Abstract
Previous reports demonstrated a relationship between proliferation potential and trilineage differentiation in mesenchymal stromal cell-derived clones generated using plastic adherence (PA-MSCs). However, there are no reports presenting a clonal analysis of the proliferative potential, differentiation potential and allosuppressive effects of human mesenchymal stromal cell subsets. In this study, we performed a clonal analysis of mesenchymal stromal cells generated from human CD271+ bone marrow mononuclear cells (CD271-MSCs). After transfection with the gene encoding green fluorescent protein, the cells were single-cell sorted and cultured for 2–4 weeks. A population doubling analysis demonstrated that 25% of CD271-MSC clones are fast-proliferating clones compared to only 10% of PA-MSC clones. Evaluation of the allosuppressive potential demonstrated that 81.8% of CD271-MSC clones were highly allosuppressive compared to only 58% of PA-MSC clones. However, no consistent correlation was observed between allosuppression and proliferative potential. Prostaglandin E2 levels were positively correlated with the allosuppressive activity of individual clones, suggesting that this molecule may be a useful predictive biomarker for the allosuppressive potential of mesenchymal stromal cells. In contrast, inhibitory studies of indoleamine 2,3 dioxygenase indicated that none of the clones used this enzyme to mediate their allosuppressive effect. Differentiation studies revealed the presence of tripotent, bipotent and unipotent CD271-MSC and PA-MSC clones which suppressed the allogeneic reaction to differing extents in vitro. In conclusion, our findings demonstrate differences between CD271-MSCs and PA-MSCs and indicate that neither proliferation potential nor differentiation potential represents a consistent predictive parameter for the immunomodulatory effects of either type of mesenchymal stromal cells.
Introduction
Mesenchymal stromal cells (MSCs) represent a very heterogeneous population of multipotent progenitor cells with a varying proliferative, differentiation and immunosuppressive potential.1 Most of the available evidence concerning the functional heterogeneity of MSCs has been obtained from clonal studies. Muraglia et al.2 reported that only one-third of the MSC clones generated from bone marrow mononuclear cells were able to differentiate into adipocytes, osteoblasts and chondrocytes (trilineage potential). Likewise, Lee et al.3 assessed adult human bone marrow MSCs at a single-cell level and found that only 7% of MSCs, which demonstrated a high proliferative potential, were able to differentiate toward multiple lineages. Most recently, Russell et al.4 reported that tripotent MSCs accounted for nearly 50% of the colony-forming cells. Another study using single-cell derived clones from parental immortalized MSCs demonstrated the presence of tri-, bi-, and uni-directional clones, suggesting that MSCs are constituted by a group of cells with different differentiation potential.5 The relationship between proliferation and trilineage potential has also been addressed using MSCs derived from tissue sources other than bone marrow. Guilak et al.6 reported that of clones generated from human adipose tissue-derived stromal cells, 21% were tripotent, 31% were bipotent, and 29% were unipotent MSCs. In addition, clonal analysis of bone marrow stromal cells from 3 patients with the degenerative disease osteoarthritis (OA) demonstrated the presence of fast-growing and slow-growing clones. All but one of the fast-growing clones were tripotent, whereas slow-growing clones displayed limited differentiation and morphological changes associated with cellular senescence.7
However, none of these studies has investigated the relationship between the proliferative and allosuppressive potential at the clonal level for PA-MSCs or any other subset of MSCs. In a previous study, we demonstrated that an MSC-subset generated from CD271+ bone marrow mononuclear cells (CD271-MSCs) was able to effectively suppress the allogeneic reaction even at low concentrations.8 In this study, we attempted to gain additional insight into the proliferative, differentiation and allosuppressive potential of this MSC-subset at the clonal level, including the molecular mechanisms involved in this process.
Methods
Immunomagnetic selection of CD271+ bone marrow mononuclear cells and generation of CD271-mesenchymal stromal cells
Bone marrow mononuclear cells (BM-MNCs) were obtained from the bone marrow aspirates of 3 healthy donors using a protocol approved by the University of Frankfurt Institutional Review Board. Briefly, two volumes of diluted bone marrow aspirate with PBS (1:2) were layered over one volume Ficoll (density 1.073 g/mL GE Healthcare, Uppsala, Sweden) and centrifuged at 700 × g for 30 min without brake. Mononuclear cells were collected from the interface, washed twice with PBS and centrifuged at 400 × g for 10 min. One proportion of the mononuclear cells was used for generation of PA-MSCs and the other portion of mononuclear cells was used for generation of CD271-MSCs, as described elsewhere.9
Evaluation of the immunosuppressive potential of MSC-derived clones
To test the immunosuppressive effect of CD271-MSC and PA-MSC derived clones on the alloantigen-driven reaction, we used mixed lymphocyte reaction (MLR). Peripheral blood mononuclear cells (PB-MNCs) from healthy unrelated donors were isolated using a Ficoll-gradient (density 1.077, Biochrom KG, Berlin, Germany), washed twice with PBS and resuspended in RPMI-1640 with 10% FBS (Invitrogen). PB-MNCs of 2 unrelated donors were cultured in black 96-well plates for six days alone (control group) or mixed with third-party, lethally irradiated (30 Gy) CD271-MSC at an MSC:PB-MNC ratio of 1:1 (1×105 MSC:1 × 105 PB-MNC). We assessed 22 clones derived from CD271-MSCs and 19 clones derived from PA-MSCs from 2 bone marrow donors. The allosuppressive potential of both CD271-MSC and PA-MSC clones generated from the same donor was tested in parallel against PB-MNCs of the same unrelated donor pair in each MLR assay. All MLRs were performed in triplicates in a 96-well plate. To evaluate levels of the allosuppression mediator PGE2, MSCs and allogeneic PB-MNCs were cultured in the presence or absence of the PGE2 inhibitor indomethacin (5 μM; Sigma, Deisenhofen, Germany). In addition, to determine the effect of indoleamine 2,3 dioxygenase (IDO) on MSC-mediated inhibiton of the alloantigen-driven reaction, we used CAY10581 (100 nM) (Biomol-Cayman, Hamburg, Germany). This molecule is a naphtoquinone derivative, and a more potent inhibitor of IDO than annulin B or 1-methyl-D-tryptohan.10 On Day 6, cells were incubated with 5-bromo-2′-deoxyuridine (BrdU) (Roche Diagnostics GmbH, Mannheim, Germany) for 24 h. The following day, relative light units (RLU/s) were measured with a luminometer 1420 Multilabel Counter Victor 3 (Perkin Elmer, Rodgau-Jügesheim, Germany). Proliferation levels of PB-MNCs were determined on Day 7 using a BrdU assay.
