Abstract
Gene therapy provides a powerful tool for regulating cellular processes and tissue repair. Minicircle (MC) DNA are supercoiled DNA molecules free of bacterial plasmid backbone elements, and have been reported to enhance prolonged gene expression compared to conventional plasmids. Despite the great promise of MC DNA for gene therapy, methods for safe and efficient MC DNA delivery remain lacking. To overcome this bottleneck, here we report the development of a poly (β-amino ester) (PBAE)-based, biodegradable nanoparticulate platform for efficient delivery of MC DNA driven by an Ubc promoter in vitro and in vivo. By synthesizing and screening a small library of 18 PBAE polymers with different backbone and end-group chemistry, we identified lead cationic PBAE structures that can complex with minicircle DNA to form nanoparticles, and delivery efficiency can be further modulated by tuning PBAE chemistry. Using human embryonic kidney 293 cells and mouse embryonic fibroblasts as model cell types, we identified a few PBAE polymers that allow efficient MC delivery at levels that are comparable or even surpassing Lipofectamine 2000. The biodegradable nature of PBAE-based nanoparticles facilitates in vivo applications and clinical translation. When injected via intraperitoneal route in vivo, MC alone resulted in high transgene expression and a lead PBAE/MC nanoparticle formulation achieved a further 2-fold increase in protein expression compared to MC alone. Together, our results highlight the promise of PBAE-based nanoparticles as promising non-viral gene carriers for MC delivery, which may provide a valuable tool for broad applications of MC DNA-based gene therapy.
Keywords: Non-viral, minicircle, gene delivery, polymer synthesis, biodegradable nanoparticles
Gene therapy holds great promise for treating both inherited and acquired diseases by delivering genes into the target cells to restore or promote specific cellular functions. Turning on or turning off specific gene targets have also been used to enhance stem differentiation, over expressing therapeutic factors for promoting tissue repair, or reprogram somatic cells to induced pluripotent stem cells.1–2 Most gene therapy approaches utilize viral vectors given their high delivery efficiency, however, broad clinical applications of viral-based gene delivery face safety concerns such as potential for insertional mutagenesis.3–4 In pursuit of an alternative safer approach, various attempts have been made to develop non-viral vehicles for gene delivery. As both plasmid DNA and cellular membrane are negatively charged, a carrier is often required to increase intracellular delivery efficiency. A variety of cationic lipid and polymers have been developed over the past two decades as non-viral transfection reagents to enhance gene delivery.5–6 Driven by the electrostatic forces, cationic polymers can condense negatively charged DNA into nanoparticles and is considered to be safer and more desirable due to its non-integrating nature.7 However, polymeric vector-based gene delivery generally suffer from substantially lower transfection efficiency compared to the viral vectors.8 Among the polymeric vectors developed so far, poly (β-amino ester)s (PBAEs) represent a family of biodegradable polymers with broadly tunable structural diversity. Given the facile synthesis procedure, large libraries of PBAEs have been synthesized and screened in a high-throughput manner to help identify novel structures that lead to highly efficient gene delivery into a broad range of cell types, and have shown excellent efficiency in vivo.9–11
In addition to the development of an effective gene carrier, the molecular structure of plasmid DNA may also be engineered to enhance gene delivery efficiency. The prokaryote cassette has been reported to have a negative influence on plasmid propagation in mammalian cells. This might be due in part to the gene silencing effects caused by the presence of CpG islands.12 To overcome such limitations, we and others have reported minicircle (MC) DNA, which are supercoiled DNA molecules free of bacterial plasmid backbone elements, such as an origin of replication and an antibiotic resistance gene. Unlike conventional plasmids, minicircles primarily consist of a eukaryotic expression cassette, and therefore do not activate exogenous silencing mechanisms to the same extent as plasmids. Recent studies have shown that minicircle vectors resulted in 10–100 folds higher transgene expression than plasmid DNA, and result in more stable ectopic transgene expression.13–14 We and others have also reported prolonged gene expression and reduced immune responses using minicircle compared to parental plasmids both in vitro and in vivo.13, 15–17 Despite the great promise of minicircle for gene delivery, in vitro transfection using minicircle DNA alone suffers from low efficiency. Previous studies have utilized electroporation or Lipofectamine 2000, a commercially available cationic lipid molecule to facilitate cellular uptake of minicircle DNA.1, 18 However, these strategies generally result in high cytotoxicity or low transfection efficiency and are unsuitable for clinical translation, therefore methods for efficient minicircle DNA delivery remain lacking. Another important molecular component of plasmids design is the promoter, and human cytomegalovirus (CMV) promoter has been widely used due to its high efficiency. However, CMV is an immediate-early promoter, hence resulting in only short term transgene expression despite its high expression levels.19–20 In contrast, the ubiquitin C (Ubc) promoter allows for prolonged gene expression, but generally are less efficient compared to CMV promoter across a range of cell types.21
The goal of this study is to develop a poly (β-amino ester)-based, biodegradable nanoparticulate platform for efficient delivery of minicircle DNA driven by an Ubc promoter in vitro and in vivo. We have chosen PBAE polymers given their biodegradable nature, facile synthesis schemes and structural tunability. While previous studies have identified PBAE structures for efficient delivery of plasmid DNA and small interfering RNA, the efficacy of PBAE for delivering minicircle DNA remains unknown. Given the substantial structural changes between plasmid DNA and minicircle, a new screening process is required to identify PBAE structures optimized for delivering minicircle DNA. We hypothesize that cationic PBAE can complex with minicircle DNA to form nanoparticles, which would protect minicircle from being degraded by nucleases, and delivery efficiency can be modulated by tuning PBAE hydrophobicity and end group chemistry. To test our hypothesis, we synthesized a small library of 18 PBAE polymers with different backbone and end-group chemistry. The ability of PBAE to protect minicircle from degradation was examined by electrophoresis and picogreen assay with or without nuclease exposure. To examine the effects of varying PBAE chemical structures on minicircle delivery efficiency, we transfected HEK293 with 18 PBAEs using MC encoding GFP as a reporter, and outcomes were evaluated with fluorescence microscopy and flow cytometry. We then characterized the biophysical properties of PBAE/MC nanoparticles that resulted in highest transfection efficiency using dynamic light scattering and scanning electron microscopy. Finally, lead nanoparticles containing luciferase encoding minicircle were injected into peritoneal cavity in mice to evaluate the efficacy of PBAE for minicircle delivery in vivo.
