Abstract
Understanding relationships between heart failure and arrhythmias, important causes of suffering and sudden death, remains an unmet goal for biomedical researchers and physicians. Evidence assembled over the last decade supports a view that activation of the multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) favors myocardial dysfunction and cell membrane electrical instability. CaMKII activation follows increases in intracellular Ca2+ or oxidation, upstream signals with the capacity to transition CaMKII into a Ca2+ and calmodulin-independeant, constitutively active enzyme. Constitutively active CaMKII appears poised to participate in disease pathways by catalyzing the phosphorylation of classes of protein targets important for excitation-contraction coupling and cell survival, including ion channels and Ca2+ homeostatic proteins, and transcription factors that drive hypertrophic and inflammatory gene expression. This rich diversity of downstream targets helps to explain the potential for CaMKII to simultaneously affect mechanical and electrical properties of heart muscle cells. Proof of concept studies from a growing number of investigators show that CaMKII inhibition is beneficial for improving myocardial performance and reducing arrhythmias. Here we review the molecular physiology of CaMKII, discuss CaMKII actions at key cellular targets and results of animal models of myocardial hypertrophy, dysfunction and arrhythmias that suggest CaMKII inhibition may benefit myocardial function while reducing arrhythmias.
Keywords: CaMKII, Arrhythmias, Heart Failure, Ion channels, Remodeling
Introduction
Despite important advances in drug and device therapy, cardiovascular disease remains a leading cause of death and suffering worldwide1. Heart failure, a condition arising from multiple causes, occurs when the myocardium is unable to pump blood in sufficient volume to meet metabolic demands. Heart failure is responsible for almost 300,000 deaths 2 and costs $39 billion dollars annually in the United States alone3. While heart failure is a mechanical problem, mostly of contraction (inotropy) but also of relaxation (lusitropy), many heart failure patients suffer from arrhythmias, an electrical problem where cell membrane excitability is inadequately controlled. Ventricular arrhythmias (ventricular tachycardia and ventricular fibrillation) and atrial arrhythmias (sinus node dysfunction, atrial tachycardia and atrial fibrillation) contribute to the increased incidence of sudden cardiac death in patients with heart failure and may themselves aggravate heart failure by disturbing the physiological relationship between heart rate and myocardial performance. Despite the clear association between heart failure and arrhythmias, it has proven difficult to develop widely applicable treatments that coordinately benefit both diseases. The association of heart failure and arrhythmias is a major challenge for clinicians, in part, because myocardial performance-enhancing inotropic drugs increase the likelihood of sudden death4 and because conventional ion channel antagonist antiarrhythmic medications are likely to impose proarrhythmic side effects on hypertrophied, scarred and failing myocardium5. Thus, an important goal for clinical cardiologists, their patients, industry, and society is to develop treatments that effectively target both heart failure and arrhythmias. It seems likely that a first step toward creating agents that can reduce arrhythmias and heart failure will be to identify cellular and molecular mechanisms that underlie both processes. This review will consider established and emergent evidence that the multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) is a signaling molecule that is uniquely positioned to promote the twin pathological phenotypes of heart failure and arrhythmias. In myocardium, CaMKII phosphorylates a diverse array of proteins involved in excitation-contraction coupling (ECC), cell death and transcriptional activation of hypertrophy and inflammation. Excessive or unchecked CaMKII activity thereby promotes core ‘downstream’ events and processes that contribute to heart failure and arrhythmias (Fig 1).
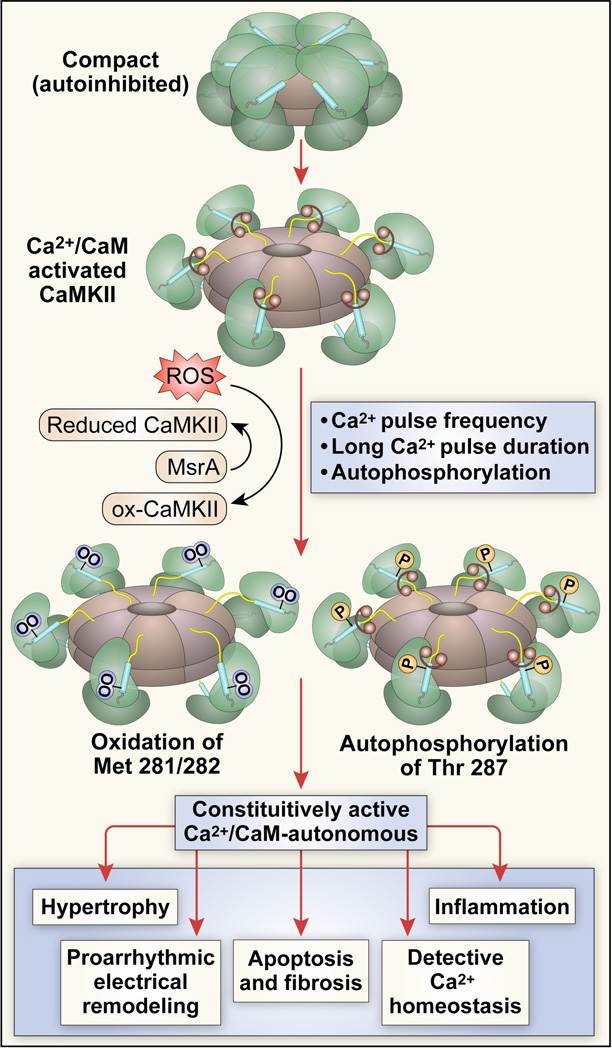
Figure 1. CaMKII becomes constitutively active by autophosphorylation and/or oxidation and constitutively active CaMKII promotes core events important for heart failure and arrhythmias.
The CaMKII holoenzyme consists of two hexameric stacked rings. Under resting conditions the catalytic subunit is conformationally constrained so that CaMKII is inactive (top). CaMKII is initially activated when Ca2+ bound calmodulin (Ca2+/CaM, dumbbell shapes) bind to the regulatory domain (blue segments) causing a more extended conformation where the catalytic domain (green) becomes accessible to substrate proteins and ATP. Sustained increases in Ca2+ lead to autophosphorylation at Thr 287, enhancing the avidity of CaM binding but also inducing a Ca2+/CaM-independent form of CaMKII after CaM unbinding. Oxidation of Met 281/282 induces a Ca2+/CaM-independent form of CaMKII without CaM trapping. Increased levels of autophosphorylated or oxidized CaMKII favor heart failure and arrhythmias, at least in part, by activating hypertrophic and inflammatory gene programs, inducing proarrhythmic electrical remodeling, increasing myocyte death and fibrosis and defective intracellular Ca2+ homeostasis.
Upstream activator signals for CaMKII
Loss of normal myocardial Ca2+ homeostasis and increased reactive oxygen species (ROS) are common ‘upstream’ signals that contribute to arrhythmias and heart failure 6,7 . However, concise molecular targets and signaling pathways connecting Ca2+ and ROS to arrhythmias and heart failure have been elusive, while antioxidant therapies have had modest or no benefit8,8,9, suggesting that improved knowledge of Ca2+ and ROS-activated signals could lead to improved therapies. CaMKII is a serine threonine kinase that is abundant in myocardium and is activated by increased intracellular Ca2+ and ROS7. There are four CaMKII gene products (α-δ), which are homologous, share activation mechanisms and are thought to co-assemble as heteromultimers. The four CaMKII isoforms are differentially expressed in various cells types. For example, CaMKII α and (β are relatively enriched in neuronal tissues and CaMKII δ and γ are predominant in myocardium. However, to our knowledge, there are no differences in catalytic domain specificity for target proteins between CaMKII isoforms. There is a hypervariable region (yellow line in Fig 2) between the association domain and the C terminus of the regulatory domain that has a nuclear localization sequence in one CaMKIIδ splice variant (CaMKIIδ3 or CaMKIIδB) but not in other CaMKIIδ splice variants (CaMKIIδ2 or CaMKIIδC)10. However, the specific role of this sequence remains unclear as recent data suggest that both CaMKIIδB and CaMKIIδC are present in nuclear and cytoplasmic compartments 11. It appears that signaling specificity for CaMKII is determined, at least in part, by the proximity of CaMKII to specific sources of Ca2+ or ROS11–13. Splice variants are relatively common in the hypervariable domain and produce different length linkers between the association and regulatory domains, leading to CaMKII variants with different sensitivities to activation by calcified calmodulin (Ca2+/CaM). CaMKII variants with shorter linkers are relatively compact and Ca2+/CaM inaccessible, while CaMKII variants with longer linkers are relatively extended resulting in a larger Ca2+/CaM accessible binding surface14. An intriguing, but untested, possibility is that CaMKII splice variants with relatively longer hypervariable domain-encoded linkers are better adapted to fight or flight physiology but are also more likely to contribute to disease, because they are more readily activated by Ca2+/CaM and ROS. Each CaMKII monomer consists of an N terminal catalytic domain and a C terminal association domain that enables assembly of the holoenzyme15. The regulatory domain resides between the catalytic and association domains (Fig 2). CaMKII is initially activated by binding Ca2+/CaM, because Ca2+/CaM binding to the C terminal aspect of the regulatory domain (i.e. the CaM-binding region) distorts and disables the negative regulatory effect of the autoinhibitory region to constrain the catalytic domain -converting inactive CaMKII to a Ca2+/CaM-dependent active enzyme. In the case of brief increases in intracellular Ca2+, under low ROS conditions, CaMKII returns to an inactive conformation after Ca2+/CaM unbinding. However, sustained increases in Ca2+/CaM allow for threonine 287 autophosphorylation (the specific numbering varies for each CaMKII isoform), leading to two important changes. The first is ‘CaM trapping’ where the binding affinity for Ca2+/CaM-CaMKII increases by ~10516 . The second is that even after Ca2+/CaM-CaMKII unbinding, CaMKII retains residual, Ca2+/CaM-independent activity17,18. Autophosphorylation of threonine 306 can prevent CaM trapping by interfering with Ca2+/CaM-CaMKII binding19. Oxidation of paired methionines (281/282) causes a similar Ca2+/CaM-independent form of CaMKII20 . In contrast to autophosphorylation, methionine oxidation does not cause CaM trapping, because oxidation of methionine (308 in CaMKIIδ) reduces the affinity of 2+ Ca2+/CaM-CaMKII binding.