The inhibitory effect of both types of MSC-derived clones on the proliferation of allogeneic MNCs was calculated as a percentage using the following formula: 100-[(proliferation of allogeneic PB-MNCs in presence of MSC/proliferation of PB-MNCs without MSCs) × 100].
Statistical analysis
The statistical significance was assessed using Prism 5 software (GraphPad Software, San Diego, CA, USA). The Mann-Whitney U test was used to compare 2 independent samples, whereas Spearman’s rank correlation coefficient was used to correlate variables. P<0.05 and r >0.6 were considered statistically significant.
Results
Clonogenic, differentiation and proliferation potential of single-cell-derived CD271-MSC clones
In this study, we asked whether MSCs generated from positively selected CD271+ bone marrow mononuclear cells (CD271-MSCs) are more homogeneous than MSCs generated from unselected BM-MNCs (PA-MSCs) at the clonal level. Both types of MSCs fulfilled the criteria to be designated as multipotent cells, as outlined by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy.11 They grew as adherent cells, they expressed typical MSC-markers (Online Supplementary Figure S1 A–B) and they were able to differentiate into adipocytes, osteoblasts and chondrocytes in vitro (Online Supplementary Figure S2). MSCs of both types from passage 1 were transfected with green fluorescent protein (GFP) and used for the generation of clones. The MSC clones derived from the GFP-transfected MSCs of both types (passage 2) were expanded in vitro and then subsequently tested for proliferative, differentiation and allosuppressive potential (Figure 1). By Day 7, single-sorted CD271-MSCs (Figure 2A) generated a small cluster of cells (Figure 2B), which proliferated over time and generated classical CFU-Fs (Figure 2C). We observed that 68±7.8% of single-sorted CD271-MSCs from passage 2 generated CFU-Fs, compared to 43.1±0.5% of PA-MSCs (P<0.04) (Figure 2D). All of the clonally derived CD271-MSCs were negative for CD45 and CD34, but they were positive for classical MSC-antigens (CD73, CD90 and CD105) and class I HLA antigens (Figure 2E). In addition to these antigens, we assessed the expression of the STRO-1 antigen and CD146, which are cell surface markers used for the prospective isolation of MSCs from the bone marrow. We found that neither CD271-MSC nor PA-MSC derived clones expressed these antigens (Figure 2E).
Figure 1.
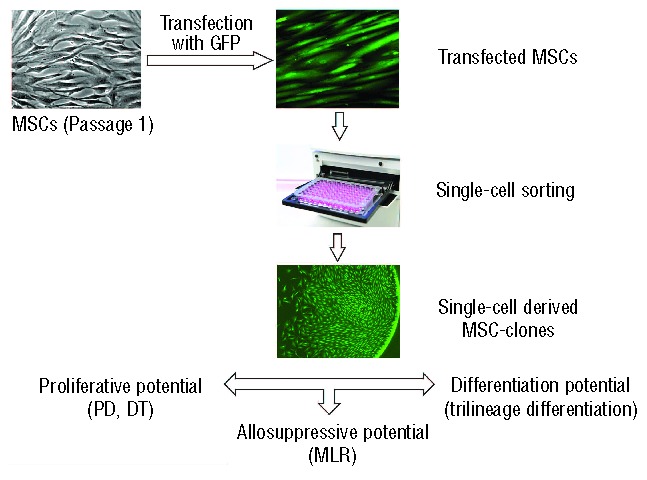
The experimental protocol used in this study. Passage 1 MSCs of both types were transfected with GFP and then single-cell sorted by FACSAria cell sorter. After 16–35 days in culture single-cell derived MSC-clones were assessed for their proliferative potential (PD: population doublings; DT: doubling time), immunosuppressive potential in mixed lymphocyte culture (MLR) and their differentiation potential along different lineages
Figure 2.