Results and Discussion
Here we synthesize and optimize PBAE-based, biodegradable nanoparticles for non-viral delivery of MC both in vitro and in vivo. Specifically we synthesized 18 PBAEs with different chemical structures (Fig. 1) and examined the effects of varying PBAE structure on nanoparticles formation and gene delivery efficiency. Our screening has identified optimal PBAE structures that allow efficient MC delivery that is comparable or more superior to Lipofectamine 2000. Given the degradable nature and low cytotoxicity associated with PBAE/MC nanoparticles, as well as the molecular design of MCs driven by a human Ubc promoter, our platform offers an attractive nanoparticulate delivery system for translating minicircle-based gene therapy for clinical applications.
Figure 1.
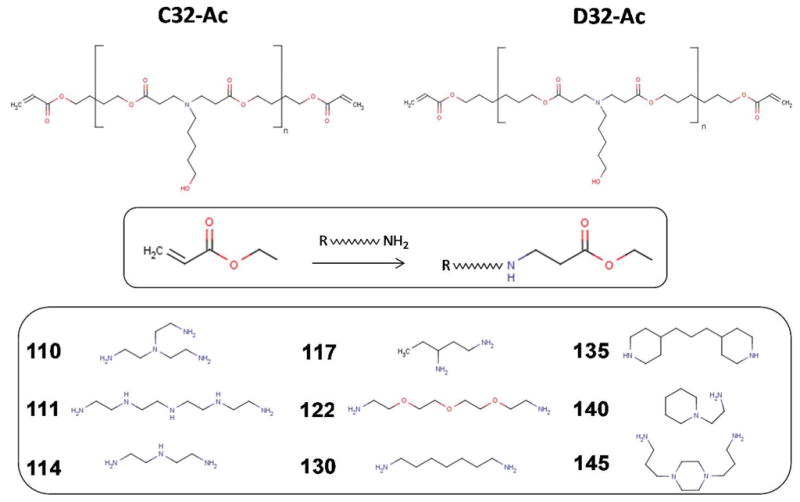
Synthesis scheme and molecular structures of 18 poly (β-amino esters). Acrylate terminated polymers (C32-Ac and D32-Ac) were first synthesized as backbone polymers, followed by end group modification with 9 different end-group chemical structures (110 to 145) to form 18 different amine-terminated poly(β-amino esters).
Synthesis and Characterization of MC/PBAE Nanoparticles
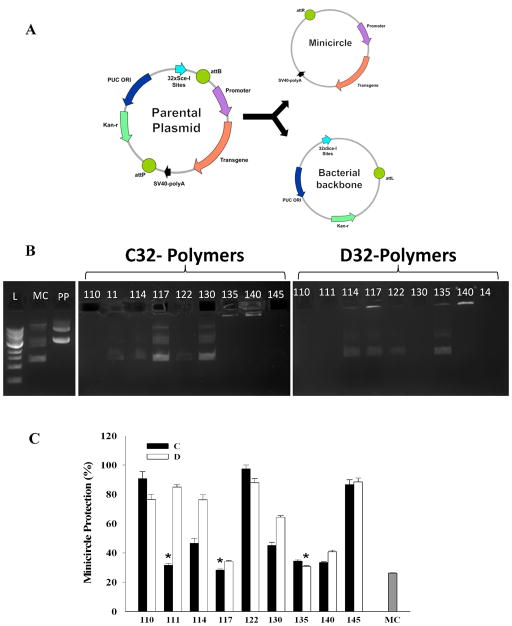
The first step in achieving successful transfection requires the formation of stable nanoparticles. The formation of a nanoparticle involves the electrostatic interaction of MC and PBAE and the subsequent self-assembly of the components into tightly wound nanoparticles. Since MCs are significantly smaller than conventional plasmids due to the removal of bacterial backbone (Fig. 2A), we first investigated the stability of both polymers C32 and D32 along with 9 different end-groups in forming nanoparticles with MCs through a gel electrophoresis assay (Fig. 2B). Free DNA such as MC or parental plasmid can travel down within the gel with notable band. For PBAE/MC groups, most C32- and D32-based polymers completely encapsulate MC DNA into nanoparticles, with no noticeable free DNA band detected. Some weak DNA bands were visible in C32-based polymers with amine end groups of 117, 130, 135 and 140, as well as D32-based PBAEs with amine end groups of 117 and 140. To further quantify the ability of PBAE to encapsulate MC into nanoparticles and thereby protect the MC from being degraded by DNAse, we also expose the 18 formulation of PBAE/MC nanoparticles to DNAse, and quantify the degree of minicircle protection using a picogreen assay to measure free DNA. (Fig. 2C) We observed a similar trend as the electrophoresis results, and the level of MC protection is mostly dictated by the PBAE end group chemistry (i.e., C32- and D32-based PBAEs that share the same end group chemistry often yielded similar MC protection efficiency). The only exceptions are PBAEs with 111 and 114 end group chemistry, in which D32-based PBAE nanoparticles generally showed much higher level of MC protection. This may be due to the increased hydrophobicity in D32-based polymers, which leads to slower degradation and possibly more stable nanoparticles. Our results demonstrate PBAEs can efficiently encapsulate MC and protect them from being degraded by nucleases in the environment.