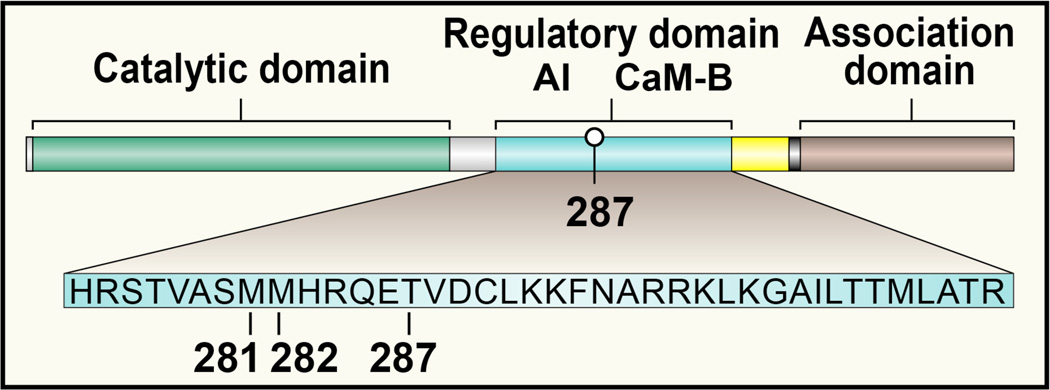
Figure 2. The domain structure of a CaMKII monomer.
Each CaMKII monomer has a C terminus association domain and an N terminus catalytic domain. The internal regulatory domain consists of a C terminal side Ca2+/CaM binding region (CaM-B) and an N terminal side autoinhibitory region (AI). Oxidation at Met 281/282 or autophosphorylation at Thr 287 prevent reassociation of the catalytic domain and the AI sequence, leading to constitutive, Ca2+/CaM-independent CaMKII activity.
The first stage of methionine oxidation (methionine sulfuxide) is enzymatically reversible by methionine sulfoxide reductase A (MsrA), while the second stage (methionine sulfone) is irreversible. At this point it is unknown how much CaMKII becomes terminally methionine oxidized in normal myocardium or in disease. MsrA deletion increases susceptibility to death and heart failure from myocardial infarction (MI)20, while MsrA over-expression prevents CaMKII oxidation and is protective against post-MI cardiac rupture in mice12. MsrA over-expression prolongs lifespan in flies (D. melanogaster)21, while MsrA knock out increases mortality in mice22 and worms (C elegans)23. These findings suggest that CaMKII is a critical target for the benefits of MsrA over-expression and for the detrimental effects of MsrA inhibition. CaMKII isoforms β, δ and γ have regulatory domain methionine pairs (the numbering varies slightly between them), while CaMKII α has a cysteine-methionine pair that appears to induce Ca2+/CaM-independent CaMKII activity, similar to oxidized methionine pairs. Although CaMKII requires Ca2+/CaM binding for initial activation, oxidized CaMKII (ox-CaMKII) resets the Ca2+-sensitivity of CaMKII so that very low levels of intracellular Ca2+ are sufficient for activation24, suggesting that conditions of high ROS may lead to increased CaMKII activity even at resting levels of Ca2+/CaM. Thus, autophosphorylation and oxidation both have the capacity to ‘lock’ CaMKII into a Ca2+/CaM-autonomous conformation with sustained activity. Oxidized, constitutively active CaMKII is now linked to heart failure20, tachyarrhythmias25–27 , bradyarrhythmias28 and post-myocardial infarction cardiac rupture,12suggesting that CaMKII is an important ROS sensor for transduction of oxidative stress into clinically-important disease phenotypes7.
Myocardial ultrastructure determines the consequences of pathological CaMKII activation
Myocardial contraction is built around an elaborate membrane ultrastructure that places sarcolemmal domains enriched with ion channels in close approximation with sarcoplasmic reticulum (SR) Ca2+ release sites (Fig 3). This ultrastructure allows for feedback between intracellular Ca2+ and sarcolemmal excitability, a complex and exquisitely tuned set of relationships that are disturbed in patients with structural heart disease. One view is that the cell membrane system that supports the ECC machinery also determines the association between heart failure, arrhythmias and sudden cardiac death. CaMKII is enriched in the vicinity of the T-tubular sarcolemmal membrane invaginations29,30 that form the ultrastructural framework for closely (~10 nm) approximating the SR Ca2+ release ryanodine receptor (RyR2) channels and sarcolemmal voltage-gated Ca2+ channels. The presence of CaMKII at CaV1.2 (the predominant voltage-gated Ca2+ channel in ventricular muscle) and at RyR2 may serve to coordinate and promote physiological activation of these ECC proteins31 to increase the force-frequency relationship (i.e. the tendency of myocardium to generate more tension at faster stimulation rates)32 and/or to engage ‘fight or flight’ acceleration of heart rate33,34. CaMKII is now recognized to bind to multiple voltage-gated ion channels (Ca2+, Na+ and K+)35, some of which will be discussed later in the context of disease. Although unproven, it seems likely that CaMKII coordinates physiological adaptations of membrane excitability to changes in intracellular Ca2+ and ROS7. The enrichment of CaMKII in particular subcellular domains29,36 suggests that CaMKII is guided by targeting mechanisms. Some protein kinases use a strategy of anchoring proteins to achieve proximity to target proteins and to other regulatory proteins, such as phosphatases. For example, A Kinase Anchoring Proteins37 and Receptors for Activated C Kinase38 provide a structural basis for targeting protein kinase A and protein kinase C in a variety of tissues, including myocardium. In contrast, there is no known family of anchoring proteins for CaMKII. Instead, CaMKII likely identifies target proteins using regions that may mimic the topology of its own regulatory domain autoinhibitory region (Figure 4). We identified short sequences with high homology to the CaMKIIδ autoinhibitory region on a wide variety of protein targets, and validated that these sequences are necessary for proarrhythmic effects of CaMKII on CaV1.239 and NaV1.536. Thus, CaMKII is enriched in at least one subcellular domain, marked by T-tubular membranes, that is a hotspot for arrhythmias and heart failure.
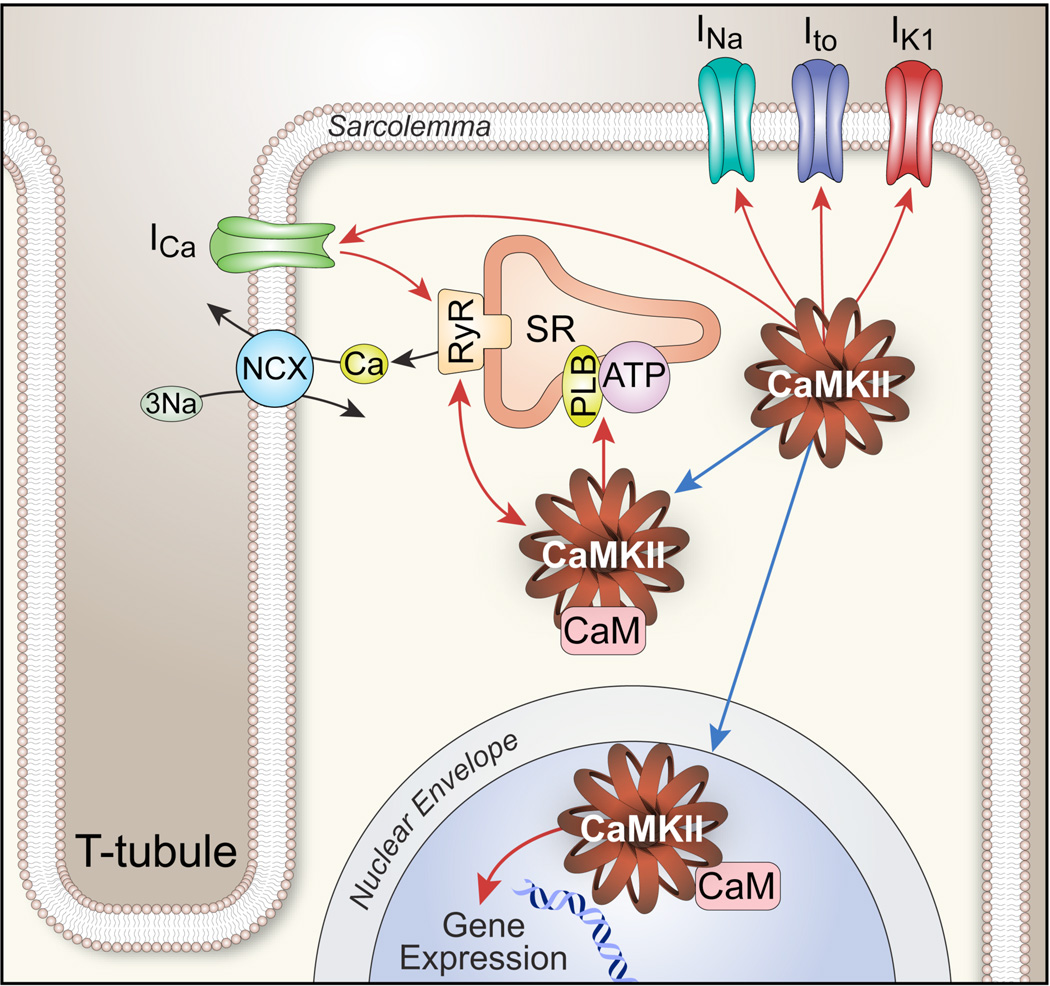
Figure 3. Myocardial cell ultrastructure creates close associations between CaMKII targets important for membrane excitability and intracellular Ca2+ homeostasis.