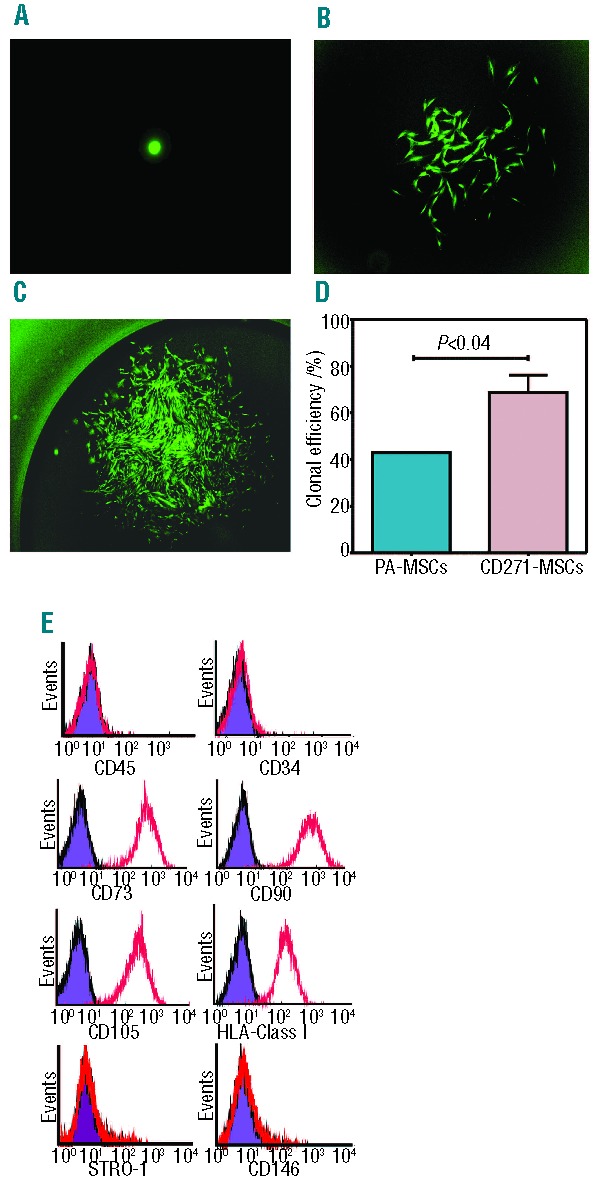
Clonogenic potential of single sorted MSCs and their phenotype. (A) On Day 1 post-sorting each well was monitored for GFP-positive cells under a fluorescence microscope, and wells containing more than one cell were excluded from the study (magnification: 100x). (B) Developing colony-forming unit-fibroblast (CFU-F) on Day 7 (magnification: 40x). (C) Confluent CFU-F colony at Day 21. These CFU-Fs were detached, expanded and then used to assess their proliferative, differentiation and allosuppressive potential (magnification 20x). (D) Clonogenic potential of single-sorted CD271-MSCs and PA-MSCs. Data are presented as a mean±SEM of single-sorted CD271-MSCs or PA-MSCs that were able to give rise to CFU-Fs. (E) Clonally-derived CD271-MSCs were negative for CD45 and CD34, while expressed typical MSC antigens (colored histograms represent isotype control, whereas red lines represent percentage of MSCs expressing the antigens).
A microscopic analysis of morphology demonstrated a qualitative difference in the composition of colonies. The majority of single-sorted CD271-MSCs generated CFU-Fs with typical spindle-shaped cell morphology. However, some clones generated CFU-Fs, which consisted of cells with either endothelial-like or osteoblast-like morphology. The morphological analysis demonstrated that CD271-MSC clones generated less endothelial-like colonies (12.2±5.4%) than PA-MSCs clones (28.3±0.35%) (P<0.007), and both types of clones generated approximately the same numbers of clones with an osteoblast-like morphology (8.9±1.8% of CD271-MSC clones and 7.5±3.4% of PA-MSC clones). Immunostaining with tissue-specific antibodies demonstrated that typical CFU-Fs containing spindle-shaped cells expressed the typical cell surface marker CD73 (Figure 3A). Endothelial-like colonies expressed von Willebrand factor but not CD31 (endothelial cell-specific markers) (Figure 3B), while osteoblast-like colonies expressed CD9 (Figure 3C). None of the colonies that were clonally-derived from either type of MSCs contained cells expressing the epithelial cell-specific marker pancytokeratin (data not shown).
Figure 3.
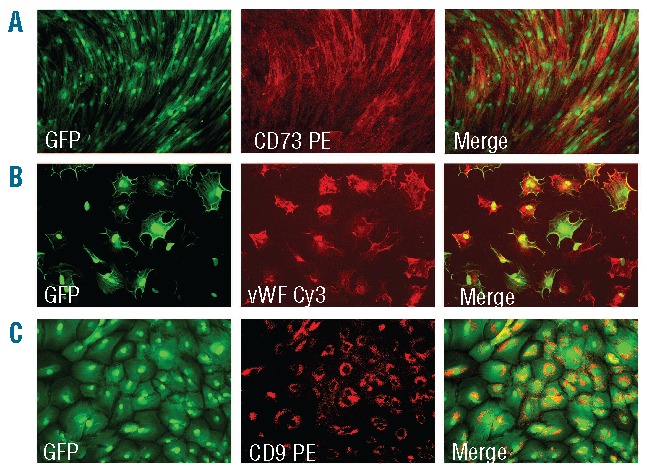
Composition of single derived colonies from CD271-MSCs. (A) Most of the clones showed a typical spindle-shaped MSC-morphology at week 3 in culture. The majority of the cells expressed high levels of CD73 (magnification: 40x). (B) A percentage of these clones revealed an endothelial-like morphology being positive for von Willebrand factor (magnification: 100x). (C) The other clones demonstrated an osteoblast-like morphology and expressed CD9 antigen (magnification: 100x).
