Abstract
Context
The genetic contribution to liability for opioid dependence is well-established; identification of the responsible genes has proved challenging.
Objective
To examine association of 1430 candidate gene single-nucleotide polymorphisms (SNPs) with heroin dependence, reporting here only the 71 SNPs in the chromosome 11 gene cluster (NCAM1, TTC12, ANKK1, DRD2) that include the strongest observed associations.
Design
Case-control genetic association study that included two control groups (lacking an established optimal control group).
Setting
Semi-structured psychiatric interviews
Participants
Australian cases (N=1459) ascertained from opioid replacement therapy (ORT) clinics, neighborhood controls (N=531) ascertained from economically disadvantaged areas near opioid replacement therapy clinics, and unrelated Australian Twin Registry (ATR) controls (N=1495) not dependent on alcohol or illicit drugs selected from a twin and family sample.
Main Outcome Measure
Lifetime heroin dependence
Results
Comparison of cases with Australian Twin Registry controls found minimal evidence of association for all chromosome 11 cluster SNPs (p≥.01); a similar comparison to neighborhood controls revealed greater differences (p≥1.8 × 10−4). Comparing cases (N=1459) with the subgroup of neighborhood controls not dependent on illicit drugs (N=340), three SNPs were significantly associated (correcting for multiple testing): ANKK1 SNP rs877138 [most strongly associated; odds ratio 1.59; 95%CI (1.32–1.92); p=9.7 × 10−7], ANKK1 SNP rs4938013 and TTC12 SNP rs7130431. A similar pattern of association was observed when comparing illicit drug-dependent (N=191) and non-dependent (N=340) neighborhood controls, suggesting that liability likely extends to non-opioid illicit drug dependence. Aggregate heroin dependence risk associated with two SNPs, rs877138 and rs4492854 (located in NCAM1), varied more than 4-fold (p= 2.74 × 10−9 for the risk-associated linear trend).
Conclusions
Our results provide further evidence of association for chromosome 11 gene cluster SNPs with substance dependence, including extension of liability to illicit drug dependence. Our findings highlight the necessity of considering drug exposure history when selecting control groups for genetic investigations of illicit drug dependence.
Family and twin studies have established that genetic factors are responsible for a substantial component of liability for opioid dependence.1,2 However, identification of the genes associated with risk has proved challenging.3 Opioid dependence is a complex trait for which many genes each likely account for a modest portion of liability. Thus far, no consistently replicated findings have emerged from genetic association studies focusing on opioid dependence. Most genetic association studies have had inadequate sample size4–10 to detect modest effects, which, combined with publication bias for positive findings, increases the likelihood of type I error. Underpowered attempts at replication are also predisposed to type II error. Finally, inconsistency in findings across studies may result from differences in important aspects of experimental design.11,12 This article examines a central component of study design, control group selection, in the context of a genetic association study of heroin dependence.
A number of issues are germane to determining the most appropriate control group for a genetic association study of heroin dependence. Genetic and environmental factors contribute to liability for heroin use13,14 and, among users, for continued use and dependence.14 Investigations in population-based twin samples have attempted to estimate the degree to which these influences are shared across the stages of this process for more commonly used substances.15,16 However, owing to the low prevalence of opioid use, abuse, and dependence, these samples lack adequate power to conduct similar examinations.17 Genes whose influence on dependence is not shared with risk for substance use would not be expected to display effects in the absence of substance exposure. Ascertainment of exposed, non-dependent individuals is complicated by the relatively low prevalence of heroin use,18–21 the lack of an identified population enriched with users who have survived the period of risk for developing dependence, the stigma associated with the drug,18,19 and high rates of progression from use to dependence due to heroin’s extreme addictivity. Heroin dependent individuals are nearly always dependent on other drugs;22,23 however, twin studies have produced widely varying estimates of the degree to which genetic and environmental risks for opioid dependence are shared with other substances.1,2,14,24 Thus, it remains unclear whether those with a history of having used but not become dependent on other illicit drugs are an adequate proxy for heroin-exposed controls. Similarly, the potential for shared genetic risk with more common phenotypes may supersede the otherwise reasonable argument25 that the use of an unassessed comparison group in genetic association studies of low prevalence diseases results in only a mild reduction in power. These issues have left investigators conducting genetic association studies of heroin dependence without an obvious best choice for the most appropriate controls. In fact, they suggest that various alternative choices may be better suited depending on whether a gene’s effects are specific to heroin dependence or shared with dependence on other drugs.
This article examines the association of single-nucleotide polymorphisms (SNPs) with heroin dependence in the Comorbidity and Trauma Study (CATS).26,27 We compare a large Australian sample of heroin-dependent cases (N=1459) receiving opioid replacement therapy (ORT) in New South Wales, Australia with two control groups: (1) controls (N=531) ascertained from economically disadvantaged neighborhoods in close proximity to ORT clinics who had little or no recreational opioid use lifetime (includes individuals dependent on alcohol and non-opioid illicit drugs and non-dependent individuals with high rates of substance exposure); and (2) controls not meeting DSM-IV criteria for lifetime alcohol or illicit drug dependence (N=1495 unrelated individuals) selected from a sample of twins and family members (non-dependent with lower rates of drug exposure). The first stage of analyses27 considered only 136 SNPs in opioid receptor genes. The second stage includes the remaining 1294 SNPs (1430 of 1536 total SNPs were retained for analyses after data cleaning). We report herein the most significant findings of these analyses involving association with SNPs in the chromosome 11 cluster of genes (neural cell adhesion molecule 1 [NCAM1; GenBank NM_000615.6], tetratricopeptide repeat domain 12 (TTC12; GenBank NM_017868.3], ankyrin repeat and kinase domain containing 1 [ANKK1; GenBank NM_178510.1], and dopamine receptor D2 [DRD2; GenBank NM_000795.3]) for which a wealth of prior studies focusing on licit substance-related outcomes have reported similar associations. Our findings exemplify the importance of considering history of drug exposure in addition to drug dependence when selecting an appropriate control group.
