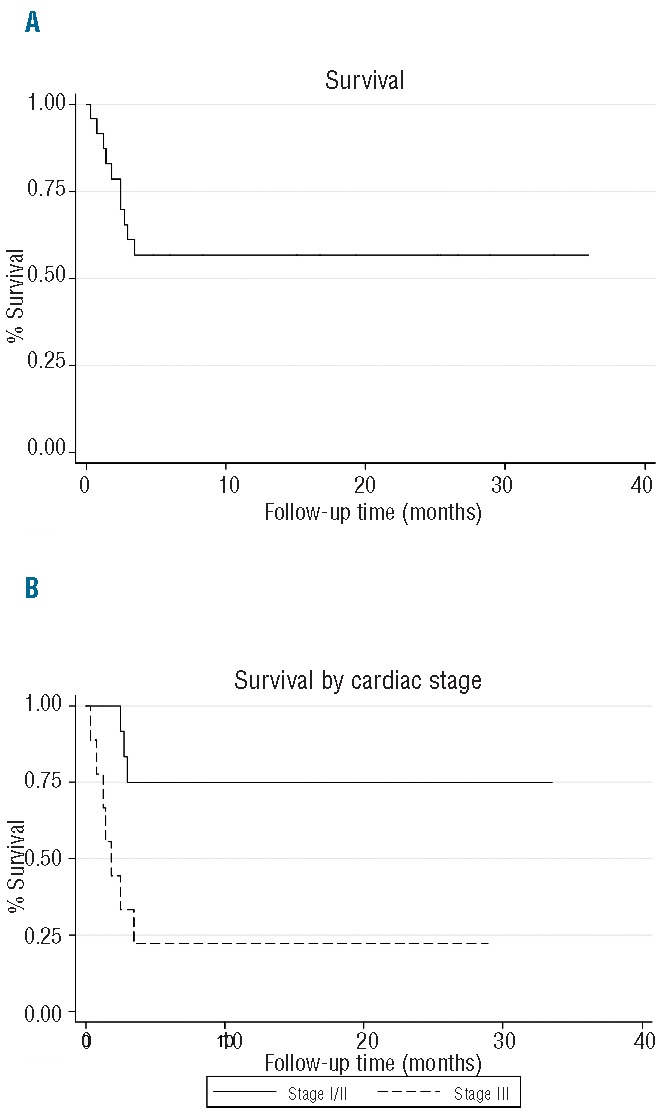
Overall survival for (A) the whole cohort of patients, (B) patients stratified by Mayo Clinic cardiac stage (P<0.005).
Figure 2.
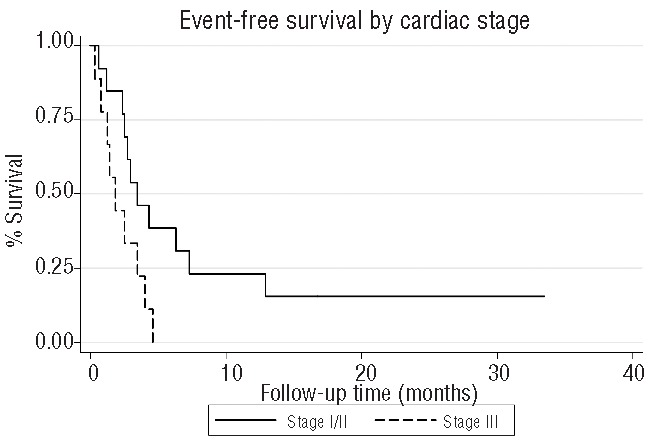
Event-free survival stratified by Mayo Clinic cardiac stage (P<0.04).
Discussion
AL treatment remains challenging because of the need to target the plasma cell clone while minimizing toxicity in patients with organ dysfunction. Overall survival has improved with the introduction of novel agents, but many patients still die within the first year of diagnosis, primarily due to AL cardiomyopathy.28 Given these limiting factors and poor outcomes, this study was conducted to evaluate an oral treatment regimen in patients with AL, including those with an advanced stage of cardiac involvement.