Activated CaMKII enhances cellular Ca2+ entry by catalyzing phosphorylation of voltage-gated Ca2+ channels (mostly CaV1.2 in ventricle) and ryanodine receptor (RyR2) Ca2+ release channels. CaV1.2 and RyR2 are brought into close association (~10 nm) by CaV1.2 enrichment on T-tubular sarcolemmal invaginations. CaMKII phosphorylation of voltage-gated Na+ channels (mostly NaV1.5 in ventricle) increases subsarcolemmal Na+ concentration that disadvantages the electrochemical driving force for Ca2+ extrusion from the cell by the Na+/Ca2+ exchanger (NCX), leading to increased intracellular Ca2+ concentration.
Figure 4. CaMKII anchoring at CaV1.2 and NaV1.5.
CaMKII associates with two important voltage-gated ion channels by catalytic domain binding to a recognition sequence that resembles the autoinhibitory region of the CaMKII regulatory domain. A sequence conserved across validated CaMKII targets is present on the β subunit of CaV1.2 (bottom panel) and on βIV spectrin, a cytoskeletal protein associated with Ankyrin G and the NaV1.5 (top panel). These sequences are required for CaMKII to regulate CaV1.2 and NaV1.5 currents.
CaMKII activity and expression in heart failure
CaMKII activation is a key part of a broader paradigm of interdependent pathological signals in heart failure and arrhythmia (also discussed under Systems Biology). Sympathetic nervous system activity is increased by a pressing need to maintain contractility in heart failure. Although activation of neurohumoral signaling pathways may be adaptive initially, chronic elevation of neurohormonal activity contributes to increased intracellular Ca2+ and ROS that are thought to promote disease progression 40. CaMKII is activated downstream to the three neurohormone agonists validated to be therapeutic targets for heart failure and arrhythmias. Most evidence for these pathways – β adrenergic receptor41, angiotensin II20 and aldosterone12 is for heart failure after myocardial infarction42–44. Mice lacking α-adrenoreceptors develop sympathetic hyperactivity-induced heart failure, increased mortality and had elevated total and threonine 287-autophosphorylated CaMKII levels compared to WT animals. Dilated cardiomyopathy, exercise intolerance and CaMKII activity in these mice were partially reversed by an angiotensin II receptor antagonist45, further suggesting that the success of angiotensin II antagonist drug therapy is at least partially due to CaMKII inhibition. Several studies have shown the role of CaMKII in the causation and progression of cardiac hypertrophy1,46–48, an independent risk factor for developing heart failure2,49. CaMKII is increased at the transcriptional level, protein level and in its activity in heart failure3,50. Heart failure occurs in mice with transgenic CaMKII over-expression4,51 and myocardial infarction causing heart failure also occurs with increases in myocardial CaMKII activity and expression in mice5,12, rabbits 6,7,52, dogs8,8,9,53 and patients7,54. CaMKII activation may be related to ventricular wall stress, as dogs with heart failure due to left bundle branch ablation and rapid pacing showed increased CaMKII activity in the lateral left ventricular wall compared to the septum. Furthermore, the increase in lateral wall CaMKII activity and expression was normalized by cardiac resynchronization (biventricular) pacing55. Although little is known about the transcriptional programs that increase CaMKII expression in heart failure, calcineurin activity and/or expression increase in animal models10,56 and patients with structural heart disease11,57,58 and calcineurin promotes myocardial expression of CaMKII11–13,59. This example may point to a broader paradigm where interdependence between various pathological signals ultimately produce severe, progressive forms of heart failure and sudden death. Transgenic myocardial overexpression of a constitutively active form of calcineurin causes severe myocardial hypertrophy, mechanical dysfunction, and premature death in mice14,60. CaMKII protein levels and activity are markedly increased in calcineurin transgenic mice compared to WT littermates and that CaMKII inhibition improved mechanical function and reduced mortality, but with minimal impact on myocardial hypertrophy15,59, suggesting that CaMKII inhibition could reduce heart failure and sudden death even in the face of forced over-expression of calcineurin. Taken together, these findings suggest that CaMKII may play a crucial role in myocardial hypertrophy and heart failure, while core, clinically-validated pathways that drive progression of heart failure and sudden death after myocardial infarction converge to activate CaMKII in myocardium.
The mechanism of CaMKII hyperactivity in heart failure is likely due to either autophosphorylation of threonine 287 or oxidation of methionines 281 and 282 (Fig 1); these post-transcriptionally modified amino acids are located in the CaMKII regulatory domain (Fig 2). Autophosphorylated CaMKII levels were elevated in mice up to 7 days after transaortic constriction, a surgical model of acute pathological left ventricular afterload16,51. Autophosphorylated CaMKII17,18 was increased in a pacing-induced rabbit model of left ventricular dysfunction and incessant ventricular tachycardia (VT storm)61. Rabbits treated chronically with a calmodulin antagonist, W-7, had improved left ventricular function, arrhythmia termination and restoration of autophosphorylated CaMKII to control levels. These recent findings provided the first functional evidence in a ‘large’ animal model that excessive CaMKII activation was causal in myopathy and arrhythmias19,61. Angiotensin II20 and aldosterone12 appear to increase ROS by activating NADPH oxidase. We found that oxidized CaMKII levels were significantly elevated in mouse heart12 and in the border zone of dog hearts days after myocardial infarction21,53, suggesting that oxidized CaMKII is a transduction signal for the increased oxidant stress that accompanies common forms of structural heart disease7,22. Intriguingly, CaMKII oxidation is reversed by MsrA and mice lacking MsrA develop increased levels of oxidized CaMKII, myocardial apoptosis, adverse left ventricular remodeling20,23, increased frequency of cardiac rupture and death after myocardial infarction12,24. In contrast, mice with myocardial transgenic MsrA over-expression show reduced mortality and are protected from aldosterone and myocardial infarction induced increases in oxidized CaMKII 12,20, suggesting that selectively targeted anti-oxidant therapy could protect against excessive CaMKII oxidation and increased mortality after myocardial infarction.
One-time static measurements of CaMKII have been made in humans with heart failure and have provided potential insights into the role of CaMKII in cardiomyopathy. CaMKIIδ mRNA and protein were significantly elevated in hearts from patients with dilated cardiomyopathy, compared to hearts from donors without structural heart disease25–27,62. Left ventricular tissue from failing human hearts with dilated, non-ischemic cardiomyopathy showed increased CaMKII activity compared to non-failing hearts, while myocardial tissue from ischemic cardiomyopathy patients had a trend towards elevation in left ventricular CaMKII activity28,63. Protein levels of cytosolic and nuclear splice variants of CaMKIIδ were higher in failing human right and left ventricle than in controls, regardless of whether cardiomyopathy was due to ischemic or non-ischemic causes12,54. We recently measured oxidized CaMKII levels in the right atrium of heart failure patients with or without sinus node dysfunction and compared them to normal controls. We found that oxidized CaMKII levels are elevated in the right atrium of heart failure patients in the vicinity of the sinoatrial node. The increase in oxidized CaMKII is particularly pronounced in heart failure patients with co-existent sinoatrial (SA) node dysfunction, compared to those with similarly severe heart failure but no SA node dysfunction 7,28. Thus, a combination of data from animal models and from failing human myocardium suggests that CaMKII activity and expression are increased in injured and failing hearts.
CaMKII target proteins
Ion channels are multi-subunit protein complexes built around a transmembrane permeation pore. A full discussion of CaMKII and ion channels is beyond the scope of this review, but is addressed more completely in other recently published reviews 29,30,64. CaMKII was first identified to increase L-type Ca2+ current (ICa), where CaMKII mediated phosphorylation induced a dynamic pattern of increasing peak current accompanied by slowing of inactivation31,65–67. However, CaMKII now appears to influence myocardial cell membrane excitability by affecting most or all known voltage-gated ion channels. What follows is a brief overview of CaMKII actions on ion channels where CaMKII actions are thought to contribute to arrhythmias.