METHODS
The Comorbidity and Trauma Study (CATS), a collaboration of investigators at Washington University School of Medicine (WUSM), Queensland Institute of Medical Research (QIMR), and National Drug and Alcohol Research Centre of the University of New South Wales (UNSW), is a case-control genetic association study of heroin dependence. Details of data collection have been previously reported.26,27 We again27 include data here from pilot study participants (25 cases and 25 neighborhood controls) for whom protocols were identical and assessment comparable.
Participants
Cases, recruited from ORT clinics in the greater Sydney, Australia, region, were required to be aged 18 years or older, to understand English, and to have participated in ORT for opioid dependence. Participants reporting recent suicidal intent or current psychosis were excluded. Individuals recruited from geographic areas in proximity to ORT clinics, termed neighborhood controls, were excluded for recreational opioid use more than five times lifetime (data were included from 23 controls who denied opioid use more than 5 times at screening but reported greater use with no dependence symptoms at interview); other inclusion and exclusion criteria were identical to those for cases. Institutional review board (IRB) approval was obtained from University of New South Wales (UNSW), Washington University School of Medicine (WUSM), Queensland Institute of Medical Research (QIMR), and area health service ethics committees governing participating clinics. Participants provided written informed consent and were reimbursed AU$50.00 for out-of-pocket expenses.
Concerns that comparisons with neighborhood controls might have inadequate power to detect effects on dependence that are shared with both drug exposure and dependence on other substances [e.g., 191 (36%) of the 531 neighborhood controls were dependent on a non-opioid illicit drug; Supplemental Table 1, http://digitalcommons.wustl.edu/psychiatry] prompted a decision to genotype a second, more broadly unaffected control group of unrelated individuals selected from the large Australian Twin Registry (ATR), including twins and family members [see28]. Inclusion criteria were institutional review board approval allowing genotyping of available DNA. Exclusion criteria were lifetime illicit drug or alcohol dependence at the prior interview. Non-nicotine-dependent individuals were preferentially selected; the prevalence of nicotine dependence (12.5%) in ATR controls is below that of the Australian population.
Assessment
Semi-structured psychiatric diagnostic interviews were completed in-person by cases and neighborhood controls; ATR controls had completed telephone interviews previously. Diagnostic sections on illicit drug and alcohol dependence were modified from the Semi-Structured Assessment for the Genetics of Alcoholism - Australia (SSAGA-OZ);29 the nicotine dependence section was modified from the Nicotine Addiction Genetics Study assessment.30 The assessments provided DSM-IV lifetime diagnoses of opioid, cannabis, sedative, stimulant, cocaine, and alcohol abuse and dependence, as well as nicotine dependence. Similar diagnoses were obtained for Comorbidity and Trauma Study (CATS) pilot project participants via the World Health Organization Composite International Diagnostic Interview.31
Marker Selection
The pair-wise option of Tagger32 (implemented in Haploview)33 with a threshold of r2≥0.8 for most genes and r2≥0.9 for high-priority candidates (e.g., opioid receptor genes) was used to select a custom set of 1536 SNPs. The set provided coverage of 72 candidate genes, 47 additional SNPs from prior reports, and 30 ancestry-informative markers (AIMs).
Genotyping
Genotyping was performed on an Illumina BeadStation using GoldenGate technology.34 Samples of DNA from CEPH trio 1334 obtained from the Coriell Cell Repository served as internal quality controls for clustering and reproducibility. Primary genotypic data analyses with Illumina BeadStudio software were followed by visual inspection and assessment of data quality and clustering.
Statistical Analyses
Data Cleaning
Details of data cleaning have been reported previously.27 In brief, SNPs were excluded owing to genotyping failure (23 SNPs), call rate less than 95% (9 SNPs), minor allele frequency less than 2% (47 SNPs), and Hardy-Weinberg equilibrium deviations (27 SNPs); 1430 SNPs were retained for analyses (Supplemental Table 2 shows the complete list). The mean call rate for 1294 non-opioid receptor SNPs remaining after data cleaning exceeded 99.9%. Samples of DNA from 1506 cases, 538 neighborhood controls, and 1500 ATR controls were genotyped. Data from samples were excluded owing to genotyping failure (1 ATR control), phenotypic-genotypic gender mismatch (1 case; 2 neighborhood controls), duplication due to participation in the project multiple times (29 cases; 3 neighborhood controls – phenotypic data from the most recent, non-pilot study interview were retained), and cryptic relatedness with identity by descent greater than 0.5 (17 cases, 4 neighborhood controls, and 4 ATR controls – individuals with the higher project identifier were excluded). The sample used for analyses consisted of 1459 cases, 531 neighborhood controls, and 1495 ATR controls.
Admixture
Cases, neighborhood controls, and ATR controls all consisted primarily of individuals of European ancestry. The former two groups also included some individuals of Asian ancestry. Principal components analyses (PCAs) were conducted using the smartpca program in the Eigensoft version 3.0 statistical software package35 to determine the appropriate admixture correction for each analysis. The kill r2 setting of 0.8 was used to remove some SNPs in high linkage disequilibrium (LD) with others in the panel with data from 1123 of the 1430 SNPs retained for PCA. Because ancestry-informative markers (AIMs) were included in the PCA, Tracy-Widom statistics could not be used to determine the number of principal components. Separate PCA indicated that comparisons of cases to neighborhood controls required no inclusion of principal components (PCs) as covariates, while that comparing cases with ATR controls found at least a trend-level significance for 4 principal components (PCs) (for details and PC plots, see the article by Nelson et al 27). For analyses that divided the neighborhood controls on the basis of lifetime licit and illicit drug dependence, a separate PCA run for each comparison found in each instance a single significant PC with the following P values: cases versus neighborhood controls not dependent on nicotine, alcohol, and illicit drugs, P = .06; cases versus neighborhood controls not dependent on alcohol and illicit drugs, P = .06; cases versus neighborhood controls not dependent on illicit drugs, P = .02; cases versus illicit drug-dependent neighborhood controls, P = .02; illicit drug-dependent versus non-dependent neighborhood controls, P = .001. Each of these analyses included a single PC as a covariate to control for admixture.