Our study confirmed the poor results seen with both conventional and novel therapies in severe cardiac amyloidosis.29–31 The 42% early death rate observed in our study was consistent with previously reported 1-year mortality rates, suggesting the continued problem with early mortality in patients with AL.28 These outcomes contrasted with the MDR study by Moreau et al., who reported a 2-year overall survival rate of 81%.21 Of note, that study enrolled a selected group of patients with an ECOG performance status of 0–1, median age of 57 years, and only 58% of patients had cardiac involvement, whereas our study included patients with ECOG performance status ≤3, median age of 67 years, and 92% of patients had cardiac involvement. The worse baseline characteristics in our population of patients resulted in deaths occurring earlier in this trial than in that by Moreau et al. While our study is more readily compared to the MDR trial by Sanchorawala et al. (median age of the patients 70 years, SWOG performance status ≤2, cardiac involvement in 69%), the median time from diagnosis to treatment was 1 month in our study as opposed to 6 months in the study by Sanchorawala et al.20 This may have contributed to the lower early death rate (19%) reported by Sanchorawala et al. compared to that in our study because the majority of amyloid deaths occur within the first 6 to 12 months after diagnosis.28
The most common toxicities seen with MDR, myelosuppression, rash, and fatigue, were comparable to those with other lenalidomide-based regimens.11,12,20–24 Both our trial and the MDR trial by Sanchorawala et al. utilized lenalidomide at a dose of 10 mg; however we observed hematologic toxicities ≥ grade 3 in a smaller proportion of patients (37.5% versus 57%).20 In contrast, only 8% of the patients in the MDR trial by Moreau et al. reported ≥ grade 3 hematologic toxicity, despite receiving a higher dose of lenalidomide.21 This difference may reflect the higher median age of the patients in the former studies (67–70 years) compared to that in study by Moreau et al. (57 years). High rates (33%) of cardiac arrhythmias and low rates of treatment completion (12.5%) were seen on this study, unlike in the two other MDR trials; this is most likely because of the large number of patients with amyloid cardiomyopathy in this study population. While cardiac arrhythmias have been more commonly reported with thalidomide in AL amyloidosis than with lenalidomide, we cannot rule out that lenalidomide may have contributed to cardiac toxicity in our study.8 However, this can be difficult to distinguish clinically from disease progression.
The reasons for study discontinuation in surviving patients were organ disease progression, cytopenias, and fatigue; this last effect may, however, be attributed to AL multi-system involvement and congestive heart failure in the majority of patients rather than to the effect of a drug.
Overall, MDR produced hematologic response rates similar to those reported in studies of immunomodulators in combination with corticosteroids with or without an alkylating agent. Thalidomide, cyclophosphamide and dexamethasone produced a 74% hematologic response rate, an impressive result for a non-transplantation treatment regimen. However, the thalidomide study included 5% of patients evaluated at the center during enrollment, raising concerns about a selection bias.9 In two previous trials of lenalidomide with dexamethasone, hematologic response rates were 41% and 47%,11,12 and so the hematologic response rate of 58% in our trial compares favorably with those in the lenalidomide trials. Furthermore, the median time to response with MDR was only 1 month versus 6 months with lenalidomide and dexamethasone, suggesting added benefit from melphalan. The hematologic response rates in our study and the MDR trial by Sanchorawala et al. were comparable to those in the MDR trial by Moreau et al., despite using lower doses of lenalidomide.20,21 The time to next treatment was short in our study because patients who did not respond to MDR or only achieved a partial response pursued alternative therapy in order to gain a greater depth of response. As a result, the effects of MDR on duration of response may be over-estimated.
Recently, the results of three additional trials on the combination of lenalidomide with cyclophosphamide and dexamethasone have been reported.22–24 MDR achieved similar hematologic response rates using a lower dose of lenalidomide. Furthermore, despite the fact that the study by Palladini et al. required that patients had a Mayo cardiac stage ≤2, there were 12 (57%) deaths on study, eight attributed to heart failure, three to sudden death, and one to liver failure, suggesting poor cardiac outcomes similar to those in our study.24
Organ responses were seen in only 8% of patients in our study and 6% of patients in the MDR trial by Sanchorawala et al., which is lower than the response rates reported by most other investigators for lenalidomide-based therapies (range, 19–50%).11,12,20–24 In particular, Moreau et al. reported a strikingly higher 50% organ response rate with MDR.21 This may be partly due to the fact that cardiac disease response can be difficult to interpret with the paradoxical elevation of BNP in the setting of lenalidomide use.32,33 However, in the 11 patients with cardiac involvement assessable for NT-proBNP off MDR treatment, only two had normalization of NT-proBNP levels. This suggests that the low organ response rate is more likely due to the high rates of early death and shortened follow up in our patient population attributable to lack of efficacy of MDR for patients with advanced cardiac amyloidosis.