An analysis of cellularity demonstrated that clones derived from CD271-MSCs or PA-MSCs contained approximately the same number of cells. CD271-MSC derived clones contained an average of 6464± 558 cells/clone (range 500–19,000 cells/clone) compared to PA-MSCs derived clones that contained 8122±1257 cells/clone (range 500–33,000 cells/clone) (Figure 4A and B). In contrast, an analysis of population doublings demonstrated that approximately 25% of CD271-MSC-derived clones (14 out of 57 clones) were fast-proliferating clones that reached the confluence within the first 16–18 days in culture, but only 10% of PA-MSC derived clones (4 out of 40 clones) were fast-proliferating clones. Approximately 61% of CD271-MSC-derived clones had a slower proliferation rate and reached confluence within 20–30 days, while 77% of PA-MSC derived clones were slow-proliferating clones (Figure 4B and C). Therefore, CD271-MSCs contain three types of clones: fast-proliferating, slow-proliferating, and very slow-proliferating. This variation in proliferation speed ensures a sustained growth of these MSCs in culture. The mean number of population doublings in CD271-MSCs-derived clones was 12.34±0.14 (Figure 4C). In addition, the time in culture needed for duplication (doubling time) was shorter for CD271-MSCs clones (1.89±0.07 days) than for PA-MSCs clones (1.98±0.06 days) (Figure 4D), but this difference was not statistically significant.
Figure 4.
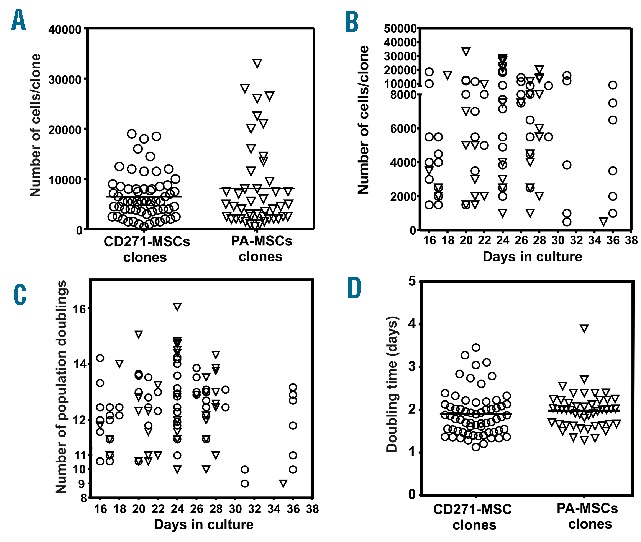
Cellularity and proliferation kinetics of single-cell derived MSC-clones. Clonally-derived MSCs of both types were harvested at different time-points and analyzed for their cellularity (A), proliferation rate during the culture (B), number of population doublings achieved within their expansion phase (C) and accordingly the time needed to duplicate themselves-doubling time (D). Circles present the CD271-MSC- derived clones, whereas triangles present the PA-MSC-derived clones.
Immunosuppressive properties of CD271-MSC clones
As MSCs have been shown to exert an immunosuppressive effect on the allogeneic reaction both in vitro and in vivo, we asked whether clonally derived MSCs possess this property and whether individual clones contribute to the heterogeneous allosuppressive effect of the population of non-cloned MSCs. An MLR analysis revealed that each clone has a different allosuppressive potential. Based on the percentage of inhibition of the PB-MNCs proliferation observed in the MLR, we classified the analyzed clones as: a) low-allosuppressive clones (inhibited the MLR up to 40%); and b) highly-allosuppressive clones (inhibited the MLR 40–100%). Four out of 22 CD271-MSC clones (18.2%) were low-allosuppressive clones, while the majority of these clones (81.8%) demonstrated a high allosuppressive effect in the MLR (range of allosuppression, 5.7–99.8%). In contrast, 42% of PA-MCS clones (8 out of 19 clones) were low-allosuppressive, while only 58% of clones were highly allosuppressive. Consistent with these data, the allosuppressive effect of CD271-MSC derived clones was significantly higher (P<0.05) than that of PA-MSC-derived clones (Figure 5A). In addition, inhibition studies using indomethacin, as a specific inhibitor of cyclooxygenase 1 and 2 (COX1 and COX2) demonstrated the presence of 3 types of clones within the CD271-MSC population: a) CD271-MSC derived clones, whose allosuppressive effect is completely mediated by PGE2, as indicated by a complete abrogation of allosuppression by indomethacin treatment (Figure 5B); b) CD271-MSC derived clones that partially use PGE2 as a mediator for the allosuppressive activity, as indicated by a partial reversal of inhibition of MNC proliferation by indomethacin (Figure 5C); and c) CD271-MSC derived clones that are PGE2-independent, as indicated by the inability of indomethacin treatment to abrogate inhibition of MNC proliferation (Figure 5D).
Figure 5.