Association
Logistic regression analyses performed in PLINK software,36 which included smartpca-derived PCs in models to control for admixture, examined the association between the log-additive effects of minor allele dosage and case status. We separately compared 1459 heroin-dependent cases (888 male, 571 female; mean [SD] age, 36.5 [8.6] years) with 531 neighborhood controls (235 male, 296 female; mean [SD] age, 34.7 [10.5] years) and 1495 ATR controls (972 male, 523 female; mean [SD] age, 45.0 [9.5] years). Because of uncertainty regarding the most appropriate control group for the current investigation and given within-group differences in the neighborhood controls observed in stage 1 analyses,27 we compared heroin-dependent cases with subgroups of neighborhood controls who were not dependent on the following: (1) any illicit drugs (N=340); (2) any illicit drugs or alcohol (N=275); and (3) any illicit drugs, alcohol, or nicotine (N=207). We also conducted a within-group comparison of neighborhood controls who were and were not illicit drug dependent. A conservative Bonferroni correction for multiple testing yielded a revised significance threshold of P = 3.9 × 10−6 (i.e., .05 /1430 SNPs/ 9 phenotypic comparisons: [1] cases vs. neighborhood controls; [2] cases vs. ATR controls; cases vs. neighborhood controls not dependent on [3] illicit drugs, [4] illicit drugs or alcohol, and [5] illicit drugs, alcohol, or nicotine; cases vs. neighborhood controls dependent on [6] illicit drugs, [7] illicit drugs or alcohol, and [8] illicit drugs, alcohol, or nicotine; and [9] neighborhood controls dependent on illicit drugs versus neighborhood controls not dependent on illicit drugs). We controlled for the allelic dosage of the most strongly associated SNP to examine whether a single signal adequately explained all observed chromosome 11 gene cluster associations. Consistent with prior reports that focused on haplotypes spanning these genes, we conducted analyses using SAS version 9.2 37 statistical software (SAS Institute, Inc.) to characterize risk (i.e., additive, dominant, or recessive) associated with each of the two SNPs for whom independent signals were found. To estimate their effects in tandem, we coded a risk level variable that was a sum of their effects. In doing so, we verified that risk associated with the alternative routes of obtaining the same risk level (e.g., one copy of the rs877138 minor allele and the rs4492854 major allele versus two copies of the former and none of the latter) did not differ significantly.
RESULTS
The comparison of cases to ATR controls found p values greater than .01 for all SNPs in the chromosome 11 gene cluster [select SNPs (i.e., primarily those more strongly associated but also including rs1800497, the Taq1A polymorphism) are shown in Table 1]. The similar comparison of cases to neighborhood controls revealed greater intergroup differences; however, the minimum p value (1.8 × 10−4 for rs7130431) was not significant with adjustment for multiple testing (comparisons for additional genotyped SNPs are shown in Supplemental Table 3; unadjusted (for ethnicity) minor allelic frequencies (MAFs) for select and additional SNPs are shown in Supplemental Table 4 and Supplemental Table 5, respectively).
Table 1.
Comparison of select SNPs in cases (N=1459) versus Australian Twin Registry controls (N=1495) and neighborhood controls (N=531) using additive models (additive models - p values are shown)
| Gene | SNP | SNP Location* | Minor allele |
P Value
|
|
|---|---|---|---|---|---|
| Heroin-dependent cases vs ATR controls1 | Heroin-dependent cases vs neighborhood controls | ||||
| NCAM1 | rs4492854 | 112488744 | G | .18 | .0073 |
| NCAM1 | rs11214546 | 112611738 | A | .054 | .31 |
| NCAM1 | rs587761 | 112615990 | A | .55 | .0046 |
| NCAM1 | rs2186798 | 112633271 | C | .045 | .056 |
|
| |||||
| TTC12 | rs2303380 | 112705919 | G | .19 | .0046 |
| TTC12 | rs10891536 | 112714638 | G | .027 | .010 |
| TTC12 | rs4938009 | 112736138 | A | .029 | .0046 |
| TTC12 | rs719804 | 112739985 | G | .085 | .14 |
| TTC12 | rs7130431 | 112743433 | A | .12 | .00018 |
| TTC12 | rs12804573 | 112746936 | G | .040 | .0014 |
|
| |||||
| ANKK1 | rs877137 | 112761540 | A | .12 | .00083 |
| ANKK1 | rs877138 | 112761718 | G | .037 | .00029 |
| ANKK1 | rs12360992 | 112768110 | C | .25 | .0014 |
| ANKK1 | rs4938013 | 112769680 | A | .069 | .00030 |
| ANKK1 | rs2734849 | 112775370 | G | .30 | .0024 |
| ANKK1 | rs2734848 | 112775584 | G | .016 | .0081 |
| ANKK1 | rs1800497 | 112776038 | T | .93 | .12 |
|
| |||||
| DRD2 | rs2234689 | 112783693 | G | .029 | .0066 |
| DRD2 | rs1554929 | 112783974 | A | .34 | .0010 |
| DRD2 | rs2440390 | 112792088 | A | .17 | .0091 |
| DRD2 | rs1076563 | 112801119 | A | .11 | .0034 |
| DRD2 | rs7125415 | 112815891 | A | .011 | .22 |
Abbreviations: ATR, Australian Twin Registry; SNP, single-nucleotide polymorphism
From NCBI build 37.2 (National Center for Biotechnology Information).
Four principal components (PCs) were included in model for admixture correction.
