Abstract
Background
Liposomes have been successfully used as delivery vehicles for anticancer drugs. Both sonication and microfluidic technologies have been used to produce liposomes. The combination of the two methods was evaluated in this study.
Materials and Methods
The microfluidic devices, mainly comprising micro-dispensers and a sonicator, were used to produce liposomal nanoparticles. Sonication was used to enhance the reduction of liposome size.
Results
Sonication significantly reduced the size of the liposomes. The particle size also decreased as the buffer to solvent flow rate ratio increased. The smallest particle sizes were achieved with a volumetric flow rate of lipids at 0.374 ml/min.
Conclusion
The microfluidic devices in combination with ultrasound are simple and may be used to produce liposomal nanoparticles with narrow size distribution.
Keywords: Liposomes, microfluidics, ethanol injection, sonication
Liposomes are spherical closed-membrane structures containing at least one lipid bilayer that surrounds an aqueous internal core. The unique versatility of lipid vesicles with respect to composition, size, and capacity for embedding and encapsulating materials has led to many applications in lab research and in industry (1). Liposomes are widely used as drug and gene delivery vehicles (2). Liposome encapsulation of anticancer drugs can sometimes reduce the rate of drug metabolism, control drug release, extend circulation time, reduce tissue toxicity and enhance cell/tissue specificity of drug delivery (3). Some liposomal drugs, such as Doxil (PEGylated liposomal doxorubicin) (4) and Daunoxome (daunorubicin citrate liposome injection) (5, 6) have already been used in clinics. Uniformity and small size of liposomes are essential for safe, and effective drug delivery.
Liposomal nanoparticles can be produced by many methods, including thin-film hydration (7, 8), ethanol injection (9), and detergent dialysis (10). Generally, the conventional bulk production of liposomes relies on self-assembly of lipids in a bulk phase. Regional fluctuation of lipid concentrations results in difficulty in controlling particle sizes and uniformity. Thus, further post-processing by extrusion or sonication is often required to reduce the sizes and break multilamellar structures of the resultant liposomes. Sonication is a simple and efficient method to reduce particle sizes of liposomes (11). The cavitation events induced by sonication can break up larger, multilamellar lipid vesicles and turn them into smaller vesicles.
Microfluidics is recently emerging as a novel technique to produce liposomal nanoparticles. Similar to the ethanol injection method, solvent dilution in a microfluidic setting is a novel and promising method for preparing liposomes. Microfluidics can manipulate liquid flows in channels with dimensions of tens to hundreds of micrometers to form homogenous reaction environments (12). The laminar flow conditions in microfluidic channels can be used to create a well-defined and predictable interfacial region between two fluids and focus fluid streams hydrodynamically to submicrometer dimensional scales for rapid mixing and patterning. Jahn et al. developed a microfluidic hydrodynamic focusing method for the control of liposome formation (13–15). Pradhan et al. devised a simple and low-cost method without advanced microfabrication to produce liposomes (16).
The goal of this study was to evaluate the effect of sonication on liposome synthesis in microfluidic channel. In our design, micro-dispensers were used as syringe pumps to control the flow rates of lipids and buffer solutions. Sonication was introduced during the liposome formation in microfluidcs by immersing a commercial available microfluidic chip in a bath sonicator. We expect that this novel strategy can dramatically reduce liposome size and allow for continuous flow production of liposomes.
Materials and Methods
Materials
Egg phosphatidylcholine (Egg PC) was purchased from Avanti Polar Lipids Inc (Alabaster, AL, USA). Cholesterol was purchased from Sigma-Aldrich Chemical Company, Inc. (Milwaukee, WI, USA). 1× PBS (pH=7.4) was purchased from Fisher Scientific (Pittsburgh, PA, USA).
Microfluidic device
The programmable Hamilton Microlab Series 300 dispenser (Hamilton Company USA, Reno, NV, USA) was adopted as a syringe pump to control flow rates. Microfluidic chip (1 mm diameter) was purchased from Translume Advanced Glass Micromachining (Ann Arbor, MI, USA). Luer fittings (FTLLP-9 Value Plastics, Inc., Ft. Collins, CO, USA) were fused to the channel openings using industrial epoxy. Masterflex Tygon laboratory tubings (EW-06429-24 Saint-Gobain; Paris, France) were attached to the fluid inlet ports of the microfluidic channel via luer connectors. Masterflex Tygon tubing with an inside diameter of 1.54 mm was used to connect the luer fluid inlet ports of the microfluidic channels to dispensers. A VWR Scientific Aquasonic sonicator (Model 50HT; ETL Testing Laboratories, Cortland, NY, USA) was used as a sonication generator.