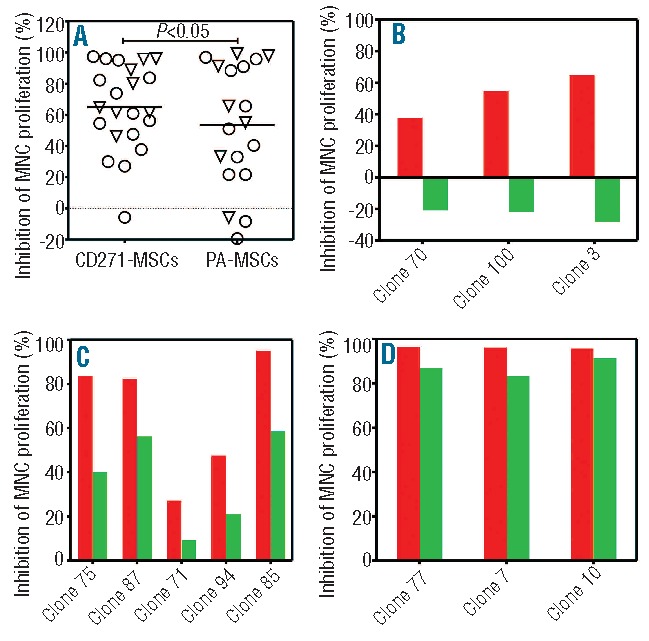
Allosuppressive potential of single-MSC derived clones and non-cloned MSCs. (A). The allosuppressive effect of clones derived from CD271-MSC and PA-MSCs which have been generated from 2 bone marrow donors. Triangles represent the MSC-clones of the first donor, whereas circles represent MSC-clones from the second donor. (B) CD271-MSC derived clones, which whose allosuppressive effects (red bars) are completely mediated by PGE2, because treatment with indomethacin abrogated completely this effect (green bars). (C) CD271-MSC derived clones which use partially PGE2 as a mediator of allosuppressive activity (red bars), while treatment with indomethacin can only partially reverse the inhibitory effect on proliferation of MNCs (green bars). (D) CD271-MSC derived clones, which are PGE2-independent, because the treatment with indomethacin as an inhibitor of PGE2 synthesis (green bars), was not able to abrogate the inhibitory potential of clones on proliferation of the mononuclear cells in the presence of MSC-clones (red bars).
Consistent with these results (Figure 5B, D), obtained from experiments when the allosuppressive effect was blocked by indomethacin, quantification of PGE2 levels in the MLR supernatants revealed that the majority of individual clones use this to mediate their allosuppressive effect. However, clones that do not use PGE2 as a mediator of their allosuppressive effect (e.g. clone 10) demonstrate a high allosuppressive potential even in the presence of low levels of PGE2. The majority of the clones with a high allosuppressive potential (more than 40% of MLR inhibition) were associated with the higher PGE2 levels than 10 ng/mL, while low-suppressive clones (less than 40% of MLR inhibition) had PGE2 levels less than 10 ng/mL (Figure 6A).
Figure 6.
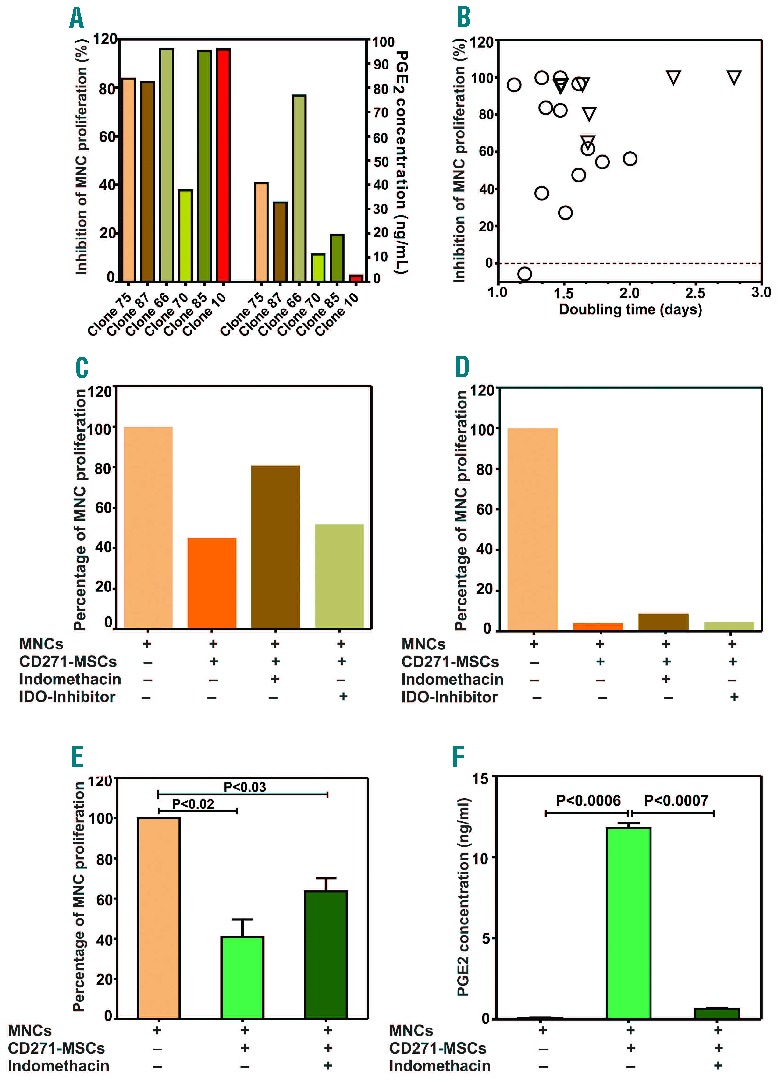
Allosuppressive and proliferation potential of the individual CD271-MSC derived clones and non-cloned CD271-MSCs. (A) In this figure is shown the relationship between the allosuppressive effect of single clones in MLR and PGE2 levels as a mediator of this effect in the appropriate supernatants (n=6 clones). (B) Relationship between the allosuppressive and proliferation potential of the individual CD271-MSC derived clones (n=20). (C) Inhibition of indoleamine 2, 3 dioxygenase (IDO) in a PGE2-dependent clone and a PGE2-independent clone (D). (E) Allosuppressive potential of non-cloned CD271-MSCs from 2 bone marrow donors, whose MSCs were used as a source for generation of the clones after single-cell sorting. Approximately 60% of this effect was mediated by PGE2, as the treatment with indomethacin reversed partially this effect. (F) PGE2 levels increased significantly (P<0.0006) when allogeneic MNCs were co-incubated with third-party CD271-MSCs, and decreased significantly after addition of indomethacin as an inhibitor of PGE2-synthesis (P<0.0007).