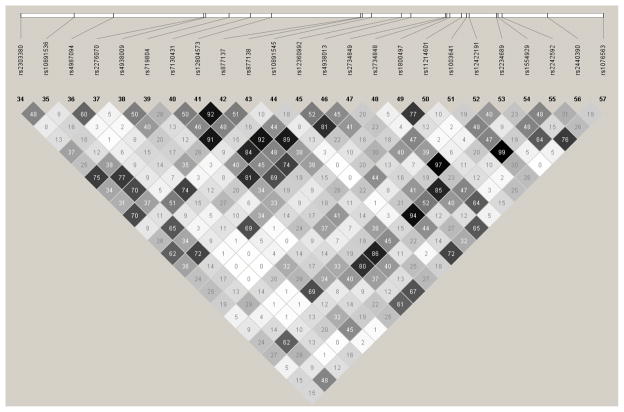
We next examined the effects of dividing neighborhood controls into subgroups based on their lifetime history of illicit and licit drug dependence (Supplemental Table 1 shows a hierarchical breakdown). We found that the association signal became stronger as the criterion for exclusion of individuals with lifetime drug dependence was more narrowly defined (Supplemental Table 6). In the comparison between heroin-dependent cases and neighborhood controls not dependent on any illicit drugs (Table 2), significant association was found for three SNPs (rs877138, rs4938013, and rs7130431) in high LD (Figure) with rs877138, the most strongly associated SNP [odds ratio [OR] = 1.59; 95%CI, (1.32–1.92); p = 9.7 × 10−7]. In contrast, for the comparison between cases and illicit drug-dependent neighborhood controls, only a single SNP reached even nominal significance. A within-neighborhood control group comparison of individuals with and without a lifetime history of illicit drug dependence found that rs877138 was again the most highly associated SNP (p= 8.0 × 10−4), indicating that liability attributable to this variant likely extends to risk for dependence on other illicit drugs.
Table 2.
Comparison (p values are shown) of single-nucleotide polymorphisms between groups using additive models (with one PC included in each for admixture correction)
| Gene | SNP | P Valuea
|
|||
|---|---|---|---|---|---|
| Heroin dependent cases (N=1459) vs neighborhood controls not dependent on illicit drug (N=340) | Heroin dependent cases (N=1459) vs illicit drug dependent neighborhood controls (N=191) | Within-neighborhood control comparison of those not dependent on illicit drugs (N=340) to those dependent (N=191) | Linkage disequilibrium (r2) of SNP with rs877138 | ||
|
| |||||
| NCAM1 | rs4492854 | .0024 | .59 | .13 | .00 |
| NCAM1 | rs11214546 | .33 | .0018 | .0027 | .00 |
| NCAM1 | rs587761 | .0097 | .15 | .59 | .02 |
| NCAM1 | rs2186798 | .19 | .16 | .81 | .00 |
|
| |||||
| TTC12 | rs2303380 | .000035 | .56 | .0027 | .70 |
| TTC12 | rs10891536 | .00050 | .88 | .028 | .37 |
| TTC12 | rs4938009 | .00050 | .89 | .022 | .35 |
| TTC12 | rs719804 | .016 | .47 | .022 | .26 |
| TTC12 | rs7130431 | .0000028 b | .78 | .0051 | .91 |
| TTC12 | rs12804573 | .000070 | .53 | .037 | .48 |
|
| |||||
| ANKK1 | rs877137 | .000024 | .56 | .023 | .51 |
| ANKK1 | rs877138 | .00000097 b | .83 | .00080 | 1.0 |
| ANKK1 | rs12360992 | .000095 | .45 | .053 | .44 |
| ANKK1 | rs4938013 | .0000013 b | .88 | .00090 | .89 |
| ANKK1 | rs2734849 | .00016 | .48 | .060 | .38 |
| ANKK1 | rs2734848 | .00083 | .93 | .023 | .38 |
| ANKK1 | rs1800497 | .047 | .92 | .22 | .15 |
|
| |||||
| DRD2 | rs2234689 | .00062 | .93 | .023 | .38 |
| DRD2 | rs1554929 | .000066 | .39 | .060 | .45 |
| DRD2 | rs2440390 | .00042 | .83 | .0083 | .25 |
| DRD2 | rs1076563 | .00020 | .57 | .051 | .27 |
| DRD2 | rs7125415 | .12 | .77 | .32 | .05 |
Abbreviation: LD, Linkage disequilibrium; PC, principal component; SNP, single-nucleotide polymorphisms
One principal component (PC) included in each for admixture correction.
Bolded values are significant with correction for multiple testing.
Figure.
Linkage disequilibrium analysis of select TTC12, ANKK1, and DRD2 gene single-nucleotide polymorphisms (r2 values are shown).
To determine whether between-group differences in drug exposure may have contributed to the lack of association found with ATR controls, we compared ATR controls to neighborhood controls not dependent on illicit drugs. We found significantly greater lifetime use for all examined illicit drug categories in non-dependent neighborhood controls; differences were most pronounced for cocaine and stimulants (see Table 3). Extending the comparison of non-dependent neighborhood controls vs. ATR controls to use more than 11 times lifetime (not shown in Table 3), a similar pattern of between-group differences was observed: stimulants, 18.2% vs. 1.4%, respectively (OR=15.64; 95%CI, 9.38–26.08); any non-cannabis illicit drug, 19.7% vs. 3.0%, respectively (OR=7.90; 95%CI, 5.30–11.78); and any illicit drug, 45.3% vs. 15.7%, respectively (OR=4.46; 95%CI, 3.45–5.76). In an assessment not used for ATR controls, 35.5% of non-dependent neighborhood controls reported having seen someone use heroin and 28.4% reported have been offered heroin. These results provide strong evidence that the neighborhood controls not dependent on illicit drugs had substantially greater levels of lifetime drug use than the ATR controls. In addition, a surprisingly large proportion of these individuals had ready access to heroin.
Table 3.