L-type Ca2+ Channels
CaMKII-catalyzed phosphorylation of L-type Ca2+ channels (CaV1.2) promotes entry into a highly active gating mode (mode 2), characterized by a high opening probability and prolonged opening events32,68. Mode 2 gating appears to underlie ICa facilitation, a dynamic pattern of increased peak ICa and slowed ICa inactivation, based on modeling studies33,34,69 and because mutations that prevent mode 2 gating also reduce ICa facilitation35,39. Mode 2 gating7,70 and ICa facilitation are hypothesized to be proarrhythmic by favoring early afterdepolarizations (EADs) 29,29,36,39,71and SR Ca2+ leak37,72, inward Na+/Ca2+ exchanger current38,73 and delayed afterdepolarizations (DADs)39,74. EADs and DADs of sufficient magnitude are able to trigger arrhythmias. Increased CaV1.2 current can contribute to action potential prolongation36,75 and mode 2 gating is particularly proarrhythmic in the setting of action potential prolongation40,76. Mode 2 gating was first identified using BayK,70, and BayK 8644 induces polymorphic ventricular tachycardia in an established rabbit model of QT interval prolongation and Torsade de Pointes77,78, suggesting that enhancement of mode 2 gating is sufficient to trigger life-threatening ventricular arrhythmias in the setting of proarrhythmic QT interval prolongation, (e.g. long QT syndromes and cardiomyopathy). L-type Ca2+ channels are formed as a constellation of subunit proteins, including the α (pore-forming) and β (regulatory) subunits. The β subunit acts as a chaperone to increase α subunit membrane expression12,79 and engages the α subunit to increase channel opening probability 30,39,42–44,80. CaMKII does not appear to affect the chaperone activity of β subunits, because WT β subunit over-expression and over-expression of β subunit mutants lacking CaMKII binding and phosphorylation sites are equally effective at increasing ICa density in cultured adult cardiomyocytes 30,39,45. CaMKII binding to β subunits is enhanced by Leu 493 and CaMKII-catalyzed phosphorylation of Thr 498 is critical for facilitation of ICa in isolated adult rat30 and rabbit39 ventricular myocytes. The Thr 498 phosphorylation site is conserved across β subunit isoforms, but was initially identified on isoform β2a. The expression of the β2a isoform is increased in failing human hearts81, a condition marked by increased CaV1.2 opening probability82, APD prolongation, loss of intracellular Ca2+ homeostasis83, EADs84 and excessive cardiomyocyte death85. Over-expression of WT β2a in adult cardiomyocytes increases ICa, induces SR Ca2+ overload, and stimulates apoptosis by a pathway that involves CaMKII82. However, over-expression of a β2a mutant lacking this CaMKII phosphorylation site (T498A) does not increase myocyte death or EADs39, supporting the hypothesis that CaMKII phosphorylation of β2a is a molecular mechanism for pathological membrane excitability and cardiomyocyte death.
Voltage-gated Na+ Channels
Failing myocardium is marked by an electrical remodeling process where reduction in net repolarizing inward current leads to action potential and QT interval prolongation. Although cardiac voltage-gated Na+ channels (mostly NaV1.5) open and close rapidly (1–10 ms), a persistent (late) component of Na+ current (INaL), appears to contribute to action potential prolongation in a rare form of the long QT syndrome (LQT3)86 and in myocytes isolated from failing hearts87. Evolving evidence suggests that CaMKII associates with and phosphorylates cardiac Na+ channels25,88. CaMKII binding motifs have been localized to the inter domain I–II linker and the C-terminal region36,88–90. Interestingly, CaMKII phosphorylation has contrasting effects on INa, as it enhances late non-inactivating INaL but also decreases the availability of NaV1.5, a phenocopy of INa alterations seen during ischemia and heart failure and in LQT390,91. CaMKII inhibition reverses heart failure-induced increases in INaL36,88, suggesting that NaV1.5 is an important target for the proarrhythmic actions of CaMKII.
Voltage-gated K+ Channels
Voltage-gated K currents (IK) are the major driving force for myocardial membrane repolarization. IK is due to multiple ion channel proteins with diverse kinetic properties. Collectively, IK plays an important role in determining the action potential configuration and duration and multiple genetic long QT syndromes linked to arrhythmic sudden death are due to biophysical and trafficking defects in IK. Furthermore, reduction in IK is a consistent feature of cardiomyopathy that contributes to proarrhythmic action potential and QT interval prolongation. CaMKII activates the transient outward K+ current (Ito)92–95 and inwardly rectifying (IK1) K+ current96. Ito is comprised of Ito,fast (KV4.2/ KV4.3) and Ito,slow (KV1.4), and site-directed mutagenesis studies have identified KV4.3 S55095 and KV1.4 Thr60297 as potential phosphorylation targets for CaMKII. Increased CaMKII activity has been shown to slow Ito inactivation and accelerate recovery, with an overall effect of shortening the action potential and decreasing the refractory period. Chronic CaMKII activation leads to increased action potential duration98, likely in part by downregulation of Ito,fast and upregulation of Ito,slow, events that occur in failing cardiomyocytes96. Heart failure is also characterized by reduction in IK1, responsible for maintaining stable resting membrane potential. Interestingly, chronic CaMKII over-expression also leads to downregulation of IK1 and conversely, chronic CaMKII inhibition increases IK1 current96. Thus, reduction in various K+ currents contributes to proarrhythmic action potential prolongation in long QT syndromes and heart failure. CaMKII over-expression also causes reduced repolarizing K+ current and CaMKII inhibition shortens action potentials41, likely by increasing K+ currents. However, CaMKII actions are complex and appear to include transcriptional and post-transcriptional effects that defy simple, comprehensive models of CaMKII activity, action potential and QT interval duration. These findings suggest that cardiomyopathic changes in K+ current are at least partially the result of increased CaMKII activity in heart failure.
Ca2+ cycling proteins
Ryanodine receptor (RyR2), phospholamban (PLN) and SR Ca-ATPase (SERCA) are well-known regulators of SR calcium uptake and release and RyR299 and PLN100 are highly validated CaMKII targets. Although the exact mechanisms are yet to be defined, it is generally agreed that in failing cardiomyocytes there is a decrease in SR Ca2+ content and SR Ca2+ release during systole 83,101–103. Low SR calcium content may be a result of decreased uptake of Ca2+ by SERCA and/or increased diastolic SR calcium release (‘leak’). Abundant evidence now supports an important role of CaMKII in promoting heart failure and arrhythmias104. RyR2 is a SR Ca2+ release channel that is activated by a trigger of Ca2+ from ICa 105. RyR2 phosphorylation by protein kinase A and CaMKII both enhance ICa and RyR2 Ca2+ release. CaMKII helps coordinate this physiological process of Ca2+-induced Ca2+ release by phosphorylation of CaV1.2 and RyR2. However, in failing myocytes the cell membrane ultrastructure supporting Ca2+-induced Ca2+ release is distorted106 and CaMKII hyperphosphorylation of CaV1.2 and RyR2 becomes arrhythmogenic. CaMKII hyperphosphorylation of serine 2814 on RyR2 promotes RyR2 Ca2+ leak and arrhythmia-triggering delayed afterdepolarizations (DADs)72 while depleting SR Ca2+ to impair inotropy99,107–110. Excessive and unbalanced β-adrenergic stimulation in heart failure could potentially activate CaMKII by cAMP-EPAC dependent and cAMP independent mechanisms to hyperphosphorylate RyR, causing SR- Ca2+ leak and arrhythmias 111–113. During the normal cardiac cycle, intracellular Ca2+ is either pumped back into the SR (by SERCA) or extruded from the cytoplasm by the Na+/Ca2+ exchange protein (NCX1). Excess Ca2+ in diastole increases the likelihood for DADs due to the electrogenic action of NCX1. Interestingly, the pathological actions of CaMKII to favor DADs in atrial114 and ventricular74 myocardium mirror the physiological role of CaMKII to promote RyR2 Ca2+ leak and increased automaticity in SA nodal pacemaker cells33,34. While the role of CaMKII in RYR dysfunction in heart failure is controversial and in early studies mice with genetically altered RyR, resistant to CaMKII phosphorylation, are not protected from heart failure32, pathological effects of CaMKII were recently shown in knock-in mice with RyR genetically modified to mimic RyR phosphorylation by CaMKII (Ser 2814 Asp)115. After aortic banding RyR (Ser 2814 Asp) mice are more likely to develop heart failure and arrhythmias. Conversely, mice engineered with CaMKII phosphorylation-resistant RyR2 (Ser 2814 Ala) were protected from heart failure and arrhythmia after aortic banding surgery. These and other findings support a view that CaMKII promotes arrhythmias and myocardial mechanical dysfunction, in part, by effects on SR Ca2+ release.
CaMKII regulates SR Ca2+ content by affecting SR Ca2+ uptake, as shown by the findings that myocardial CaMKII inhibition reduces SR Ca2+ content in ventricular myocytes116 and sinoatrial nodal pacemaker cells33. PLN appears to be a target for CaMKII effects on SR Ca2+ content because knock out of PLN reverses the reduction in SR Ca2+ content in mice with myocardial CaMKII inhibition117. PLN is a negative regulator of SERCA118 but PLN phosphorylation by PKA (at serine 16) or CaMKII (at threonine 17) can reduce the inhibitory efficacy of PLN, leading to increased SERCA activity. Loss of PLN increases SR Ca2+ filling in mice with cardiomyopathy and sudden death due to over-expression of CaMKII119. However, PLN ablation leads to premature death and mitochondrial Ca2+ over-load, suggesting that increasing SR Ca2+, at least in the presence of excessive CaMKII activity, is disadvantageous. Whether or not CaMKII directly catalyzes SERCA phosphorylation and its physiological significance remain controversial120,121. Thus, CaMKII appears to act as a coordinating signal that favors SR Ca2+ flux, by enhancing SR Ca2+ uptake and release. These actions are likely important for myocardial ECC but are corrupted in cardiomyopathy and contribute to myocardial dysfunction and arrhythmias.