Liposome preparation and characterization
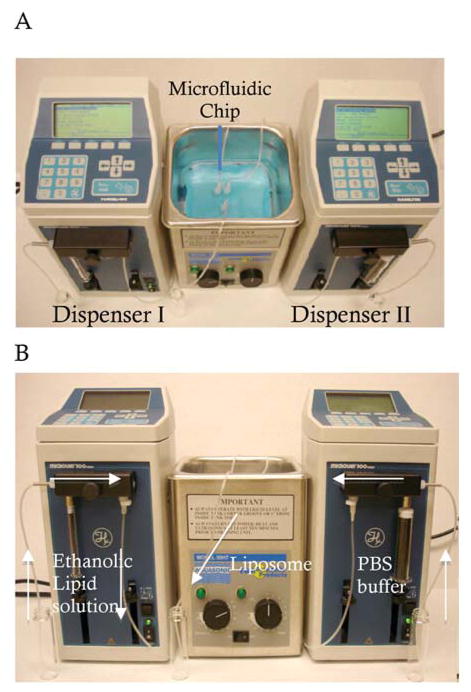
As shown in Figure 1, our microfluidic device for the production of liposomes mainly consists of two micro-dispensers and one ultrasonic bath processor. The micro-dispenser was used for a syringe pump to inject ethanolic lipid solution and PBS buffer solution, and is able to control flow rates in a programmable manner. The microfluidic chip was immersed in the water-bath of the ultrasonic processor (frequency: 50~60 Hz) to obtain ultrasonic energy (Figure 1A).
Figure 1.
Photographs of the microfluidic device with a bath sonicator for production of liposomes. A, top view; B, side view.
The liposomes were formed with Egg PC and cholesterol at a weight ratio of 6:1. One ml ethanolic lipid solution (3 mg/ml) and 10 ml 1×PBS were loaded into the 1 ml and the 10 ml gas-tight syringes of dispenser I and II, respectively. Correspondingly, the dissolved lipid solution and PBS buffer solution were injected into microfluidic channel through inlet 1 and inlet 2, followed by mixing at the interface of the microfluidic channel (Figure 2). Microfluidic synthesis of liposomes with and without sonication was examined at different flow rates and flow rate ratios. The flow rate ratio is defined as the volumetric PBS flow rate to the volumetric ethanol flow rate. In this study, with a flow rate ratio at 10, five flow rates of PBS (2.78 ml/min, 3.24 ml/min, 3.74 ml/min, 4.12 ml/min and 5.88 ml/min) were examined. The flow rate ratio was studied from 8 to 12 when the flow rate of the ethanolic lipid solution was kept at 3.74 ml/min.
Figure 2.
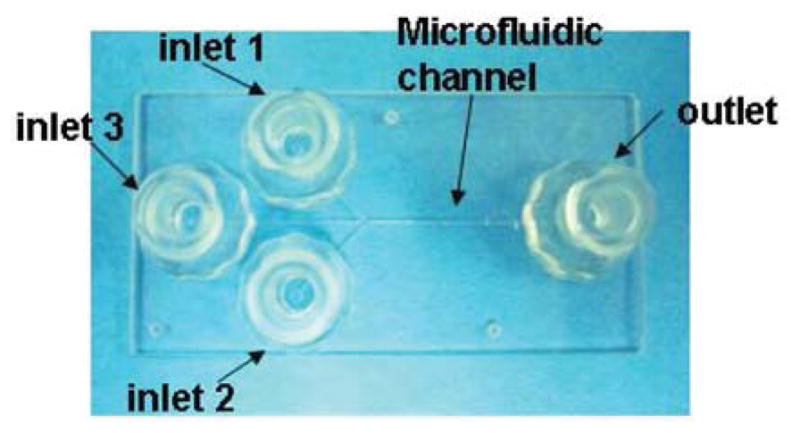
Photographs of the microfluidic chip with 3 inlets and 1 outlet.
The size of the liposomes was characterized by a NICOMP 370 Particle Size Analyzer (Particle Sizing Systems, Santa Barbara, CA, USA). The mean sizes of resultant liposomes are presented as volume-weighted average diameters.