Figure 2.
(A) Schematic diagram of minicircle cleavage from parental plasmid. (B) Electrophoresis showing nanoparticle protection. L=ladder (1Kb), MC=Minicircle, PP=Parental plasmid. Nanoparticles are located in wells within the agarose gel. Fluorescence within the well or lack of signal within the gel indicates nanoparticle formation, hence transport of minicircle through the gel indicates inadequate nanoparticle formation. (C) Quantitative measurement of minicircle protection. Nanoparticles were treated with DNAse followed by quantification of DNA content by PicoGreen analysis. The decrease in DNA content due to degradation is expressed as a ratio of the non-treated nanoparticles. Data is presented as mean ± standard deviation. * represents no statistical difference relative to MC (p<0.05).
In vitro Transfection Efficiency of MC/PBAE Nanoparticles
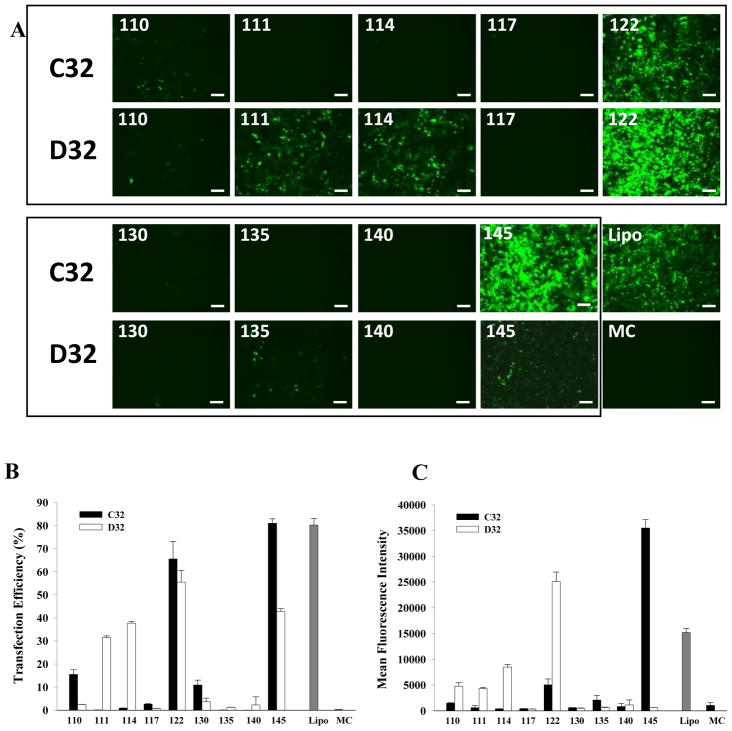
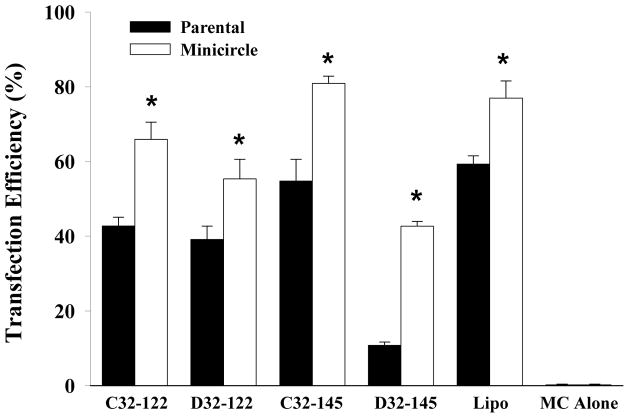
We then assessed the ability of PBAE/MC nanoparticles to transfect HEK293 cells using 18 nanoparticle formulations containing MC encoding GFP DNA, which allowed direct visualization of transfection efficiency by fluorescence microscopy and quantification by flow cytometry. Cells transfected with Lipo/MC or MC alone were included as controls. Fluorescence images showed that transfection efficiency differs significantly depending on the chemistry of the PBAE (Fig. 3A). A few leading PBAE structures (C32-122, D32-122 and C32-145) resulted in 70–80% positively transfected cells, which is comparable to the positive control group transfected with Lipofectamine 2000 (Fig. 3B). These PBAE/MC nanoparticles also showed high efficiency in protecting MC DNA from degradation. However, nanoparticle stability is not solely responsible for the observed high transfection efficiency, as some other stable PBAE nanoparticles (e.g., D32-110) resulted in very low transfection efficiency. We also noted that most PBAE pairs with the same end group chemistry resulted in substantially different transfection efficiency, suggesting that transfection efficiency is influenced by additional parameters such as polymer hydrophobicity and NP stability. One exception is C32-122 and D32-122, which both resulted in over 50% transfection efficiency for MC. Interestingly, C32-122 has also been previously identified to be a leading transfection agent for plasmid DNA delivery22, and this is the first time C32-122 has been examined for MC delivery. We also quantified mean fluorescent intensity, which is a measure of total protein production. In this regard, two PBAE/MC nanoparticles (D32-122 and C32-145) demonstrated a substantially higher level of GFP intensity compared to Lipofectamine control, despite their comparable level of percentage of cells being transfected (Fig. 3C). Transfection efficiency describes the percentage of cells that have been transfected, regardless of the difference in the level of protein production among individual cells. In contrast, mean fluorescence intensity takes into account of the difference in protein production by individual cells, and normalize that by the total number of cells. Therefore, mean fluorescence intensity is a better prediction of the level of protein production post-transfection. To ensures that the differences in transfection efficiency were not caused by potential toxicity from polymeric nanoparticles, we also examined the effects of PBAE/MC nanoparticle on cell viability under the optimized transfection doses. Our results showed most PBAE/MC nanoparticle formulations did not negatively affect cell viability compared to untreated cells alone (Fig. 4). The only exception was D32-110, which showed slightly lower cell viability (~80%). Previous studies on using PBAE for plasmid DNA delivery required use of PBAE with weight ratios up to 50:1 to reach optimal transfection efficiency, which may also cause increased cell death.23 In our study, substantially less PBAE was required (10:1) to form stable nanoparticles with MCs due to the smaller size resulting from removal of the bacterial cassette. This offers an additional advantage of using PBAE for MC delivery given the minimal toxicity effects for cells.