Our study is the first to assess lenalidomide in combination with an alkylating agent and dexamethasone in a population that included a large proportion (92%) of patients with AL cardiomyopathy, high-risk cardiac disease, and poor performance status. Both our study and the study by Sanchorawala et al. showed that these agents can produce hematologic responses in combination at lower doses. However, while we confirmed the hematologic response rates observed by other investigators using similar regimens, MDR did not alter outcomes for patients with high-risk cardiac disease. Notably, there was no difference in survival outcomes based on depth of hematologic response. This is likely due to the fact that MDR was toxic and ineffective for patients with advanced cardiac amyloidosis, as indicated by a 42% early death rate, 33% rate of cardiac arrhythmias, median duration of therapy limited to three cycles, and an 8% organ response rate. These data, and the results reported by Sanchorawala et al. suggest that this drug combination should not be used in this population. These findings are consistent with prior results of treatment with melphalan and dexamethasone with or without thalidomide in patients with advanced cardiac involvement.29–31 Trials of additional novel agents and new drug combinations are, therefore, necessary in this subset of patients. Recent retrospective analyses have shown impressive response rates and survival outcomes after treatment with cyclophosphamide in combination with the proteasome inhibitor bortezomib and dexamethasone, which should be confirmed prospectively, particularly in patients with advanced cardiac stage amyloidosis.34,35
Acknowledgments
This study was supported by research funding from Celgene to ML and SS.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349(6):583–96 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone and melphalan, prednisone and colchicine. N Engl J Med. 1997;336(17):1202–7 [DOI] [PubMed] [Google Scholar]
- 3.Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain (AL) amyloidosis. Leukemia. 2012; 26(11):2317–25 [DOI] [PubMed] [Google Scholar]
- 4.Skinner M, Anderson J, Simms R, Falk R, Wang M, Libbey C, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicines versus colchicine only. Am J Med. 1996;100(3):290–8 [DOI] [PubMed] [Google Scholar]
- 5.Palladini G, Perfetti V, Obici L, Caccialanza R, Semio A, Adami F, et al. Association of melphalan and high-dose dexamethasone is effective and well-tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103(8):2936–8 [DOI] [PubMed] [Google Scholar]
- 6.Sanchorawala V, Skinner M, Quillen K, Finn KT, Doros G, Seldin DC. Long-term outocome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007; 110(10):3561–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357(11):1083–93 [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Lacy MQ, Rajkumar SV, Geyer SM, Witzig TE, Fonesca R, et al. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid. 2003;10(4):257–61 [DOI] [PubMed] [Google Scholar]
- 9.Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Wakins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109(2):457–64 [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JB, Dredge K, Dallies AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–22 [DOI] [PubMed] [Google Scholar]
- 11.Dispenzieri A, Lacy MQ, Zeldenrust SR, Hayman SR, Kumar SK, Geyer SM, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007; 109(2):465–70 [DOI] [PubMed] [Google Scholar]
- 12.Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109(2):492–6 [DOI] [PubMed] [Google Scholar]
- 13.Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkin PN, Perfetti V, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28(6):1031–7 [DOI] [PubMed] [Google Scholar]
- 14.Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Blade J, et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4): 865–73 [DOI] [PubMed] [Google Scholar]
- 15.Lamm W, Willenbacher W, Lang A, Zojer N, Muldur E, Ludwig H, et al. Efficacy of the combination of bortezomib and dexamethasone in systemic AL amyloidosis. Ann Hematol. 2011;90(2):201–6 [DOI] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomized controlled trial. Lancet. 2006;367(9513):825–31 [DOI] [PubMed] [Google Scholar]