Comparison of Australian Twin Registry controls (N=1495) with neighborhood controls not dependent on illicit drugs (N=340)
| Control group | Prevalence of illicit drug use by control group | ||||||
|---|---|---|---|---|---|---|---|
| Cannabis | Stimulant | Opioid | Sedative | Cocaine | Any illicit | Any non-cannabis | |
| ATR | 44.4 % | 6.8 % | 7.4 % | 3.5 % | 2.3 % | 49.8 % | 15.0 % |
| Nondependent Neighborhood | 71.1 % | 44.4 % | 10.6 % | 12.9 % | 25.9 % | 79.1 % | 50.6 % |
| Odds-ratio (95% CI) | 4.22 (3.21–5.54) | 10.91 (8.14–14.63) | 1.49 (1.00–2.22) | 4.12 (2.71–6.28) | 14.57 (9.63–22.03) | 3.81 (2.88–5.05) | 5.78 (4.48–7.46) |
Abbreviations: ATR, Australian Twin Registry; OR, odds ratio
Inclusion of rs877138 as a covariate in the comparison of heroin-dependent cases to neighborhood controls not dependent on illicit drugs yielded suggestive evidence (p<2.5 × 10−3) of a second signal involving rs4492854, an NCAM1 SNP. Further analyses supported a dominant model for liability associated with this SNP’s major allele (OR=1.65; 95%CI 1.26–2.17). An examination of aggregate risk associated with these two SNPs [i.e., the number of rs877138 minor alleles (additive) plus the presence of a copy of the rs4492854 major allele] in data from cases and non-dependent neighborhood controls found that risk varied more than 4-fold on the basis of these two SNPs (Table 4). The proportion of heroin-dependent individuals (shown as the column percentage) was observed to increase with this measure of aggregate risk; the p value for the risk-associated linear trend is 2.74 × 10−9.
Table 4.
Comparison of cases (N=1459) with neighborhood controls not dependent on illicit drugs (N=340)
| Group | Risk level indicates number of rs877138 minor alleles plus presence of a rs4492854 major allele | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Non-dependent neighborhood controls* | 47 (32.4%) | 168 (21.8%) | 107 (15.2%) | 18 (10.1%) |
| Heroin dependent* | 98 (67.6%) | 603 (78.2%) | 598 (84.8%) | 160 (89.9%) |
| Odds-ratio (95% CI) | 1.00 --- |
1.69 (1.15–2.49) | 2.62 (1.75–3.93) | 4.30 (2.36–7.84) |
Mantel-Haenszel chi-square = 35.40 (df =1); p=2.74 × 10−9 (linear trend);
Number of individuals are shown with column percentage in parentheses
COMMENT
Our data provide strong evidence that ANKK1 and TTC12 SNPs are associated with dependence on heroin and other illicit drugs. These results are an important extension of previously reported associations that largely involved nicotine- or alcohol-related outcomes.38–48 Our findings emphasize the necessity of considering both drug exposure and dependence history when selecting a control group for genetic association studies focusing on drug dependence.
Depending on the control group to which we compared heroin-dependent cases, the magnitude of observed associations varied markedly. The comparison of cases with ATR controls found scant evidence of association. Although p values exceeded 0.01 for all SNPs, rs877138 (p=.037) was among those nominally associated. Greater evidence of association was observed in the comparison of cases to neighborhood controls; however, no p value for any SNP was within an order of magnitude of the significance level required to correct for multiple testing. Our analyses that divided neighborhood controls into subgroups based on lifetime history of licit and illicit drug dependence found that the association signal became stronger as the exclusion was defined more narrowly to exclude only individuals with lifetime history of illicit drug dependence.
Three consistent findings emerged with this division. First, the comparison of heroin-dependent cases to non-dependent neighborhood controls found three SNPs with p values significant after correction for multiple testing. Second, the comparison of cases to illicit drug-dependent neighborhood controls was remarkable for the complete lack of even nominally-significant differences for TTC12 and ANKK1 SNPs. Third, the comparison of non-dependent to illicit drug-dependent neighborhood controls found a pattern of association nearly identical to the comparison of the former with cases, with the smaller size of the latter sample limiting overall power. A post-hoc comparison of non-dependent neighborhood controls with a combined group of heroin-dependent cases and illicit drug dependent controls found significance improved incrementally for the association with rs877138 (p=6.4 × 10−7). Our results provide strong evidence that a block of ANKK1 and TTC12 SNPs in high LD is associated with heroin and other illicit drug dependence. The large difference in the strength of association observed in the comparison with non-dependent neighborhood controls (individuals with high exposure to illicit drugs, either via use or from residing in environments with widespread drug availability) versus ATR controls (individuals not dependent on alcohol or illicit drugs, with significantly lower illicit drug exposure) strongly suggests that this liability likely represents risk of dependence contingent on drug exposure. Our findings raise an intriguing possibility that non-dependent highly substance-exposed controls might be particularly informative for attempts to identify polymorphisms associated with drug dependence liability.
The three SNPs (rs877138, rs4938013, and rs7130431) for which we observed significant association are located in a region spanning ANKK1 and TTC12. High LD between SNPs in these genes prevents determination, without additional sequencing, of the gene primarily contributing to liability. For example, rs877138 is located in the 5′ flanking region 2005 base pairs upstream from the first exon of ANKK1 but is in complete LD with several intronic TTC12 SNPs. Both rs4938013, an exonic ANKK1 SNP resulting in a synonymous substitution, and rs7130431, an intronic TTC12 SNP, are in high LD with rs877138 (respective r2 values of .898 and .912), consistent with a single association signal. Although nominally significant association extends to the Taq1A polymorphism (rs1800497) and DRD2 SNPs, analyses that controlled for allelic dose of rs877138 found no evidence of an independent signal involving this functional polymorphism or any other ANKK1, TTC12, or DRD2 SNP. Since prior studies6,11,49–53 that reported an association of rs1800497 with opioid dependence genotyped few or no additional ANKK1 SNPs, the signal they observed may have resulted from similar LD. The only association not attributable to LD in our sample involved rs4492854, an NCAM1 SNP, for which risk was best explained by a dominant model. Owing to the complete lack of LD between rs877138 and rs4492854, we instead examined risk associated with these SNPs in tandem rather than the haplotype-based analyses used in prior investigations38,40,42,44 and found greater than 4-fold variation in risk (OR = 4.30; 95%CI, 2.36–7.84) across combinations of these two SNPs.