Figure 3.
(A) Fluorescent images of HEK cells transfected with 18 different PBAE nanoparticles (C32- or D32 polymers with 9 different amine end group) containing a minicircle encoding GFP. Cells transfected using Lipofectamine 2000 (Lipo) or minicircle alone without transfection agent (MC) were included as controls. (B) Transfection efficiency of 18 PBAE nanoparticles shown by percentage of GFP positive cells, which is quantified by flow cytometry. (C) Mean fluorescent intensity of HEK cells transfected with 18 PBAE nanoparticles determined by flow cytometry. Lipo=Lipofectamine 2000, MC=Minicircle. Data is presented as mean ± standard deviation.
Figure 4.
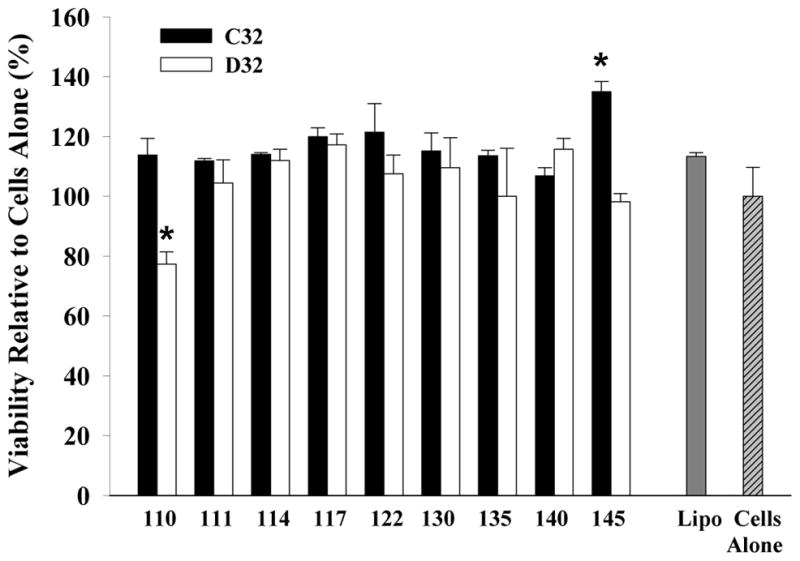
Cell viability as determined by the CellTiter assay. Cells were transfected with MC encoding for GFP. Data is presented as mean ± standard deviation. * indicates statistical difference to cells alone (p<0.05).
Biophysical Characterization of Nanoparticles
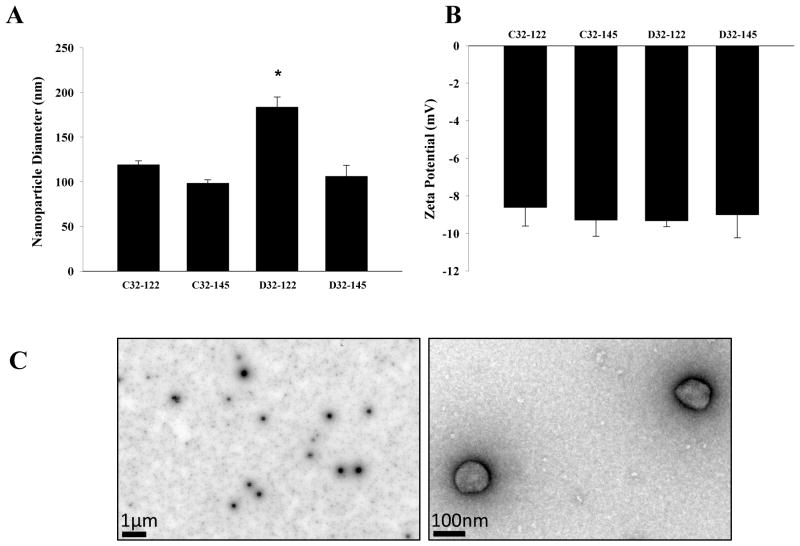
To further investigate the biophysical properties of PBAE nanoparticles that demonstrated high efficiency of minicircle delivery, we measured the particle size and zeta potential of four nanoparticles which showed highest transfection efficiency (C32-122, C32-145, D32-122 and D32-145) by dynamic light scattering. The particle size of 4 leading PBAE nanoparticles demonstrated a fairly broad distribution ranging from 91 nm (for C32-145 and D32-145) to 192 nm (D32-122) (Fig. 5A). This size range is comparable to previous reports on PBAE nanoparticles for plasmid delivery.23 It has been suggested that smaller particle size may lead to increased transfection.24 Given the comparable transfection efficiency among our lead nanoparticles with different particle size, our results suggest that transfection efficiency of our PBAE/MC nanoparticles are not solely dominated by particle size. Different than the particle size, all lead PBAE/MC nanoparticles demonstrated comparable surface charge in medium containing 10% serum, which range from −7.5 mV to −11.02 mV (Fig. 5B) as measured by zeta potential. To further consolidate the biophysical results observed, we took one of the polymers D32-122 and subjected it to transmission electron microscope analysis. TEM imaging confirmed stable PBAE/MC nanoparticle formation through self-assembly of MC and PBAE, with nanoparticle size consistent with the dynamic light scattering result (Fig. 5C).
Figure 5.