- 17.Facon T, Mary J, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–18 [DOI] [PubMed] [Google Scholar]
- 18.Palumbo A, Falco R, Corradini P, Falcone A, Di Raimondo F, Giuliani N, et al. Melphalan, prednisone and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA – Italian Multiple Myeloma Network. J Clin Onc. 2007; 25(28):4459–65 [DOI] [PubMed] [Google Scholar]
- 19.Kumar SK, Lacy MQ, Hayman SR, Stewart K, Buadi FK, Allred J, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol. 2011;86(8):640–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchorawala V, Patel J, Sloan M, Shelton A, Zeldis J, Seldin D. Melphalan, lenalidomide and dexamethasone for the treatment of immunoglobulin light chain amyloidosis: results of a phase II trial. Haematologica. 2013;98(5):789–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau P, Jaccard A, Benboubker L, Royer B, Leleu X, Bridoux F, et al. Lenalidomide in combination with melphalan and dexamethasone in patients with newly-diagnosed AL amyloidosis: a multicenter phase 1/2 dose escalation study. Blood. 2010; 116(23):4777–82 [DOI] [PubMed] [Google Scholar]
- 22.Kumar SK, Hayman S, Buadi F, Roy V, Lacy M, Gert M, et al. Lenalidomide, cyclophosphamide, and dexamethasone for ligh-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119(21):4860–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Pamboukas C, Boletis I, et al. A phase 1/2 study of lenalidomide with low-dose oral cyclophosphamide and low-dose dexamethasone in AL-amyloidosis. Blood. 2012;119(23):5384–90 [DOI] [PubMed] [Google Scholar]
- 24.Palladini G, Russo P, Milani P, Foli A, Lavatelli F, Nuvolone M, et al. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica. 2013;98(3):433–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gertz M, Comenzo R, Falk R, Fermand J, Hazenberg B, Hawkins P, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol. 2005;(79):319–28 [DOI] [PubMed] [Google Scholar]
- 26.Common Terminology for Adverse Events Version 3.0. U.S. Department of Health and Human Services. National Institutes of Health. National Cancer Institute; Published August 9, 2006 [Google Scholar]
- 27.Palladini G, Dispenzieri A, Gertz M, Kumar S, Wechalekar A, Hawkins P, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012; 30(36):4541–9 [DOI] [PubMed] [Google Scholar]
- 28.Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman S, Buadi F, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011; 86(1):12–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebovic D, Hoffman J, Levine BM, Hassoun H, Landau H, Goldsmith Y, et al. Predictors of survival in patients with systemic light-chain amyloidosis and cardiac involvement initially ineligible for stem cell transplantation and treated with oral melphalan and dexamethasone. Br J Haematol. 2008; 143(3):369–73 [DOI] [PubMed] [Google Scholar]
- 30.Dietrich S, Schonland SO, Benner A, Bochtler T, Kristen AV, Beimler J, et al. Treatment with intravenous melphalan and dexamethasone is not able to overcome the poor prognosis of patients with newly diagnosed systemic light chain amyloidosis and severe cardiac involvement. Blood. 2010;116(4):522–8 [DOI] [PubMed] [Google Scholar]
- 31.Palladini G, Russo P, Lavatelli F, Nuvolone M, Albertini R, Bosoni T, et al. Treatment of patients with advanced cardiac AL amyloidosis with oral melphalan, dexamethasone, and thalidomide. Ann Hematol. 2009; 88(4):347–50 [DOI] [PubMed] [Google Scholar]
- 32.Tapan U, Seldin DC, Finn KT, Fennessey S, Shelton A, Zeldis JB, et al. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. 2010;116(23):5071–72 [DOI] [PubMed] [Google Scholar]
- 33.Dispenzieri A, Dingli D, Kumar SK, Rajkumar SV, Lacy MQ, Hayman S, et al. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010; 85(10):757–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikhael J, Schuster S, Jimenez-Zepeda V, Bello N, Spong J, Reeder CB, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19): 4391–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venner C, Lane T, Foard D, Rannigan L, Gibbs S, Pinney J, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119(19):4387–90 [DOI] [PubMed] [Google Scholar]