Inflammation and hypertrophy
There is substantial body of literature that links inflammation to cardiovascular disease, including heart failure and arrhythmias. Human patients with atrial fibrillation have higher blood levels of C-reactive protein, interleukin-6 (IL-6) and −8 (IL-8), compared to normal controls. Human atrial biopsies from patients with atrial fibrillation and animal models with atrial fibrillation have inflammatory infiltrates, suggesting an association between atrial fibrillation and inflammation. Markers of inflammation such as high sensitivity C reactive protein122 and chemokines, like IL-8 and MCP-1, are elevated in patients with sudden death and ventricular fibrillation 123, suggesting life-threatening ventricular arrhythmias and sudden death are favored by a pro-inflammatory state. CaMKIV affects inflammatory responses by aiding T-cell development, regulating IL-2 activity in T-cells and by modulating the activity of transcription factors124. However, the role of CaMKII in promoting inflammatory responses in myocardium is a newer finding. The α-isoform of CaMKII is present in macrophages where it promotes toll-like receptor (TLR) 4, 9, 3-triggered production of IL-6, tumor necrosis factor-alpha, and interferon-alpha/beta (IFN-alpha/beta) 125. CaMKII also regulates the localization of MHC-class II proteins in human dendritic cells 126. The pro-inflammatory actions of CaMKII in conventional inflammatory cells appear to be recapitulated, at least in part, in cardiomyocytes. We used a microarray to identify a cluster of pro-inflammatory genes regulated by CaMKII in cardiomyocytes, by comparing mRNA levels between control mice and mice with myocardial-delimited CaMKII inhibition due to expression of a CaMKII inhibitor peptide (AC3-I) that resembles the CaMKII regulatory domain, at baseline and after myocardial infarction. We found that complement factor B (CFB), a critical component of the alternative complement fixation pathway, was induced in cardiomyocytes and contributed to membrane injury by assembly into a membrane attack complex. Genetic inhibition of complement factor B reduced mortality and protected against adverse structural remodeling in hearts after MI127. The increase in CFB expression in myocardium by CaMKII is mediated through NF-κB, which has been shown to be activated by CaMKII in neuronal cells128 . More recently, we found that oxidized CaMKII is increased in response to lipopolysaccharide (LPS) a TLR-4 agonist in myocardium129 , suggesting that CaMKII activity is increased by inflammatory signals that increase oxidative stress, while at the same time CaMKII can act as a feed forward signal to augment transcription of inflammatory genes.
We used AC3-I mice with myocardial-targeted CaMKII expression as a probe to test the breadth of CaMKII-regulated transcription after myocardial infarction. We found that nearly half of the significantly up- or down-regulated mRNAs interrogated by our 8,500 element array were regulated by CaMKII expression three weeks after myocardial infarction, suggesting CaMKII activation has important consequences for transcriptional reprogramming after myocardial infarction127 . Because myocardial CaMKII inhibition appears to be protective in the setting of MI, at least in mice12,20,41, it seems likely that the preponderance of CaMKII-modulated transcriptional events after MI are ultimately maladaptive. To date, we have examined two CaMKII targets identified in this gene array in detail. The first was increased CFB expression that was down stream to CaMKII-dependent increases in NF-κB activity127. We also identified increased expression of Mmp9, a gene encoding matrix metalloproteinase 9 (MMP9). Recently, we found that aldosterone infusion led to non-genomic activation of CaMKII by oxidation and oxidized CaMKII enhanced Mmp9 expression causing increased mortality due to cardiac rupture by activating the transcription factor myocyte enhancer factor 2 (MEF2) 12. When aldosterone was infused in mice, to levels measured in high risk patients with heart failure resulting from myocardial infarction, at the time of myocardial infarction surgery we found excessive early mortality that was mostly the result of cardiac rupture. Although invading cells are a major source of MMP9, we found that mice with myocardial-delimited expression of AC3-I or MsrA were significantly protected from myocardial rupture, suggesting that CaMKII-dependent transcription of Mmp9 in heart muscle cells made a critical contribution to enhancing vulnerability to post-myocardial cardiac rupture. Three studies now show that CaMKII activation is critical for pathological responses of each of the therapeutically validated neurohumoral pathways that promote heart failure and sudden death: β adrenergic receptor signaling 41, angiotensin II signaling20, and aldosterone12.
Electrocardiographic left ventricular hypertrophy is an independent risk factor for sudden cardiac death130. The relationship of left ventricular hypertrophy to supraventricular arrhythmias like atrial fibrillation is also well established. The relationship between hypertrophy and arrhythmias could be favored by relative myocardial ischemia caused by the thickened ventricular wall, modified myocardial substrate related to fibrosis, and electrophysiological alterations in the hypertrophied myocardial cell. CaMKII appears to play an important role in ischemic and pressure overload induced hypertrophy, primarily by transcriptional regulation of hypertrophic genes. CaMKII mediated phosphorylation of class II HDACs, especially HDAC4 and HDAC5, derepress MEF-2 mediated gene expression131. CaMKδ phosphorylates HDAC4 preferentially compared to HDAC5, leading to activation of hypertrophic genes. CaMKIIδ knock out mice with pressure overload due to aortic banding have attenuated cardiac hypertrophy and less phosphorylated HDAC4 compared to controls46. Other labs have also shown that while HDAC5 phosphorylation does not change in CaMKIIδ knock out mice subjected to pressure overload compared to controls, and that these mice are protected from progressive left ventricular dilatation, myocardial decompensation and heart failure but not from hypertrophy132. Calcineurin, a Ca2+ and calmodulin-dependent phosphatase, is a powerful prohypertrophic signal by dephosphorylating the nuclear factor for activated T cells (NFAT), leading to increased nuclear localization of NFAT and transcription of hypertrophic genes60. On one hand, CaMKII decreases transcription of calcineurin, a regulator of the NFAT transcription factor involved in cardiac hypertrophy 133. On the other hand, calcineurin over-expression increases myocardial CaMKII expression. We found that CaMKII inhibition in mice with transgenic myocardial over-expression of calcineurin reduced cardiac arrhythmias, improved myocardial function and reduced mortality without a major effect on hypertrohpy 59. Taken together, we interpret these findings to suggest that a complex interplay between hypertrophic, inflammatory and likely yet to be identified transcriptional pathways are activated directly or indirectly by CaMKII. The potential complexity of these interactions is highlighted by a recent study showing that the role of CaMKII to promote pathological hypertrophy appears to be antagonized by protein kinase A (PKA), a protein kinase that is responsive to cyclic AMP and that is activated by catecholamines. Although mice with transgenic myocardial PKA over-expression showed myocardial hypertrophy134, PKA leads to an inhibitory, CaMKII-insensitive, cleavage product of HDAC4 that antagonizes hypertrophic actions of CaMKII135. Despite these complexities, we anticipate that the net effect of CaMKII in these pathways varies over time and differentially couples to upstream pathological stress signals, but in the aggregate down stream CAMKII actions are maladaptive and so are candidate targets for future antiarrhythmic and anti-cardiomyopathic therapies.
Cell death and Fibrosis
CaMKII induced apoptosis appears to play an instrumental role in both the development of heart failure after myocardial infarction and in the transition from compensated hypertrophy to decompensated cardiomyopathy in non-ischemic animal models. CaMKII induces apoptosis by an array of downstream mechanisms, some of which involve activation of pro-apoptotic proteases and others that involve transcriptional control on pro-apoptotic pathways. CaMKII can activate AP24, a pro-apoptotic protease, leading to DNA fragmentation136. CaMKII can also directly phosphorylate and activate pro-apoptotic factor BCL10. In addition, CaMKII can increase expression of pro-apoptotic genes that are downstream of the MAP kinase kinase TAK1137, and ASK 1138. Recently, CaMKII has been implicated in mitochondria dependent pro-death pathways. Activation of CaMKII by a CaV1.2 and SR Ca2+ release dependent pathway increases mitochondrial-triggered myocyte death82. Endoplasmic reticulum (ER) stress triggers an increase in cytosolic calcium and ROS that activates CaMKII, at least in part by oxidation, which can associate with mitochondria where it promotes release of cytochrome C, loss of mitochondrial membrane potential and mitochondrial dependent apoptosis139. CaMKII-dependent phosphorylation of PLN triggers mitochondria-dependent cell death140, consistent with the effects of PLN knock out in CaMKIIδ transgenic mice where mitochondrial Ca2+ and myocardial apoptosis are increased119. Transgenic mice over-expressing mActin develop dilated cardiomyopathy with four chamber enlargement, cardiac dysfunction, increased total and auto-phosphorylated CaMKII, increased expression of p53 and increased cardiomyocyte death. These maladaptive changes were ameliorated by CaMKII inhibition with KN-93 or interbreeding with AC3-I transgenic mice141. Our group showed that mice with myocardial CaMKII inhibition due to transgenic expression of AC3-I were resistant to apoptosis by angiotensin II infusion20, and 2 hours and 5 hours after myocardial infarction116. The AC3-I transgenic mice were protected from myocardial infarction-induced systolic dysfunction compared to WT and transgenic control mice41. In a Langendorff-perfused isolated heart preparation, ischemia-reperfusion injury caused an increase in CaMKII activity, increased caspase 3 activity and myocardial apoptosis, all of which were ameliorated by the addition of CaMKII inhibitory drug KN-93142. We interpret these findings, in diverse models, to suggest that CaMKII-triggered apoptosis contributes to adverse left ventricular remodeling and mechanical dysfunction.