(A) The size (z-average) and (B) zeta potential of PBAE/MC nanoparticles that demonstrated the highest level of transfection efficiency. Measurements were performed using a Zetasizer Nano Z (Malvern). PBAE/MC nanoparticles were suspended in fully supplemented DMEM medium containing 10% fetal bovine serum to reach a final MC concentration of 12 μg/ml. (C) Transmission electron microscope image of PBAE/MC nanoparticles formed using D32-122 and minicircle.
Transfection Efficiency in Mouse Embryonic Fibroblasts
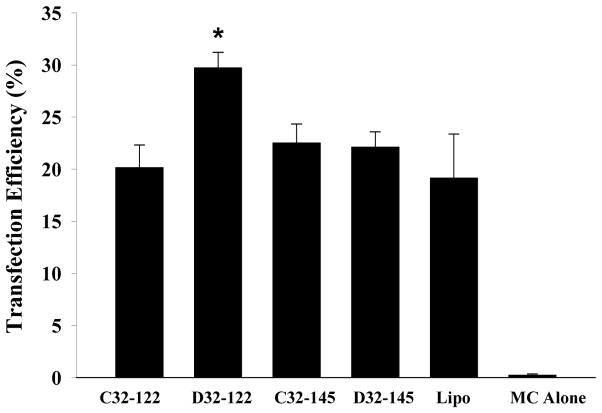
To further examine the ability of lead PBAE/MC nanoparticles to transfect other cell types, mouse embryonic fibroblasts (MEFs) were transfected using optimized PBAE/MC nanoparticles including C32-122, C32-145, D32-122 and D32-145. MEFs were also transfected with Lipofectamine 2000 as a positive control. We observed transfection efficiency up to 30% (D32-122), which was 10% higher than that of Lipofectamine as well as comparable transfection efficiency of all the other polymers (Fig. 6). Interestingly, although C32-145 achieved highest transfection in HEK293 cells, D32-122 was the most efficient polymer for MEFs transfection pointing out the need to screen polymers for cell specific transfections.
Figure 6.
Transfection efficiency in mouse embryonic fibroblasts (MEFs) as determined by flow cytometry using lead PBAE/MC nanoparticles encoding GFP. * indicates statistical difference relative to lipofectamine 2000 (Lipo) (p<0.05).
Increased Gene Expression using Minicircles over Parental Plasmids
The advantage of using MC as a vector for gene delivery was confirmed by comparing transfection efficiency using nanoparticles formed by PBAE/MC or PBAE/parental plasmid from which the MC was derived. PBAE/MC-mediated transfection consistently resulted in higher transfection efficiency in HEK cells than transfection efficiency achieved using PBAE/parental plasmids (Fig. 7). A similar trend was observed using Lipofectamine as the gene carrier, where MC also resulted in higher transfection efficiency. An even great increase was observed with D32-122/MC providing a 50-fold increase in mean fluorescent intensity (Fig. S1B), suggesting MC as a better vector for efficient gene delivery over parental plasmid. Our finding is consistent with a previous report on human melanoma cells, where MC resulted in 12-fold increase in gene expression than parental plasmid at equimolar concentration.25
Figure 7.
Transfection efficiency of HEK cells transfected with minicircle or parental plasmid encoding for GFP. Transfection efficiency was determined by FACS analysis. Data is presented as mean ± standard deviation. * indicates statistical difference within each group (p<0.05).
In vivo Minicircle Delivery
While some polymer-mediated non-viral nanoparticles demonstrate high transfection efficiency in vitro, they often lose their ability to transfect in vivo due to the presence of various serum proteins and enzymes. PBAE polymers have been used in the past for in vivo delivery of plasmid DNA in a wide range of animal models. 10–11, 26–27 Delivery routes include intravenous, intraperitoneal, intratumoral and intradermal. To demonstrate the efficiency of PBAEs for MC delivery in vivo, we have chosen an intraperitoneal route of delivery, which allows expression in multiple intraperitoneal organs with one single injection.
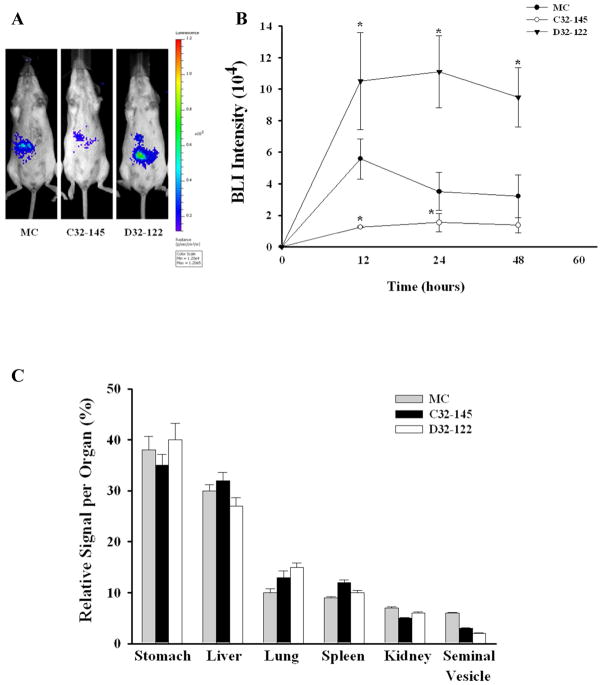
To examine the potential of PBAE for MC delivery in vivo, we injected two formulations of PBAE/MC nanoparticles based on C32-145 and D32-122 for delivering MC through an intraperitoneal route. To facilitate real-time monitoring of in vivo transfection efficiency, we have chosen a minicircle encoding luciferase gene as a reporter. MC/C32-145 or MC/D32-122 (30 μg per injection) were injected i.p. and MC alone was also delivered directly as a control. The mice were imaged 12, 24 and 48 hours after injection and bioluminescence was quantified. While MC alone lacks the ability to transfect cells in vitro, previous studies have shown that MC can achieve excellent efficiency in vivo, which is also what we observed in our study.15, 17 One of our PBAE polymer, D32-122 further enhanced luciferase production by 2-fold relative to MC alone at 24 hr. This trend persisted when measured at 48 hr, suggesting D32-122 as a promising polymeric vector for enhanced MC delivery in vivo (Fig. 8A, B). We speculate that such increase in transfection efficiency may be, at least in part, due to the increased hydrophobicity of D32-122, which in turn led to more stable nanoparticles and prolonged protection and availability of MC for increased transfection efficiency in vivo. We also observed that transfection efficiency of polymer based transfection in vivo cannot be solely predicted from the transfection efficiency results in vitro. For example, while C32-145 resulted in highest efficiency in vitro, the level of protein production in vivo was lower than that achieved with MC alone (Fig. 8A). To gain a better understanding of the biodistribution of polymer-MC transfection after i.p. administration, whole organs were harvested from the mice at 24 hr and individual luminescence readings were performed on each organ. In a trend similar to that of whole body imaging, D32-122 mediated a higher expression in all organs compared to DMSO (except the kidney and seminal vesicle) whereas C32-145 was not as efficient as DMSO (stomach and liver) (Figure S3). Biodistribution of the nanoparticles was similar with and without PBAE across all organs (Fig. 8C).