In addition, we asked whether the proliferation potential of CD271-MSC clones is predictive of their allosuppressive effect. Analysis of doubling time of individual clones, as an indicator of their proliferative potential, indicated that the allosuppressive potential of the CD271-MSC clones does not directly correlate with their proliferation potential (Figure 6B) (i.e. there are slow-growing clones which highly suppress the allogantigen-driven reaction and vice versa).
To identify other mediators in partially PGE2-dependent and PGE2-independent clones, we used a highly specific IDO-inhibitor (CAY10581) in order to investigate the role of IDO in the observed alloantigen-driven suppression. Our results demonstrate that the IDO-inhibitor was unable to reverse the allosuppressive effect of clones (Figure 6C and D).
Remarkably, the mean allosuppressive potential of non-cloned CD271-MSCs generated from 2 bone marrow donors that were used for generation of clones (Figure 6E) was nearly the same (60%) as the mean of allosuppressive potential of individual clones generated from these MSCs (Figure 5A). This allosuppressive effect was only partially reversed by the PGE2 synthesis inhibitor indomethacin (Figure 6E) and was associated with a significant increase (P<0.0006) in PGE2 levels (Figure 6F). A similar pattern was observed for the PA-MSC derived clones (data not shown).
Trilineage differentiation potential of clones and its relationship to the allosuppressive effect
In this study, we asked whether CD271-MSC derived clones possess trilineage differentiation potential. We differentiated 15 clones derived from CD271-MSCs and 10 clones derived from PA-MSCs (Online Supplementary Methods). We found that 13.3% of CD271-MSC derived clones were tripotent, with the ability to differentiate into adipocytes, osteoblasts and chondrocytes (AOC). Twenty percent of the clones differentiated into osteoblasts and chondrocytes (OC) (bipotent), and 7% differentiated only into adipocytes and osteoblasts (AO). Approximately 13% of CD271-MSC-derived clones were unable to differentiate into any lineage (Figure 7A). The clones that generated more cells were assessed not only for their differentiation potential study but also for their allosuppressive potential (Figure 7B and C). Our data demonstrated that there is no correlation between differentiation and allosuppressive potential of clones derived from either CD271-MSCs or PA-MSCs. We observed tripotent, bipotent and unipotent clones that could effectively suppress the allogeneic reaction in vitro.
Figure 7.
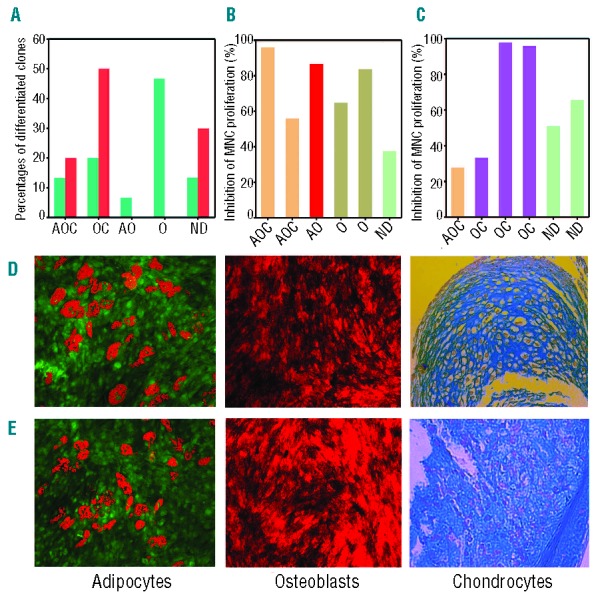
Trilineage differentiation of MSC-derived clones and their allosuppressive potential. (A) Clones generated from CD271-MSC (n=15) and PA-MSCs (n=10) were cultured in induction media for adipocytic, osteoblastic and chondrocytic differentiation. Green bars represent the clones derived from CD271-MSCs, red bars represent the clones derived from PA-MSCs. A: adipocytic; O: osteoblastic; C: chondrocytic; ND: non-differentiated. In addition to trilineage differentiation, six CD271-MSC derived clones (B) and six PA-MSC derived clones (C) were assessed for their allosuppressive potential in mixed lymphocyte reaction (MLR). (D) Representative examples for trilineage differentiation of CD271-MSCs and PA-MSC derived clones (E) into adipocytes, osteoblasts and chondrocytes. Magnification used for microscopic examination of adipocytes was 20x, for osteoblasts 10× and for chondrocytes 40x.