Statistical analysis
The data of experimental groups with sonication and without sonication were subjected to a pair-wise t-test.
Results
The effect of flow rate ratio on microfluidic synthesis of liposomes in the absence and presence of sonication
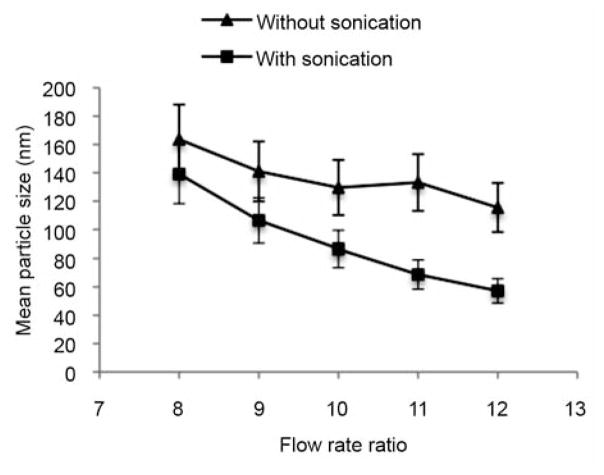
Figure 3 shows the effect of the flow rate ratio on the particle size of liposomes synthesized in microfluidics. The particle sizes of liposomes prepared with the assistance of sonication were significantly reduced compared to the liposomes prepared without sonication. When the flow rate ratio increased from 8 to 12 in the presence of sonication, the mean particle size of resultant liposomes remarkably decreased from ~150 nm to ~50 nm. A similar trend was also observed in the microfluidic synthesis without sonication. Thus, the particle size of liposomes synthesized in microfluidics decreases with an increase in flow rate ratio.
Figure 3.
Flow rate ratio versus particle size of liposomes synthesized in microfluidics with or without sonication.
The effect of microfluidic flow rates on particle size of resultant liposomes in the absence and presence of sonication
Figure 4 shows the relationship of particle sizes versus flow rate in the absence and presence of sonication. It is obvious that the particle size decreased when sonication was used. At the flow rate of 3.74 ml/min the particle size decreased by 50%. The smallest mean size of the liposomes (66.27 nm) was achieved at ethanolic lipid flow rate of 0.374 ml/min with sonication. As seen in Figure 4, the relation between the flow rate and particle size was not linear. When the flow rate of PBS solution was at 3.74 ml/min, the particle size was the smallest with and without sonication. As the flow rate increased from 2.78 ml/min to 3.74 ml/min, liposome size decreased from 168.3 nm to 66.27 nm. In contrast, as the flow rate increased from 3.74 ml/min to 5.88 ml/min, the mean liposome size increased, with a liposomal size range of 66.27 nm to 189.9 nm.
Figure 4.
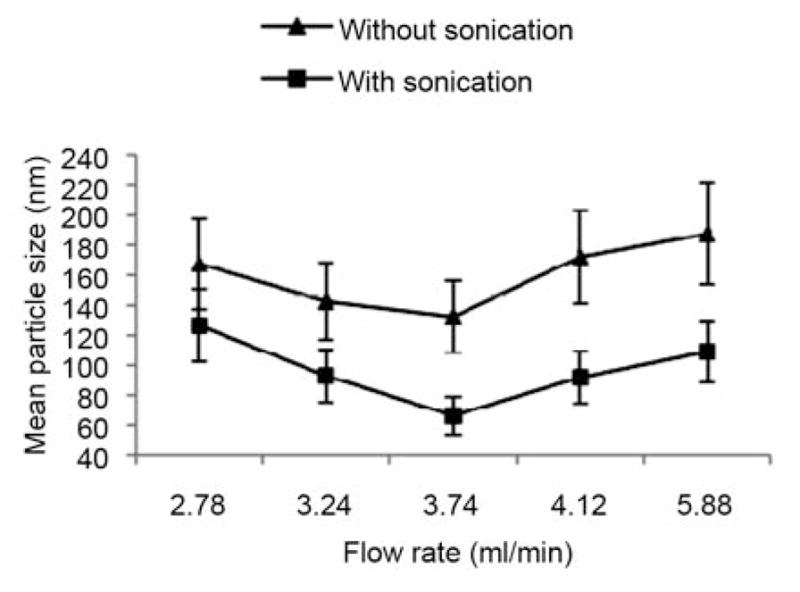
Flow rates versus particle size of liposomes prepared in microfluidics with or without sonication.