Figure 8.
In vivo delivery of minicircle (MC) alone or MC complexed by PBAE injected via intraperitoneal route. (A, B) MC encoding luciferase was used and mice were imaged 12, 24 and 48 hours post-injection. (C) Biodistribution of luciferase expression in all harvested organs for MCs delivered with DMSO, C32-145 or D32-122. Data was presented as mean + standard error. * indicates statistical significance (p<0.05).
Conclusions
In summary, here we report the development of PBAE-based, biodegradable nanoparticles as efficient vehicles for delivering Ubc driven minicircle DNA using a combinatorial chemistry approach. By screening a total of 18 synthesized PBAEs with varying chemical structures, we identified lead PBAE structures that resulted in markedly increased MC delivery efficiency both in vitro and in vivo. Our results confirmed that PBAE can effectively complex MC into nanoparticles, and protect the MC from being degraded by environmental nucleases. Furthermore, our results support our hypothesis that MC delivery efficiency can be modulated by tuning PBAE hydrophobicity and end group chemistry. Using HEK and MEF cells as model cell types, we identified a few PBAE polymers that allow efficient MC delivery at levels that are comparable or even surpassing Lipofectamine 2000. Unlike lipofectamine 2000, which is non-degradable, the biodegradable nature of PBAE-based nanoparticles facilitates in vivo applications and clinical translation. While MC alone allows high level of transgene expression in vivo, we demonstrate a further 2-fold increase in efficiency using lead PBAE/MC nanoparticles. Together, our results highlight the promise of PBAE-based nanoparticles as novel non-viral gene carriers for MC delivery.
Materials and Methods
Preparation of Minicircles
Minicircles (MCs) are the product of site-specific intramolecular recombination between the attB and attP sites driven by bacteriophage FC31 integrase. The DNA fragments containing enhanced green fluorescent protein (MC-GFP) or firefly luciferase (MC-Fluc) were bluntly ligated between the attB and attP sites of the parental plasmid. MCs were prepared as described previously.17 Briefly, ZYCY10P3S2T cells (E. coli was a kind gift from Mark Kay, Stanford University) were transformed with parental plasmids. Cells from one transformed colony were inoculated into 5 mL of luria-bertani (LB) (Sigma-Aldrich, St. Louis, MO) solution containing Kanamycin (50 μg/mL) (Sigma-Aldrich) and incubated at 37°C with shaking at 250 rpm. Following a 4 hour incubation, the bacteria was amplified by combining 100 μl of culture to every 400 mL LB containing Kanamycin (50 μg/mL) and incubation was continued for 16 hours. The next day, a minicircle induction mix comprising of 400 mL fresh LB, 16 mL 1N sodium hydroxide (Sigma-Aldrich) and 0.4 % (w/v) L-arabinose (Sigma-Aldrich) were added to the overnight culture and allowed to grow at 32°C for another 8 hours. MCs were then isolated using a Plasmid Plus Maxi Kit (Qiagen, Valencia, CA) according to manufacturer’s protocol.
Synthesis of Poly (β-amino esters) with Structural Diversity
Acrylate terminated poly (β-amino ester) (PBAE) was synthesized as described previously.23 To synthesize C32-Ac and D32-Ac respectively, 1.533 g of 5-amino-1-pentanol (32) (Alfa Aesar, Ward Hill, MA) was combined with 3.255 g of butanediol diacrylate (C) or hexanediol diacrylate (D) and stirred overnight at 90°C. C32-Ac and D32 were used as polymer backbone and each was modified with 9 different end-group amines, producing a total of 18 different PBAEs. To introduce different end-group chemistry, 9 different types of amines monomers (Fig. 1) (10 mM) were mixed with 5 g of acrylate-terminated C32 or D32 in the presence of tetrahydrofuran (Fisher Scientific, Houston, TX) and stirred overnight. The products were then precipitated with diethyl ether and dissolved in anhydrous DMSO (Fisher Scientific) (100 mg/mL). All materials were stored at −20°C until further use. The resulting polymers were divided into 2 subgroups: C32 and D32, each containing 9 end-modified polymers as shown in Fig. 1.
Formation of PBAE/MC Nanoparticles
To form nanoparticles containing minicircles, PBAE and MC’s were mixed together (10:1 in weight ratio) in 25 mM sodium acetate (Fisher Scientific) and incubated at room temperature for 10 minutes before use. Preliminary studies indicated that increasing PBAE:MC weight ratio did not enhance transfection efficiency (Fig S2). Control nanoparticles were formed using Lipofectamine 2000 (Life Technologies, Grand Island, NY) according to manufacturer’s protocol. Briefly, MCs and Lipofectamine 2000 were mixed (1:2.5 in weight ratio) in OptiMEM® (Life Technologies) and incubated at room temperature for 15 minutes before use.