CaMKII is activated by catecholamine stimulation, and initially it was thought that CaMKII activation was due exclusively to increased intracellular Ca2+ that followed protein kinase A-dependent phosphorylation of Ca2+ homeostatic proteins. However, Zhu et al showed that β adrenergic receptor stimulation with isoproterenol can promote cardiomyocyte cell death by activating CaMKII, independent of PKA in vitro143. We found AC3-I mice were significantly resistant to isoproterenol-induced apoptosis in vivo. This resistance to isoproterenol-induced apoptosis was reduced in mice with myocardial AC3-I expression, but lacking PLN, a negative regulator of sarcoplasmic reticulum Ca2+ uptake116, by Ca2+ loading the SR even when CaMKII was inhibited. β adrenergic receptor type 1 knock out mice are protected from increased CaMKII activity, and cell death after myocardial infarction144, consistent with findings that show CaMKII binds selectively to type 1 β adrenergic receptors13. Recently, an alternative pathway for activating CaMKII after myocardial infarction has been shown by our group. This pathway involved the activation of CaMKII by oxidation of the methionine 281/282 residues in the regulatory domain. We found that myocardial infarction or angiotensin II caused increased oxidized CaMKII leading to cell death and mechanical dysfunction20. Subsequently other investigators showed that angiotensin II caused myocyte death mediated by ROS- dependent CaMKII activation causing p38 MAPK activation24. Thus, a variety of upstream signals converge on CaMKII to increase apoptosis, mechanical dysfunction and premature death.
The role of CaMKII to increase cell death may be linked to arrhythmias. We recently found that CaMKII activation can cause cellular arrhythmias and death by phosphorylating an accessory β subunit of the voltage-gated Ca2+ channel CaV1.2. CaMKII-catalyzes phosphorylation of threonine 498 on β2a, leading to increased Ca2+ current, increased sarcoplasmic reticulum Ca2+, arrhythmia-initiating afterdepolarizations and cell death. Both afterdepolarizations and cell death could be reduced or prevented by CaMKII inhibition, inhibition of sarcoplasmic reticulum Ca2+ release or by elimination of the β2a CaMKII phosphorylation site39. Mice with transgenic myocardial over-expression of CaMKIIδ show increased myocardial apoptosis, afterdepolarizations, arrhythmias and premature death145. We interpret these findings to suggest that pro-death actions of excessive CaMKII activity are likely to promote heart failure and contribute to pro-arrhythmic tissues substrates.
Sinus node physiology and dysfunction
The rhythm in the mammalian heart is set by autonomous excitation of the specialized pacemaker cells in the SA node. In conditions requiring increased cardiac output, the heart ramps up its beating rate while maintaining the rhythm. Until recently, the spontaneous action potentials in SA node cells were thought to be exclusively governed by a hyperpolarization activated cyclic nucleotide gated channels (HCN4)146. In the last two decades our understanding of this process has been refined by experimental evidence describing the role of intracellular calcium cycling in pacemaker activity. Coordinated action between SERCA and RyR in SAN cells causes rhythmic calcium oscillations, setting up a series of events leading to a net positive charge in the cell in late diastole, culminating in action potential initiation and enhanced automaticity147. Localized calcium release events (LCRs) were shown by Vinogradova et al to be dependent on CaMKII activity, because inhibition of CaMKII by AIP, an inhibitory peptide, or KN-93 led to decreased ICa,L amplitude and SAN action potential formation148. We recently showed that CaMKII plays an essential role in increasing heart rate in response to “fight or flight,” mediating catecholamine induced heart rate increases by increasing SR calcium content, SR Ca2+ release and inward INCX, leading to enhancement of the diastolic depolarization rate (DDR). In contrast, CaMKII did not appear to affect the HCN4 mediated pacemaker current (If). We also showed that mice with genetic CaMKII inhibition failed to increase diastolic calcium sparks, SR calcium and DDR in response to isoproterenol, even though their resting heart rates were similar to wild type mice33. Recent work from our lab shows that CaMKII activity is not only important in β adrenergic receptor stimulation induced heart rate response, but is also necessary for catecholamine independent chronotropic competence. We activated Ca2+-dependent pacing mechanisms directly by applying the L-type Ca2+ channel agonist Bay K8644 directly to SAN cells and Langendorff-perfused isolated hearts. BayK 8644 increased heart rate in a dose-dependent fashion over a similar dynamic range as isoproterenol, but without increasing If, suggesting that Ca2+-dependent mechanisms were fully capable of generating physiological fight or flight heart rate responses. SAN cells and isolated hearts from mice with genetic CaMKII inhibition were refractory to heart rate increases and had reduced late diastolic intracellular calcium release and INCX when treated with BayK 8644 compared to WT controls, even though Bay K increased ICa equally in all SAN cells 34. These findings suggested that CaMKII contributes to heart rate by a series of events that promote SR Ca2+ release and INCX in SAN cells.
While CaMKII activity is essential for the proper functioning of the cardiac pacemaker cells, excess CaMKII has deleterious effects resulting in SA node dysfunction. Recently we showed that angiotensin II, a neurohormone found in excess in heart failure, when infused into mice, increased SA nodal oxidized CaMKII, increased SA node cell death and decreased resting HR and activity related heart rate increases28. Interestingly, human patients with heart failure and SA node dysfunction had increased oxidized CaMKII in the right atria compared to patients with heart failure but no SA node dysfunction. Mice with genetic cardiac CaMKII inhibition and mice lacking a critical subunit (p47) of important NADPH oxidases were protected from angiotensin II induced SA node cell death, fibrosis and dysfunction, indicating that NADPH oxidase mediated oxidation of CaMKII plays an essential role in angiotensin II induced toxicity in the SA node. We also showed that SA node targeted CaMKII inhibition was sufficient to protect against SA node effects of angiotensin II infusion. We developed a computational model that showed that angiotensin II-induced cell death in the SA node caused defective impulse formation and propagation, by creating a source-sink mismatch due to loss of SA node cells relative to surrounding atrial myocardium, leading to sinus node dysfunction (Fig 5). These data suggest that although CaMKII is critical for cardiac pacemaking and maintenance of a normal sinus rhythm, excess CaMKII, especially in conditions of increased oxidant stress, such as heart failure and/or angiotensin II infusion, promotes cardiac arrhythmias.
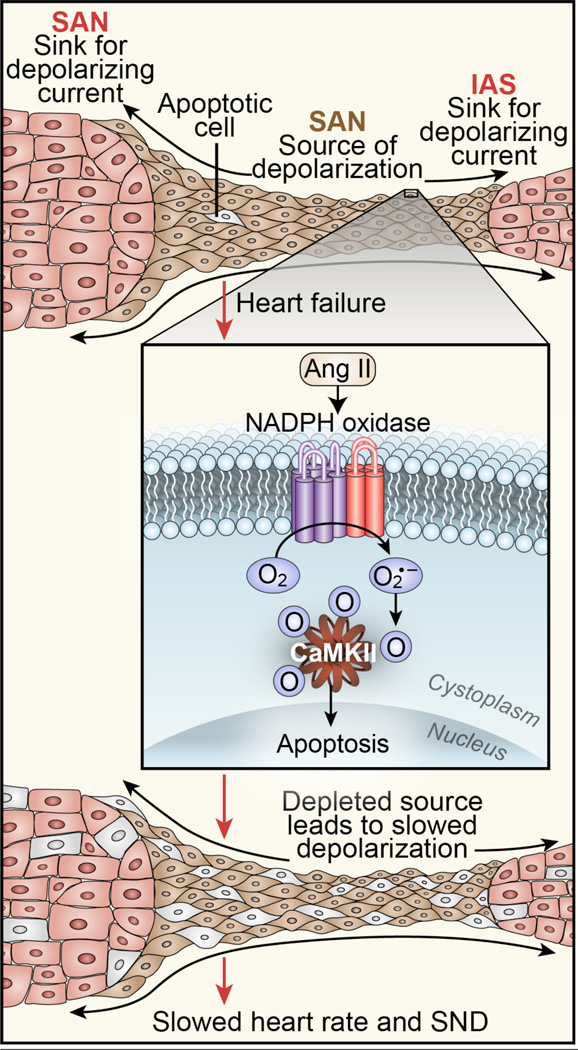
Figure 5. Oxidized CaMKII leads to SA node cell death and SA node dysfunction.
Under physiological conditions (top) the SA node initiates each heart beat by inducing an inward current of sufficient magnitude to depolarize surrounding atrial myocardium with high fidelity. Activation of NADPH oxidase by angiotensin II leads to CaMKII activation by oxidation and excessive oxidized CaMKII leads to SA node cell apoptosis. SA node dysfunction occurs when SA node cell loss beyond a critical threshold prevents high fidelity capture of surrounding myocardium (bottom).