Our results are broadly consistent with a literature38–47 in which, led by the efforts of Gelernter, Kranzler, and colleagues, attention has shifted from DRD2 to ANKK1 and TTC12 as the genes in this region most strongly associated with substance dependence. They initially conducted a family-based association study38 of tobacco dependence in a largely polysubstance-dependent sample drawn from sets of European American (EA) and African American (AA) affected (cocaine or opioid dependent) sibling pairs. The strongest association in pooled African American and European American data included ANKK1 and TTC12 SNPs in a moderate to high LD block that overlaps our main findings [e.g., their top hits included rs4938012 (p=8 × 10−6) and rs4938013 (p=3 × 10−5)]. Of note, DSM-IV nicotine dependence is more highly correlated with other DSM-IV substance dependence diagnoses than is Fagerström score (for which they found much weaker association). Further analyses implicated a 4-SNP haplotype spanning TTC12 and ANKK1. They later focused40 on alcohol dependence in two European American samples, finding only nominally significant associations for individual SNPs; haplotype-based analyses observed significant associations centering on TTC12, NCAM1, and ANKK1. A subsequent examination42 found that risk associated with most of these haplotypes was for comorbid alcohol and illicit drug dependence rather than alcohol dependence alone. In Collaborative Study on the Genetics of Alcoholism (COGA) family data,39 nominal associations for alcohol-related phenotypes were found in an overlapping ANKK1 region (including both rs877138 and rs4938012). A Collaborative Study on the Genetics of Alcoholism genome-wide association study (COGA GWAS)45 found that rs10502172, a more upstream TTC12 SNP, was nominally associated with alcohol dependence (p=7.0 × 10−4). A Finnish population-based birth cohort study46 reported strong association of SNPs within a haplotype block stretching from TTC12 to DRD2 (including rs877138; p=.001) with smoking at age 14 years; rs10502172 (p=9.1 × 10−6) was most highly associated. These SNPs were more weakly associated with smoking at age 31 years. Overall, our association signal extensively overlaps those of reports focusing on nicotine- and alcohol-related phenotypes;38,39,46 the weaker, independent association with the NCAM1 SNP rs4492854 is consistent with prior reports.40,42 Our investigation provides strong evidence of association of individual ANKK1 and TTC12 SNPs with heroin and other illicit drug dependence, replicating findings in a prior report.42 Despite these converging findings, considerable variation in the intensity and location of association was found across reports.38–40,42–46 Differences in examined phenotype likely contributed to this variance because alcohol- and nicotine-related outcomes differ but are significantly correlated.
The protein encoded by ANKK1, a member of the receptor interacting protein serine/threonine kinases, is believed to play a role in signal transduction that includes activation of transcription factors in response to environmental factors.54 ANKK1 is reportedly expressed in radial glia during development and in astrocytes within the adult brain.55 ANKK1 expression is upregulated by administration of apomorphine, a dopamine agonist, and is temporally associated with DRD2 expression in developing mice.54–56 These findings, coupled with the genes’ proximity, have led investigators to posit that the protein encoded by ANKK1 may play an important role in the alterations in dopaminergic signaling following drug exposure central to the addiction process.54 TTC12, also expressed in the brain, contains a tetratricopeptide repeat structure known to facilitate protein-protein binding and for which effects on steroid hormone receptors have been reported.57 Further investigation will be necessary to characterize more clearly the roles these genes may play in the pathophysiology of illicit drug dependence.
Several limitations must be considered when interpreting our findings. Because our cases were ascertained entirely from New South Wales ORT clinics, generalizability to samples of individuals not currently in treatment or from other areas will need to be demonstrated. Our primary findings emerged after dividing neighborhood controls into subsamples based on history of lifetime drug dependence; thus, replication in other similarly ascertained samples would provide important confirmation. It is possible that population stratification could have contributed to our findings. Cases and control groups primarily included individuals of European ancestry. Although we did observe ethnicity differences, the most substantial were between cases and ATR controls. We conducted PCA prior to each comparison and, when appropriate, included PCs to control for population stratification. Post hoc comparisons of cases to non-dependent neighborhood controls that included four PCs as covariates produced similar results. Another correlated phenotype (e.g., a component of temperament) could be responsible for the current association findings and those previously reported. While this possibility is difficult to exclude, examinations that have incorporated aspects of temperament have produced mixed results.41,48 Despite the considerably larger size of our sample (more than three-fold larger than most prior association studies of heroin dependence), it is possible that we may have failed to detect significant associations because of limited power (i.e. type II error). Similarly, the smaller size of the neighborhood control subsamples either could be limiting significant differences found in comparisons with cases or between subgroups or could be resulting in spurious associations (i.e., type I error). Finally, the reductions in sample size produced by the more stringent exclusion criteria (i.e., including alcohol or tobacco dependence along with illicit drug dependence) used for the neighborhood control subgroups may have contributed to the weaker observed associations by decreasing power.
In summary, we provide evidence that ANKK1 and TTC12 SNPs are strongly associated with substance dependence, substantially overlapping findings from other reports.38–47 Our focus on illicit drug dependence is an important extension of scope beyond that of prior studies. Additional investigations (e.g., deep sequencing) can characterize more definitively the polymorphisms most highly associated with heroin and other illicit drug dependence and determine the gene responsible for the observed association. Finally, our findings highlight the importance of considering substance exposure history when selecting the most appropriate control group for genetic investigations of substance dependence and raise an intriguing possibility that non-dependent, highly substance-exposed controls might prove particularly informative.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants R01 DA017305 (Dr. Nelson) and R01 DA23668 (Dr. Agrawal) from the National Institute on Drug Abuse and by the National Drug and Alcohol Research Centre and the Australian National Health and Medical Research Council (Dr. Degenhardt).
Footnotes
Author Contributions: Dr. Nelson had full access to all data in the study, performed the statistical analyses, and takes responsibility for the integrity of the data and accuracy of data analysis.
Conflict of Interest Disclosures: None of the authors have a financial or personal conflict of interest.