Particle Size and Zeta Potential Measurements
To prepare PBAE/MC nanoparticles for biophysical characterization, PBAE/MC nanoparticles were suspended in fully supplemented DMEM medium containing 10% fetal bovine serum to reach a final MC concentration of 12 μg/mL (mass refers to MC content), and loaded into a disposable capillary cell (Malvern, Westborough, MA). Measurement of the nanoparticle size distribution and zeta potential were performed using a Zetasizer Nano ZS (Malvern).
Transmission Electron Microscopy
Transmission electron microscopy (TEM) imaging was performed to assess the shape and size of PBAE/MC nanoparticles. Formed nanoparticles were deposited on glow discharged 300 mesh carbon/formvar coated Cu grids (Ted Pella, Redding, CA). Grids were then washed with 1% (w/v) uranyl acetate in distilled water and air dried overnight. Samples were examined on a TEM operating at 120 kV (Jeol1230, Jeol, Portland, OR).
Gel Electrophoresis and Minicircle Protection Assay
The ability of PBAEs to encapsulate MC DNA into nanoparticles was evaluated by gel electrophoresis, in which only free (un-encapsulated) MC DNA can move in the gel and be visualized, whereas MC entrapped in nanoparticles will stay in the loading well. PBAE/MC nanoparticles (80 ng) were loaded in a 1.2% agarose gel containing 0.012% ethidium bromide and electrophoresis was performed at 60 V for 30 minutes. A 1 kb ladder was used as reference. After electrophoresis, the resulted DNA migration bands were visualized with a BioSpectrum®AC Imaging System (Ultra-Violet Products, Upland, CA).
To further assess the ability of PBAE nanoparticles to protect MC DNA from nuclease degradation, PBAE/MC nanoparticles were treated with 1 U/μL DNase I (Life Technologies) at 37°C for 30 minutes. Samples without DNAse treatment were included as controls. Following incubation, 100 μL of Quant-iT™ PicoGreen (Life Technologies) was added to each sample to measure free DNA. Fluorescence intensity was determined using a plate reader (Spectramax M2e, Molecular Devices, CA) at an excitation of 480 nm and emission of 520 nm. The percentage of minicircle protection was defined as .
Cell Culture and Viability
Human embryonic kidney cells (HEK293) and mouse embryonic fibroblasts (MEFs) were maintained in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin (Life Technologies) at 37°C in a humidified atmosphere of 5% carbon dioxide. To examine cell viability post-transfection using PBAE/MC nanoparticles, HEK293 cells were transfected with PBAE/MC nanoparticles (2.4 μg MC DNA per 75,000 cells) and cell viability was assessed at day 2 using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to manufacturer’s protocol. Percentage of cell viability was normalized to cells cultured alone without nanoparticles.
In vitro MC Delivery using PBAE
To evaluate the ability of 18 PBAEs in delivering MC DNA, we used HEK293 cells, a commonly used model cell type for gene delivery and MC encoding GFP as a reporter. HEK293 cells were seeded at a concentration of 75,000 cells/well in 48-well plates pre-coated with 0.1% gelatin. For transfection with mouse embryonic fibroblasts (MEFs), cells were seeded at a concentration of 18,000 cells/well in a 48 well plate. Cells were left overnight for attachment, and then treated with complete DMEM culture medium containing 10% FBS and PBAE/MC nanoparticles. After 4 hr incubation, NPs were removed and replaced with fresh culture medium. Transfection efficiency and mean fluorescence intensity were measured by fluorescence-activated cell sorting (FACS) 48 hours after initial transfection. Briefly, cell culture medium was removed and cells were washed once with PBS. Cells were detached by the addition of 0.25% Trypsin-EDTA (Life Technologies). The resuspended cells were transferred to a v-bottom 96 well plate and centrifuged for removal of trypsin. Cells were then resuspended in 75 μL of PBS containing 2% (v/v) FBS and 0.1% (v/v) propidium iodide (Life Technologies). FACS analysis was performed on a BD LSR II with High Throughput Sampler (HTS) (BD Biosciences, San Jose, CA, USA). The GFP positive/PI negative cells were counted as positively transfected cells. The data was analyzed by Flowjo software (Tree Star, OR, US).
In vivo Delivery of Minicircle using PBAE and Bioluminescence Imaging
To evaluate the efficacy of PBAE/MC nanoparticles for gene expression in vivo, nanoparticles containing MC encoding luciferase were injected into the peritoneal cavity of 8-week old female FVB mice (30 μg MC per injection prepared as described for in vitro transfection, n=5). Briefly, nanoparticle solution was mixed with 66 μL of glucose (30 % w/v) to bring the total volume to 400 μL. MC alone controls were included as control. In vivo bioluminescence imaging was performed using an IVIS imaging system and LIVING IMAGE acquisition and analysis software (Xenogen, Alameda, CA). Mice were anesthetized with 1.5% isoflurane and subsequently injected i.p. with luciferin (150 μg per g of body weight). Whole-body images were acquired 10 minutes after luciferin administration and were continued until signals were observed to decrease.
Supplementary Material
Acknowledgments
The authors would like to thank the American Heart Association (10SDG2600001), Donald E. and Delia B. Baxter Foundation, the McCormick Faculty Award, and the Stanford Bio-X Interdisciplinary Initiative grant for funding. S.G. O. and J. C. W. would like to acknowledge the National Institutes of Health (R01 HL095571 and R01 HL093172) for funding.