Discussion
Herein, we have demonstrated an ultrasound-enhanced microfluidic system for producing liposomal nanoparticles. In this system, the micro-dispensers were used as syringe pumps to control flow rates. To obtain sonication for liposome synthesis in microfludics, a commercially available microfluidic chip was placed in the water-bath of a bath sonicator. Our design efficiently combined the advantages of microfluidic and sonication technologies.
When sonication was applied to the formation of the liposomes in microfluidics, a significant decrease in particle size was observed (Figure 3 and 4). A pair-wise t-test was conducted between the data of liposome size with and without sonication, giving a p-value of 0.0038 for the data in Figure 3 and 0.0013 for these in Figure 4. This trend was preserved at all data points. The process of sonication may generate many cavitation events, which enhance the dispersion of lipid molecules and therefore reduce the liposome size.
The experimental study suggests that the flow rate ratio affects the particle size of the liposome formed in microfluidics (Figure 3). According to the theory of Lasic et al. (17), lipids dissolved in an organic solvent transform into intermediate bilayer phospholipid fragments (BPF). Thus, diffusion of water and alcohol during the liposome formation in microfluidics causes thermodynamic instabilities at the edges of BPFs, inducing bending and closing of the BPFs (13). Changes in the flow rate ratio result in variable stream widths of the focused solvent stream. As the ratio increases, the stream width of the organic solvent decreases, which provides a central region with higher organic solvent concentration. Based on the intermediate BPF assumption, one possible explanation for our data is that higher lipid-ethanol concentrations can stabilize the structure of BPFs, which causes more BPFs to link with each other, thus resulting in larger liposomes. On the contrary, the ethanol content in the thin solvent stream under a high flow rate is more diluted, limiting the formation of BPFs, thus resulting in smaller liposomes.
Conclusion
An ultrasound-enhanced microfluidic method was developed to produce liposomes. The mean size of liposomes can easily be controlled by this method. Compared to other techniques for liposome preparation, this novel method is simple and more efficient, and is especially suitable for lab-scale use. This can also easily be scaled up to industrial scale by using larger pumps and arrays of microfluidic chips. The combination of sonication and microfluidics may be applied to the production of other nanoparticles.
Acknowledgments
This work was supported by the National Institute of Health Grant R01 CA135243 and R21 CA131832.
References
- 1.Gregoriadis G. Liposome Technology. New York: Taylor & Francis/Informa; 2006. [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5(1):25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- 5.Pan XQ, Lee RJ. In vivo antitumor activity of folate receptor-targeted liposomal daunorubicin in a murine leukemia model. Anticancer Res. 2005;25(1A):343–346. [PubMed] [Google Scholar]
- 6.Forssen EA, Coulter DM, Proffitt RT. Selective in vivo localization of daunorubicin small unilamellar vesicles in solid tumors. Cancer Res. 1992;52(12):3255–3261. [PubMed] [Google Scholar]
- 7.Amselem S, Gabizon A, Barenholz Y. Optimization and upscaling of doxorubicin-containing liposomes for clinical use. J Pharm Sci. 1990;79(12):1045–1052. doi: 10.1002/jps.2600791202. [DOI] [PubMed] [Google Scholar]
- 8.Bhalerao SS, Harshal AR. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev Ind Pharm. 2003;29(4):451–467. doi: 10.1081/ddc-120018380. [DOI] [PubMed] [Google Scholar]
- 9.Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, Hope MJ, Scherrer P, Cullis PR. Stabilized plasmid-lipid particles: construction and characterization. Gene Therapy. 1999;6(2):271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 11.Szoka F, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Ann Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 12.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 13.Jahn A, Reiner JE, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Preparation of nanoparticles by continuous-flow microfluidics. J Nanopart Res. 2008;10(6):925–934. [Google Scholar]
- 14.Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23(11):6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- 15.Jahn A, Vreeland WN, Gaitan M, Locascio LE. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J Am Chem Soc. 2004;126(9):2674–2675. doi: 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan P, Guan J, Lu D, Wang PG, Lee LJ, Lee RJ. A facile microfluidic method for production of liposomes. Anticancer Res. 2008;28(2A):943–947. [PubMed] [Google Scholar]
- 17.Lasic DD. The mechanism of vesicle formation. Biochem J. 1988;256(1):1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]