Atrial Fibrillation
Emerging evidence suggests that CaMKII may play an important role in modulating signaling pathways leading to atrial fibrillation, a rhythm that is particularly common in patients with heart failure149and SA node dysfunction150. CaMKII is activated by upstream signaling pathways including catecholamines 41,151, the renin-angiotensin-aldosterone system (RAAS)12,20 and reactive oxygen species20,25,28 that are critical to structural and electrical remodeling in heart failure152 and atrial fibrillation153. Most fundamental evidence of the role of CaMKII in atrial fibrillation comes from mouse models where molecular hypotheses can be directly tested. However, it is important to examine this evidence in the context of the clinical disease and data acquired from clinical and translational studies. Atrial myocytes from patients with atrial fibrillation express elevated levels of CaMKII92,114 and show increased phosphorylation of downstream target proteins RyR2154 and PLN155. Interestingly, atrial myocytes from patients with atrial fibrillation also show increased spontaneous Ca2+ spark frequency that is normalized by CaMKII inhibition156.
In many cases, AF is thought to originate in pulmonary vein ostia in the left atrium157 and in isolated rabbit pulmonary vein tissue isoproterenol stimulates non-sustained spontaneous arrhythmias158, that are suppressed by the CaMKII inhibitor, KN-93158. Transgenic mice expressing the CaMKII inhibitory peptide AC3-I41 or knock-in mice with CaMKII resistant RyR2 Ser 2814 Ala114 have proved to be useful in testing the role of CaMKII and SR calcium release in atrial fibrillation without potential off-target actions of currently available small molecule CaMKII inhibitors. RyR2 R176Q/+ knock-in mice have enhanced diastolic SR Ca2+ leak and rapid atrial pacing increases phosphorylation of RyR2 Ser 2814, a validated CaMKII site, leading to significantly higher atrial fibrillation as compared to wild type mice114. Mice with cardiac-specific overexpression of CREM-IbΔC-X develop spontaneous atrial fibrillation and atrial myocytes isolated from these mice show increased phosphorylation of RyR2 at Ser 2814159. FKBP12.6 is a protein that is hypothesized to stabilize RyR2 and mice lacking FKBP12.6 are prone to spontaneous and pacing induced atrial fibrillation160. Cross-breeding FKBP12.6 knock-out mice with Ser 2814 Ala knock-in mice inhibits the SR calcium leak normally present in FKBP 12.6−/− atrial myocytes and decreases atrial fibrillation160. Taken together, these studies suggest an important role of CaMKII activation and subsequent RyR2 phosphorylation in contributing to atrial fibrillation.
Ventricular arrhythmias
CaMKII inhibition, through pharmacological and/or transgenic means, has been shown to prevent or suppress ventricular arrhythmias in a variety of animal models of both acquired and congenital cardiac disease. In fact, the anti-arrhythmic benefit of targeted disruption in CaMKII signaling extends across a relatively broad range of disorders with unique phenotypic presentations.
Congenital arrhythmia disorders
CaMKII has emerged as a novel anti-arrhythmic target in several forms of congenital heart disease that for the most part confer arrhythmia susceptibility by interfering with normal intracellular Ca2+ cycling. Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia syndrome characterized by stress-induced arrhythmias in a structurally normal heart without any electrocardiographic indications under resting conditions. Genetic mutations in RyR2 encoding for the cardiac SR Ca2+ release channel are the primary cause of CPVT. The role of CaMKII in regulating Ca2+ cycling and its involvement in the β adrenergic receptor signaling cascade make it a logical therapeutic target in CPVT. Consistent with this hypothesis, pharmacological CaMKII inhibition has recently been shown to completely eliminate stress-induced arrhythmias and triggered activity in the R4496C mouse model of CPVT161.
Timothy syndrome (also referred to as long-QT syndrome 8) is a rare autosomal dominant disease caused by mutations in L-type Ca2+ channel (Cav1.2) and characterized by ventricular tachycardia and sudden death162. At the cellular level, Cav1.2 Timothy syndrome mutations result in impaired Cav1.2 voltage-dependent inactivation and inappropriate Ca2+ entry that, in turn, activates intracellular signaling cascades, including CaMKII. In some critical aspects CaMKII activation phenocopies Timothy syndrome by promoting action potential prolongation and arrhythmias98,163. Recent studies expressed a Cav1.2 Timothy syndrome mutant (Gly 406 Arg) in adult rat ventricular myocytes to examine the therapeutic potential of CaMKII inhibition in Timothy syndrome. Consistent with the proposed link between increased Ca2+ entry, CaMKII activation and arrhythmias, CaMKII inhibition normalized action potential duration, SR calcium content and SR calcium transients and prevented arrhythmogenic afterdepolarizations in myocytes expressing the Timothy syndrome mutant CaV1.2164. These findings support the idea that CaMKII represents a promising therapeutic target for preventing arrhythmias associated with Timothy syndrome, similar to CPVT. Furthermore, it is interesting to consider the likelihood that, in a broader sense, genetic arrhythmias associated with inappropriate Ca2+ cycling (e.g. CPVT, Timothy syndrome, other long-QT syndromes) may respond well to CaMKII inhibition.
Common forms of acquired ventricular arrhythmias
Of course, arrhythmias linked to defects in Ca2+ signaling are not restricted to rare congenital forms of heart disease and CaMKII inhibition has been shown to prevent ventricular arrhythmias in a variety of animal models of acquired cardiac disease. Early studies showed that CaMKII inhibition prevented arrhythmias and afterdepolarizations in transgenic mice with cardiac hypertrophy induced by overexpression of constitutively active CaMKIV163. For uncertain reasons the CaMKIV transgenic mice also have elevated levels of myocardial CaMKII. Similarly, improved left ventricular function and decreased arrhythmias were observed with CaMKII inhibition in calcineurin-overexpressing mice with severe cardiomyopathy59. Finally, CaMKII inhibition prevents cellular afterdepolarizations and isoproterenol-induced arrhythmias in vivo in transgenic mice over-expressing CaMKIIδC with hypertrophic and dilated cardiomyopathy51,145. Going forward, it will be interesting to dissect the specific contributions of individual CaMKII targets to better understand the beneficial effects of CaMKII inhibition in hypertrophy/heart failure. For example, recent work has shown that transgenic mice lacking the CaMKII phosphorylation site on the ryanodine receptor SR Ca2+ release channel are resistant to pacing-induced ventricular arrhythmias following aortic banding surgery115. Consistent with this finding, CaMKII inhibition has been shown to reduce RyR phosphorylation and SR Ca2+ leak in non-ischemic rabbit model of heart failure72. CaMKII inhibition has also been shown to be effective in rabbit chronic AV block models of acquired long-QT and electrical storm61,165.
The anti-arrhythmic benefit of CaMKII inhibition has also been explored in ischemic heart disease. Importantly, transgenic inhibition of CaMKII prevents structural remodeling after myocardial infarction41. Furthermore, elevated levels of autophosphorylated and oxidized CaMKII have been reported in a well-established canine model of arrhythmias due to myocardial infarction. Parallel computational studies indicate that CaMKII overactivity contributes to the arrhythmogenic substrate after myocardial infarction with CaMKII inhibition improving Ca2+ cycling and voltage-gated Na+ channel function in the infarct border zone53,166. Taken together these studies in both non-ischemic and ischemic animal models highlight the therapeutic anti-arrhythmic potential of CaMKII across a broad spectrum of acquired cardiac disease states.
Systems biology and CaMKII signaling
As we attempt to elucidate the critical pathways linking mechanical and electrical dysfunction in the failing heart, it will be important to consider alternative approaches that are well-suited for studying large and multi-scalar systems. Mathematical modeling has been applied for over half a century to provide important insight into fundamental processes governing complex biological systems. Recent advances in biology (particularly in genetics) along with technological advances in computing have elevated the profile of systems level approaches capable of integrating and analyzing large networks of data167. With its vast network of substrates and interacting proteins, CaMKII is an attractive target for a systems biology approach that combines “wet bench” science with computational biology and mathematical modeling to develop a quantitative understanding of CaMKII signaling pathways53,69,166,168–177. Furthermore, the multimeric nature of the CaMKII holoenzyme and its multiple modes of regulation (e.g. Ca2+/calmodulin, autophosphorylation, oxidation) lend themselves to a computational approach that is capable of answering questions difficult or even impossible to address in an experiment.
Systems biology has already generated important insights into the behavior and function of CaMKII in excitable cells. Early mathematical modeling studies demonstrated that CaMKII at the neuronal post-synaptic density is a critical mediator of long-term memory172,173,176. Specifically, these studies showed that due to its unique structural and biophysical properties, CaMKII operates as a molecular “switch” that can store information in a stable manner. While early computational studies focused on the role of CaMKII in neuronal function, recent efforts have analyzed CaMKII dynamics and function in heart. Notably, mathematical models of CaMKII activity have been successfully incorporated into whole-cell models of the cardiac action potential to investigate CaMKII function in the broader context of the intact cell53,69,166,169,171,174,175,178. These detailed models have advanced the field by helping to integrate the extensive and growing literature on CaMKII regulatory network in heart, and by increasing our understanding of CaMKII function in both normal and diseased states. For example, computational models have predicted an important role for CaMKII in the normal response of cardiac excitability and contractility to changes in pacing rate53,69,166,171,174,178,179. In fact, computer simulations have shown that CaMKII is ideally suited to detect pacing frequency (e.g. heart rate), and may effectively store a cell’s pacing history, similar to its role in neurons. The key features that allow CaMKII to sense pacing are its sensitivity to Ca2+/CaM and the ability for subunits to phosphorylate neighboring subunits (autophosphorylation). These studies address a critical outstanding question related to CaMKII in heart. Namely, with mounting evidence supporting a view of CaMKII as a purveyor of dysfunction in disease, why do cardiomyocytes express CaMKII at all? Dynamic modeling is just one area in which systems level approaches have contributed to our understanding of CaMKII function in heart. Computational biology has also helped identify potential new CaMKII targets and interacting proteins36. A computational screen of the human genome using a consensus CaMKII binding sequence found in the b2a subunit of the L-type Ca2+ channel generated a select list of potential binding partners, including cytoskeletal, mitochondrial, nuclear and membrane proteins36. One of these proteins, the actin-associated polypeptide bIV-spectrin, was validated as a CaMKII anchoring protein (CaMKAP) in vivo that targets CaMKII to the cardiomyocyte intercalated disc36.