Footnotes
Fluorescent images and further FACS analysis on minicircle vs. parental plasmid transfection are available in the supplementary information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, et al. A Nonviral Minicircle Vector for Deriving Human iPS Cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauta A, Seidel C, Deveza L, Montoro D, Grova M, Ko SH, Hyun J, Gurtner GC, Longaker MT, Yang F. Adipose-derived Stromal Cells Overexpressing Vascular Endothelial Growth Factor Accelerate Mouse Excisional Wound Healing. Mol Ther. 2013;21:445–455. doi: 10.1038/mt.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, Forster M, Stocking C, Wahlers A, Frank O, et al. Murine Leukemia Induced by Retroviral Gene Marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 4.Baum C, Kustikova O, Modlich U, Li Z, Fehse B. Mutagenesis and Oncogenesis by Chromosomal Insertion of Gene Transfer Vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 5.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, et al. A Versatile Reducible Polycation-based System for Efficient Delivery of a Broad Range of Nucleic Acids. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Russ V, Wagner E. Influence of the Molecular Weight of Bioreducible Oligoethylenimine Conjugates on the Polyplex Transfection Properties. AAPS J. 2009;11:445–455. doi: 10.1208/s12248-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundstrom K. Latest Development in Viral Vectors for Gene Therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 8.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and Development of Polymers for Gene Delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A Polymer Library Approach to Suicide Gene Therapy for Cancer. Proc Natl Acad Sci U S A. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green JJ, Zugates GT, Tedford NC, Huang YU, Griffith LG, Lauffenburger DA, Sawicki JA, Langer R, Anderson DG. Combinatorial Modification of Degradable Polymers Enables Transfection of Human Cells Comparable to Adenovirus. Adv Mater. 2007;19:2836–2842. [Google Scholar]
- 11.Zugates GT, Peng W, Zumbuehl A, Jhunjhunwala S, Huang YH, Langer R, Sawicki JA, Anderson DG. Rapid Optimization of Gene Delivery by Parallel end-modification of Poly(beta-amino ester)s. Mol Ther. 2007;15:1306–1312. doi: 10.1038/mt.sj.6300132. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZY, He CY, Meuse L, Kay MA. Silencing of Episomal Transgene Expression by Plasmid Bacterial DNA Elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA Vectors Devoid of Bacterial DNA Result in Persistent and High-level Transgene Expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 14.Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A New DNA Vehicle for Nonviral Gene Delivery: Supercoiled Minicircle. Gene Ther. 1997;4:1341–1349. doi: 10.1038/sj.gt.3300540. [DOI] [PubMed] [Google Scholar]
- 15.Huang M, Nguyen P, Jia F, Hu S, Gong Y, de Almeida PE, Wang L, Nag D, Kay MA, Giaccia AJ, et al. Double Knockdown of Prolyl Hydroxylase and Factor-inhibiting Hypoxia-inducible Factor with Nonviral Minicircle Gene Therapy Enhances Stem Cell Mobilization and Angiogenesis after Myocardial Infarction. Circulation. 2011;124:S46–54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, et al. MicroRNA-210 as a Novel Therapy for Treatment of Ischemic Heart Disease. Circulation. 2010;122:S124–131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel Minicircle Vector for Gene Therapy in Murine Myocardial Infarction. Circulation. 2009;120:S230–237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of Adult Human Induced Pluripotent Stem Cells using Nonviral Minicircle DNA Vectors. Nat Protoc. 2011;6:78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byun HM, Suh D, Jeong Y, Wee HS, Kim JM, Kim WK, Ko JJ, Kim JS, Lee YB, Oh YK. Plasmid Vectors Harboring Cellular Promoters can Induce Prolonged Gene Expression in Hematopoietic and Mesenchymal Progenitor Cells. Biochem Biophys Res Commun. 2005;332:518–523. doi: 10.1016/j.bbrc.2005.04.155. [DOI] [PubMed] [Google Scholar]
- 20.Gazdhar A, Bilici M, Pierog J, Ayuni EL, Gugger M, Wetterwald A, Cecchini M, Schmid RA. In vivo Electroporation and Ubiquitin Promoter--a Protocol for Sustained Gene Expression in the Lung. J Gene Med. 2006;8:910–918. doi: 10.1002/jgm.911. [DOI] [PubMed] [Google Scholar]
- 21.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic Comparison of Constitutive Promoters and the Doxycycline-Inducible Promoter. PLoS One. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunshine JC, Akanda MI, Li D, Kozielski KL, Green JJ. Effects of Base Polymer Hydrophobicity and End-Group Modification on Polymeric Gene Delivery. Biomacromolecules. 2011;12:3592–3600. doi: 10.1021/bm200807s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunshine J, Green JJ, Mahon KP, Yang F, Eltoukhy AA, Nguyen DN, Langer R, Anderson DG. Small-molecule End-groups of Linear Polymer Determine Cell-type Gene-delivery Efficacy. Adv Mater. 2009;21:4947–4951. doi: 10.1002/adma.200901718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of Surface Charge, Particle Size and Morphological Properties of Chitosan-TPP Nanoparticles Intended for Gene Delivery. Colloids Surf B Biointerfaces. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Kobelt D, Schleef M, Schmeer M, Aumann J, Schlag PM, Walther W. Performance of High Quality Minicircle DNA for in vitro and in vivo Gene Transfer. Mol Biotechnol. 2013;53:80–89. doi: 10.1007/s12033-012-9535-6. [DOI] [PubMed] [Google Scholar]
- 26.Park HJ, Lee J, Kim MJ, Kang TJ, Jeong Y, Um SH, Cho SW. Sonic Hedgehog Intradermal Gene Therapy using a Biodegradable Poly(beta-amino esters) Nanoparticle to Enhance Wound Healing. Biomaterials. 2012;33:9148–9156. doi: 10.1016/j.biomaterials.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Green JJ, Langer R, Anderson DG. A Combinatorial Polymer Library Approach Yields Insight into Nonviral Gene Delivery. Acc Chem Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.