Additional Contributions: The authors would like to thank Anthony Caracella, BSc, for his work in sample receipt and preparation; Megan Campbell, BAppSC, for project coordination; Lisa Bowdler, BAppSc,and Sara Smith, BBiomedSci, for their efforts in sample genotyping; and Michelle Torok, MSocSc, Elizabeth Conroy, PhD, Elizabeth Moore, PhD, Caitlin McCue, BHSc and Cherie Kam, RN, BAppSC for assistance with data collection.
References
- 1.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 2.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55(11):967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103(7):1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Xu K, Deng H, Cai G, Liu J, Liu X, Wang R, Xiang X, Zhao J, Murray RM, Sham PC, Collier DA. Association analyisis of the dopamine D4 gene exon III VNTR and heroin abuse in Chinese subjects. Mol Psychiatry. 1997;2(5):413–416. doi: 10.1038/sj.mp.4000310. [DOI] [PubMed] [Google Scholar]
- 5.Duaux E, Gorwood P, Griffon N, Bourdel MC, Sautel F, Sokoloff P, Schwartz JC, Ades J, Lôo H, Poirier MF. Homozygosity at the dopamine D3 receptor gene is associated with opiate dependence. Mol Psychiatry. 1998;3(4):333–336. doi: 10.1038/sj.mp.4000409. [DOI] [PubMed] [Google Scholar]
- 6.Lawford BR, Young R, Nobel EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D2 dopamine receptor A1 allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96(5):592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Zhu ZH, Liu X, Hu X, Zhao J, Sham PC, Collier DA. Association analysis of polymorphisms in the DRD4 gene and heroin abuse in Chinese subjects. Am J Med Genet. 2000;96(5):616–621. doi: 10.1002/1096-8628(20001009)96:5<616::aid-ajmg6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbergh DJ, Rodriguez LA, Hivert E, Schiller JH, Villareal G, Pugh EW, Lachman H, Uhl GR. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: no interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am J Med Genet. 2000;96(5):678–683. doi: 10.1002/1096-8628(20001009)96:5<678::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Szeto CYK, Tang NLS, Lee DTS, Stadlin A. Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport. 2001;12(6):1103–1106. doi: 10.1097/00001756-200105080-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ray R, Doyle GA, Crowley JJ, Buono RJ, Oslin DW, Patkar AA, Mannelli P, DeMaria PA, Jr, O’Brien CP, Berrettini WH. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr Genet. 2005;15(4):295–298. doi: 10.1097/00041444-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Perez de los Cobos J, Baiget M, Trujols J, Sinol N, Volpini V, Banuls E, Calafell F, Luquero E, del Rio E, Alvarez E. Allelic and genotypic associations of DRD2 TaqI A polymorphism with heroin dependence in Spanish subjects: a case control study. Behav Brain Funct. 2007;3:25. doi: 10.1186/1744-9081-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7(7):720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am J Med Genet. 2000;96(5):665–670. [PubMed] [Google Scholar]
- 14.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. Br J Psychiatry. 1999;175:351–356. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Neale MC, Jacobson KC, Prescott CA, Kendler KS. Illicit drug use and abuse/dependence: modeling of two-stage variables using the CCC approach. Addict Behav. 2005;30(5):1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 18.Hall WD, Ross JE, Lynskey MT, Law MG, Degenhardt LJ. How many dependent heroin users are there in Australia? Med J Aust. 2000;173(10):528–531. doi: 10.5694/j.1326-5377.2000.tb139321.x. [DOI] [PubMed] [Google Scholar]
- 19.Law MG, Lynskey M, Ross J, Hall W. Back-projection estimates of the number of dependent heroin users in Australia. Addiction. 2001;96(3):433–443. doi: 10.1046/j.1360-0443.2001.9634337.x. [DOI] [PubMed] [Google Scholar]
- 20.Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, Lynskey M, Wiessing L, Mora ME, Clark N, Thomas J, Briegleb C, McLaren J GBD illicit drug use writing group. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug Alcohol Depend. 2011;117(2–3):85–101. doi: 10.1016/j.drugalcdep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Degenhardt L, Hall W. The extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 22.Shand FL, Degenhardt L, Slade T, Nelson EC. Sex differences amongst dependent heroin users: histories, clinical characteristics and predictors of other substance dependence. Addict Behav. 2011;36(1–2):27–36. doi: 10.1016/j.addbeh.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shand FL, Slade T, Degenhardt L, Baillie A, Nelson EC. Opioid dependence latent structure: two classes with differing severity? Addiction. 2011;106(3):590–598. doi: 10.1111/j.1360-0443.2010.03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendler KS, Karkowski L, Prescott CA. Hallucinogen, opiate, sedative and stimulant use and abuse in a population-based sample of female twins. Acta Psychiatr Scand. 1999;99(5):368–376. doi: 10.1111/j.1600-0447.1999.tb07243.x. [DOI] [PubMed] [Google Scholar]
- 25.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shand FL, Degenhardt L, Nelson EC, Mattick RP. Predictors of social anxiety in an opioid dependent sample and a control sample. J Anxiety Disord. 2010;24(1):49–54. doi: 10.1016/j.janxdis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL, Henders AK, Wallace L, Todorov AA, Schrage AJ, Madden PAF, Degenhardt L, Martin NG, Montgomery GW. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol. doi: 10.1111/j.1369-1600.2012.00445.x. published online 2012 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansell NK, Agrawal A, Whitfield JB, Morley KI, Gordon SD, Lind PA, Pergadia ML, Montgomery GW, Madden PAF, Todd RD, Heath AC, Martin NG. Can we identify genes for alcohol consumption in samples ascertained for heterogeneous purposes? Alcohol Clin Exp Res. 2009;33(4):729–739. doi: 10.1111/j.1530-0277.2008.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 30.Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA, Maunu H, Heikkila K, Morley KI, Rice JP, Todd RD, Kaprio J, Peltonen L, Martin NG, Goate AM, Madden PAF. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet. 2007;80(5):856–866. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33(2):80–88. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- 32.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37(11):1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 33.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 34.Peters K, Wiltshire S, Henders AK, Dragovic M, Badcock JC, Chandler D, Howell S, Ellis C, Bouwer S, Montgomery GW, Palmer LJ, Kalaydjieva L, Jablensky A. Comprehensive analysis of tagging sequence variants in DTNBP1 shows no association with schizophrenia or with its composite neurocognitive endophenotypes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1159–1166. doi: 10.1002/ajmg.b.30741. [DOI] [PubMed] [Google Scholar]