Discussion
As MSCs are a heterogeneous population of multipotent cells with regard to their proliferation, differentiation and immunosuppressive potential, clonal analysis is an ideal method to investigate the mechanisms that underlie the functional heterogeneity of these cells. Previous studies demonstrated that not all clones generated either from bone marrow mononuclear cells2,4 or primary MSCs3,5 are endowed with the potential to differentiate into adipocytes, osteoblasts and chondrocytes. This trilineage potential correlated with the proliferative potential of the tested clones. However, these studies used MSCs generated only by plastic adherence (PA-MSCs). In this study, we asked whether MSCs generated from CD271+ bone marrow mononuclear cells (CD271-MSCs) are a more homogeneous cell population with regard to their differentiation, proliferative, and allosuppressive potential at the clonal level.
Morphological analysis revealed that the majority of colonies derived from CD271-MSC clones consisted of cells with typical spindle-shaped morphology. In addition, 8.9±1.8% of the colonies expressed CD9, a concomitant marker for early mineralization stage,12 while 12.2±5.4% of them expressed endothelial-specific marker von Willebrand factor and lacked CD31. These data are consistent with the findings of Oswald et al.13 who demonstrated that MSCs are able to differentiate into cells with the phenotypic and functional features of endothelial-like cells after their stimulation with a cocktail of cytokines in vitro. To the best of our knowledge, our data are the first to suggest the presence of single progenitors within the MSC population that are intrinsically committed to endothelial and osteoblast lineages, even in the absence of tissue-inductive media.
Numerous reports demonstrated that CD271-MSCs have a significantly higher proliferative potential than PA-MSCs.14,15 We asked whether this functional property of CD271-MSCs could be confirmed at the clonal level. CD271-MSCs from passage 2 gave rise to a significantly higher number of CFU-Fs than PA-MSCs (P<0.04), indicating that these MSCs have a higher clonogenic potential, as has been previously reported for the whole population of CD271-MSCs.9,14 Proliferative studies presented here also demonstrated that a significantly higher number of clones (25%) derived from CD271-MSCs were highly proliferative than clones (10%) derived from PA-MSCs. The percentage of highly proliferative clones generated from CD271-MSCs was similar to the number of clones generated from PA-MSCs, as reported by Muraglia et al.2 In addition, the number of highly proliferative clones generated from PA-MSCs in our study was consistent with the findings of Lee et al.,3 but in contrast to data reported by Russell et al.,4 who found that tripotent PA-MSCs accounted for nearly 50% of the colony-forming cells.
An emerging body of evidence indicates that PA-MSC possess immunomodulatory properties and may play specific roles as immunomodulators in the maintenance of peripheral tolerance, transplantation tolerance, autoimmunity and tumor evasion, as well as fetal-maternal tolerance.16–18 However, the precise mechanisms underlying MSC-mediated immunosuppression remain largely undefined. Soluble factors such as hepatocyte growth factor (HGF), transforming growth factor (TGF)-β,19,20 indoleamine 2,3-dioxygenase (IDO),21 IL-2 and IL-10,22 prostaglandin E2 (PGE2),23–25 soluble HLA-G,26 nitric oxide (NO),27 immune cells (regulatory T cells),28 as well as contact dependent mechanisms,29,30 have been involved in MSC-mediated immunosuppression. In a previous study8 we demonstrated that CD271-MSCs elicited a potent allosuppression even at low concentrations, and that the majority of the immunosuppressive effect of CD271-MSC is mediated via PGE2, but not via HLA-G, NO or IL-10 molecules. In addition, we demonstrated that a subset of naive Tregs may contribute further to the allosuppressive activity of CD271-MSC. To date, no study has reported a clonal analysis of the allosuppressive activity of human MSC-subsets, although one study reported a clonal analysis of mouse MSCs.31 To gain additional insight into this functional property of MSCs, we assessed the allosuppressive potential of CD271-MSCs at the clonal level and investigated soluble molecules that might be involved in this process. Our results indicated that approximately 82% of CD271-MSC clones were highly allosuppressive in the MLR, compared to approximately 60% of clones derived from PA-MSCs. Consistent with this result, the mean allosuppressive effect of CD271-MSC-derived clones was significantly higher than that of PA-MSC-derived clones (P<0.05). We previously reported that PGE2 is the major mediator of the allosuppressive effect of non-cloned CD271-MSCs.8 In this study, inhibition of PGE2 synthesis by the cyclooxygenase 1 and 2 inhibitor indomethacin revealed for the first time that there are 3 types of CD271-MSC clones: a) clones in which the allosuppressive effect was completely mediated by PGE2; b) clones in which the inhibition of the alloantigen-driven reaction was partially mediated by PGE2; and c) clones in which the allosuppressive effect is independent of PGE2. Quantification of PGE2 revealed a positive correlation between PGE2 levels and the allosuppressive activity of individual clones. However, further studies with a larger number of clones are needed to determine whether PGE2 levels can be used as a predictive biomarker or potency assay for the allosuppressive potential of MSCs. In addition to PGE2, IDO has been reported as an important mediator of the allosuppressive effect of MSCs.21 The molecular mechanism that underlies this effect is thought to involve tryptophan degradation by the interferon-gamma (IFN-gamma)-induced expression of this enzyme. In contrast to the available data, Gieseke et al.32 demonstrated that human MSCs with an IFN-gamma receptor 1 (R1) defect inhibited the proliferation of HLA-mismatched PBMCs to a similar extent as control MSCs. The IFN-gammaR1-deficient MSCs displayed no detectable mRNA for IDO in the presence of recombinant human IFN-gamma or in co-culture with HLA-mismatched PBMCs. Considering these controversial data, we asked whether IDO is involved in the allosuppressive effect of CD271-MSC- or PA-MSC-derived clones. A highly specific inhibitor of IDO failed to reverse the inhibitory effects of MSCs on the alloantigen-driven reaction which suggests that clones derived from either type of MSCs fail to use IDO as an allosuppressive mediator. Previous studies demonstrated a positive correlation between the proliferative and the trilineage potential of clones.2–4 Our results indicate the presence of tripotent, bipotent and unipotent CD271-MSC and PA-MSC clones which suppressed the allogeneic reaction to differing extents in vitro.
Taken together, the clonal analysis presented in this study demonstrated that CD271-MSCs are functionally heterogeneous and differ from PA-MSCs with regard to their proliferative, differentiation and allosuppressive potential. Our results also indicate that neither proliferation potential nor differentiation potential represent a consistent predictive parameter for the immunomodulatory effect of both types of MSCs. In addition, elevated PGE2 levels in MLR may serve as a useful biomarker for the selection of MSCs for clinical use in the treatment of graft-versus-host disease and other inflammatory disorders.
Acknowledgments
The authors also express their gratitude to Frankfurter Stiftung für Krebskranke Kinder (Frankfurt, Germany) for their kind financial support (SK), and are indebted to Mr. T. Merovci from the Biopharmaceutical Institute Georg-Speyer-Haus (Frankfurt, Germany) for his excellent technical assistance in single-cell sorting.
Footnotes
The Online version of this paper contains a Supplementary Appendix.
Funding
The authors would like to thank the Else Kröner-Fresenius-Stiftung (2011_A186) for funding of this study. MG, HB,UK,TK and PB are supported by the LOEWE Center for Cell and Gene Therapy Frankfurt/Main funded by Hessisches Ministerium für Wissenschaft und Kunst (HMWK) (funding reference number: III L 4-518/17.004, 2010).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113(9):2806–12 [DOI] [PubMed] [Google Scholar]
- 2.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113( Pt 7):1161–6 [DOI] [PubMed] [Google Scholar]
- 3.Lee CC, Christensen JE, Yoder MC, Tarantal AF. Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells. Exp Hematol. 2010;38(1):46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28(4):788–98 [DOI] [PubMed] [Google Scholar]
- 5.Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295(2):354–61 [DOI] [PubMed] [Google Scholar]
- 6.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206(1):229–37 [DOI] [PubMed] [Google Scholar]
- 7.Mareddy S, Crawford R, Brooke G, Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007; 13(4):819–29 [DOI] [PubMed] [Google Scholar]
- 8.Kuci Z, Kuci S, Zircher S, Koller S, Schubert R, Bonig H, et al. Mesenchymal stromal cells derived from CD271(+) bone marrow mononuclear cells exert potent allosuppressive properties. Cytotherapy. 2011; 13(10):1193–204 [DOI] [PubMed] [Google Scholar]
- 9.Kuci S, Kuci Z, Kreyenberg H, Deak E, Putsch K, Huenecke S, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95(4):651–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Malachowski WP, DuHadaway JB, LaLonde JM, Carroll PJ, Jaller D, et al. Indoleamine 2,3-dioxygenase is the anti-cancer target for a novel series of potent naphthoquinone-based inhibitors. J Med Chem. 2008;51(6):1706–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315–7 [DOI] [PubMed] [Google Scholar]
- 12.Hanagata N, Li X. Osteoblast-enriched membrane protein IFITM5 regulates the association of CD9 with an FKBP11-CD81-FPRP complex and stimulates expression of interferon-induced genes. Biochem Biophys Res Commun. 2011;409(3):378–84 [DOI] [PubMed] [Google Scholar]
- 13.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84 [DOI] [PubMed] [Google Scholar]
- 14.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–91 [DOI] [PubMed] [Google Scholar]
- 15.Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, Discepoli G, et al. Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy. 2009;11(2):153–62 [DOI] [PubMed] [Google Scholar]
- 16.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312(12):2169–79 [DOI] [PubMed] [Google Scholar]
- 17.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006; 36(10):2566–73 [DOI] [PubMed] [Google Scholar]
- 18.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506 [DOI] [PubMed] [Google Scholar]
- 19.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43 [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004; 60(3):307–15 [DOI] [PubMed] [Google Scholar]
- 21.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–21 [DOI] [PubMed] [Google Scholar]
- 22.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305(1):33–41 [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4): 1815–22 [DOI] [PubMed] [Google Scholar]
- 24.Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316(19):3109–23 [DOI] [PubMed] [Google Scholar]
- 25.Najar M, Raicevic G, Boufker HI, Fayyad KH, De Bruyn C, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010; 264(2):171–9 [DOI] [PubMed] [Google Scholar]
- 26.Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, et al. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87(9 Suppl):S62–S66 [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109(1):228–34 [DOI] [PubMed] [Google Scholar]
- 28.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–25 [PubMed] [Google Scholar]
- 29.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003; 171(7):3426–34 [DOI] [PubMed] [Google Scholar]
- 30.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005; 105(5):2214–9 [DOI] [PubMed] [Google Scholar]
- 31.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17(3):240–8 [DOI] [PubMed] [Google Scholar]
- 32.Gieseke F, Schutt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, et al. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007;110(6):2197–200 [DOI] [PubMed] [Google Scholar]