- 35.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAS Institute, Inc. . The SAS System for Windows, Version 9.2. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 38.Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, Kranzler HR, Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15(24):3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- 39.Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, Budde JP, Goldstein EL, Kaplan D, Edenberg HJ, Nurnberger J, Jr, Hesselbrock V, Schuckit M, Kuperman S, Tischfield J, Porjesz B, Begleiter H, Bierut LJ, Goate A. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin Exp Res. 2007;31(10):1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16(23):2844–2853. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
- 41.Ponce G, Hoenicka J, Jiménez-Arriero MA, Rodríguez-Jiménez R, Aragüés M, Martín-Suñé N, Huertas E, Palomo T. DRD2 and ANKK1 genotype in alcohol-dependent patients with psychopathic traits: association and interaction study. Br J Psychiatry. 2008;193(2):121–125. doi: 10.1192/bjp.bp.107.041582. [DOI] [PubMed] [Google Scholar]
- 42.Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcohol Clin Exp Res. 2008;32(12):2117–2127. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34(2):319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 44.David SP, Mezuk B, Zandi PP, Strong D, Anthony JC, Niaura R, Uhl GR, Eaton WW. Sex differences in TTC12/ANKK1 haplotype associations with daily tobacco smoking in Black and White Americans. Nicotine Tob Res. 2010;12(3):251–262. doi: 10.1093/ntr/ntp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, Todd Webb B, Tischfield JA, Porjesz B, Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34(5):840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ducci F, Kaakinen M, Pouta A, Hartikainen AL, Veijola J, Isohanni M, Charoen P, Coin L, Hoggart C, Ekelund J, Peltonen L, Freimer N, Elliott P, Schumann G, Järvelin MR. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol Psychiatry. 2011;69(7):650–660. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelernter J. Developmental perspective on the role of genes in smoking risk. Biol Psychiatry. 2011;69(7):616–617. doi: 10.1016/j.biopsych.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasiakogia-Worlley K, McQuillin A, Lydall GJ, Patel S, Kottalgi G, Gunwardena P, Cherian R, Rao H, Hillman A, Gobikrishnan N, Douglas E, Qureshi SY, Jauhar S, Ball D, O’Kane A, Owens L, Dedman A, Sharp SI, Kandaswamy R, Guerrini I, Thomson AD, Smith I, Dar K, Morgan MY, Gurling HM. Lack of allelic association between markers at the DRD2 and ANKK1 gene loci with the alcohol-dependence syndrome and criminal activity. Psychiatr Genet. 2011;21(6):323–324. doi: 10.1097/YPG.0b013e3283458a68. [DOI] [PubMed] [Google Scholar]
- 49.Shahmoradgoli Najafabadi M, Ohadi M, Joghataie MT, Valaie F, Riazalhosseini Y, Mostafavi H, Mohammadbeigi F, Najmabadi H. Association between the DRD2 A1 allele and opium addiction in the Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2005;134B(1):39–41. doi: 10.1002/ajmg.b.30117. [DOI] [PubMed] [Google Scholar]
- 50.Barratt DT, Coller JK, Somogyi AA. Association between the DRD2 A1 allele and response to methadone and buprenorphine maintenance treatments. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(4):323–331. doi: 10.1002/ajmg.b.30319. [DOI] [PubMed] [Google Scholar]
- 51.Doehring A, Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Harder S, Lötsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19(6):407–414. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- 52.Hou QF, Li SB. Potential association of DRD2 and DAT1 genetic variation with heroin dependence. Neurosci Lett. 2009;464(2):127–130. doi: 10.1016/j.neulet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 53.López-Castromán J, Vaquero-Lorenzo C, Perez-Rodriguez MM, Diaz-Hernandez M, Fernandez-Piqueras J, Saiz-Ruiz J, Baca-Garcia E. Gender effect on association between DRD2 polymorphism and substance dependence in a Spanish sample. Drug Alcohol Depend. 2009;101(3):210–212. doi: 10.1016/j.drugalcdep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Garrido E, Palomo T, Ponce G, García-Consuegra I, Jiménez-Arriero M, Hoenicka J. The ANKK1 protein associated with addictions has nuclear and cytoplasmic localization and shows a differential response of Ala239Thr to apomorphine. Neurotox Res. 2011;20(1):32–39. doi: 10.1007/s12640-010-9219-6. [DOI] [PubMed] [Google Scholar]
- 55.Hoenicka J, Quiñones-Lombraña A, España-Serrano L, Alvira-Botero X, Kremer L, Pérez-González R, Rodríguez-Jiménez R, Jiménez-Arriero MA, Ponce G, Palomo T. The ANKK1 gene associated with addictions is expressed in astroglial cells and upregulated by apomorphine. Biol Psychiatry. 2010;67(1):3–11. doi: 10.1016/j.biopsych.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Lucht M, Samochowiec A, Samochowiec J, Jasiewicz A, Grabe HJ, Geissler I, Rimmbach C, Rosskopf D, Grzywacz A, Wysiecka JP, Tybura P, Brzuchalski B, Bieńkowski P. Influence of DRD2 and ANKK1 genotypes on apomorphine-induced growth hormone (GH) response in alcohol-dependent patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):45–49. doi: 10.1016/j.pnpbp.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 57.Schülke JP, Wochnik GM, Lang-Rollin I, Gassen NC, Knapp RT, Berning B, Yassouridis A, Rein T. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS One. 2010;5(7):e11717. doi: 10.1371/journal.pone.0011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.