Systems biology has also identified important roles for CaMKII in abnormal cell function in the diseased heart. Specifically, computational models have predicted a role for CaMKII overactivity in creating a substrate for arrhythmias in both congenital and acquired heart disease39,53,69,164,166,169,179. Following myocardial infarction, extensive ion channel and action potential remodeling is a hallmark of infarct border zone regions where arrhythmias are highly localized. Computer models have helped identify CaMKII overactivity (downstream of increased oxidation and autophosphorylation) as a nexus for pro-arrhythmic changes in mechanical and electrical function53,166. Similarly, other studies have shown that CaMKII may promote inappropriate membrane depolarizations (afterdepolarizations) that serve as arrhythmia triggers through direct phosphorylation of the L-type Ca2+ channel and/or RyR2 SR Ca2+ release channel39,179,180. More recently, computational modeling has shown that loss of SA node cells secondary to CaMKII overactivity can produce SA node dysfunction by changing the source-sink relationship between the primary pacemaker and surrounding atrial tissue28. Thus, models illustrate the multi-factorial way in which CaMKII can regulate not only the trigger but also the substrate for life-threatening arrhythmias.
Going forward, systems biology will undoubtedly play a critical role in addressing some of the important unanswered questions in CaMKII pathophysiology. For example, how does CaMKII coordinate the heart’s “fight-or-flight” response to β adrenergic receptor stimulation? It is clear that CaMKII regulates the response of working myocardium to increases in heart rate, but how does CaMKII regulate heart rate itself? How is fidelity of CaMKII signaling maintained within the cell? Dedicated CaM tethered to substrates is one potential mechanism for controlling CaMKII signaling175 . Alternatively, CaMKAPs such as bIV-spectrin may define subcellular CaMKII signaling domains36. To what extent do these pathways fine-tune CaMKII regulation within the cell? How does CaMKII transform from an adaptive to maladaptive signaling node in disease? Clearly, there is overlap between the cellular pathways involved in regulating normal heart function and disease. What determines the breaking point when a physiological CaMKII response becomes detrimental? Can we eliminate or mitigate the negative elements of CaMKII signaling while preserving heart function (e.g. response to β adrenergic receptor stimulation)? Finally, with the ever-expanding list of CaMKII targets, mathematical models will become increasingly important for understanding broader roles for CaMKII in cardiomyocytes (e.g. apoptosis, mitochondrial energetics and pacemaking), as well as in other organ systems (e.g. brain, vasculature, pancreas).
Summary
Understanding of the roles for CaMKII in physiology and disease in heart has advanced dramatically over the past decade. Accumulated information is now sufficient to show CaMKII is a validated molecular mechanism and a potential therapeutic target for heart failure and arrhythmias. Much remains to be learned about how CaMKII integrates oxidant stress and Ca2+ to target specific proteins in cardiovascular and other systems. Findings in the cardiovascular system may be relevant to other diseases in other systems where elevated ROS and disrupted Ca2+ homeostasis are features of disease.
Acknowledgements
We are grateful for artistic contributions of Mr. Shawn Roach.
Sources of funding: This work was funded in part by National Institutes of Health (NIH) Grants R01 HL 079031, R01 HL 096652, and R01 HL 070250, the University of Iowa Research Foundation and the Fondation Leducq Transatlantic Alliance for CaMKII Signaling. P.D. Swaminathan’s salary was supported by a Kenneth M. Rosen Fellowship and Max Schaldach Fellowship from the Heart Rhythm Society and a University of Iowa Cardiovascular Center Interdisciplinary Research Fellowship. A. Purohit’s salary was supported by AHA postdoctor- al fellowship grant 10POST3620047. T.J. Hund has support from NIH grant R00 HL 096805 and the Gilead Sciences Research Scholars Program in Cardiovascular Disease.
Non-standard Abbreviations and Acronyms
- Ca2+/CaM
Calcified Calmodulin
- CFB
Complement factor B
- CPVT
Catecholaminergic polymorphic ventricular tachycardia
- DAD
Delayed afterdepolarization
- DDR
Diastolic depolarization rate
- EAD
Early afterdepolarizations
- ECC
Excitation-contraction coupling
- HR
Heart rate
- MMP9
Matrix metalloproteinase 9
- MsrA
Methionine sulfoxide reductase A
- NFAT
Nuclear factor for activated T cells
- ox-CaMKII
Oxidized Calmodulin Kinase II
- PLN
Phospholamban
- ROS
Reactive oxygen species
- RyR2
Ryanodine receptor
- SA Node
Sinoatrial node
- SERCA
Sarcoplamic endoplamic reticulum calcium ATPase
- SR
Sarcoplasmic reticulum
Footnotes
Disclosures: Dr Anderson is a co-founder of Allosteros Therapeutics and an inventor on patents claiming to treat heart failure and arrhythmias by CaMKII inhibition.
References
- 1.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on High Blood Pressure Research, Quality of Care Outcomes Research Interdisciplinary Working Group, Functional Genomics and Translational Biology Interdisciplinary Working Group. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research. Circulation. 2008;117(19):2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 2.Members WG, Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J Executive Summary: Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Committee OBOTAHAS Subcommittee SS Roger VL Turner MB Disease OBOTAHAH Group SSW. Circulation. 2011;123(4):459–463. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Brown T, Carnethon M. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 4.Krell MJ, Kline EM, Bates ER, Hodgson JM, Dilworth LR, Laufer N, Vogel RA, Pitt B. Intermittent, ambulatory dobutamine infusions in patients with severe congestive heart failure. Am. Heart J. 1986;112(4):787–791. doi: 10.1016/0002-8703(86)90475-8. [DOI] [PubMed] [Google Scholar]
- 5.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324(12):781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 6.Tomaselli GF, Barth AS. Sudden cardio arrest: oxidative stress irritates the heart. Nat Med. 2010;16(6):648–649. doi: 10.1038/nm0610-648. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol. Rev. 2011;91(3):889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P, Vitamin E. supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342(3):154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J. Cell Biol. 1994;126(4):839–852. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S, Gray CBB, Miyamoto S, Bers DM, Brown JH. Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ. Res. 2011;109(12):1354–1362. doi: 10.1161/CIRCRESAHA.111.248401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He BJ, Joiner M-LA, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17(12):1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangmool S, Shukla AK, Rockman HA. beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J. Cell Biol. 2010;189(3):573–587. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao LH, Stratton MM, Lee I-H, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, Kuriyan J. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell. 2011;146(5):732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoelz A, Nairn AC, Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol. Cell. 2003;11(5):1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256(5060):1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 17.Lou LL, Lloyd SJ, Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(24):9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. The Journal of Biological Chemistry. 1986;261(19):8581–8584. [PubMed] [Google Scholar]
- 19.Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. The Journal of Biological Chemistry. 1993;268(10):7163–7170. [PubMed] [Google Scholar]
- 20.Erickson JR, Joiner M-LA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan H, Tang XD, Chen M-L, Joiner M-LA, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu C-F, Hoshi T, Chen M-L, Joiner MA, Heinemann SH. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minniti AN, Cataldo R, Trigo C, Vasquez L, Mujica P, Leighton F, Inestrosa NC, Aldunate R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell. 2009;8(6):690–705. doi: 10.1111/j.1474-9726.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 24.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ. Res. 2009;105(12):1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 25.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive Oxygen Species-Activated Ca/Calmodulin Kinase II{delta} Is Required for Late INa Augmentation Leading to Cellular Na and Ca Overload. Circ. Res. 2011;108(5):555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L-H, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ. Res. 2009;104(1):79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita N, Lee JH, Bapat A, Fishbein MC, Mandel WJ, Chen PS, Weiss JN, Karagueuzian HS. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am. J. Physiol. Heart Circ. Physiol. 2011;301(1):H180–H191. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner M-LA, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen P-S, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J. Clin. Invest. 2011;121(8):3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am. J. Physiol. 1999;276(6 Pt 2):H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 30.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham A-JL, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol. Cell. 2006;23(5):641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation-contraction coupling. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proceedings of the National Academy of Sciences. 2010;107(22):10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XHT, Mohler PJ, Song LS, Anderson ME. From the Cover: Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proceedings of the National Academy of Sciences. 2009;106(14):5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, Singh MV, Hall DD, Koval OM, Luczak ED, Joiner MLA, Chen B, Wu Y, Chaudhary AK, Martins JB, Hund TJ, Mohler PJ, Song LS, Anderson ME. Catecholamine-Independent Heart Rate Increases Require Ca2+/Calmodulin-Dependent Protein Kinase II. Circ Arrhythm Electrophysiol. 2011;4(3):379–387. doi: 10.1161/CIRCEP.110.961771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson ME. Sticky fingers: CaMKII finds a home on another ion channel. Circ. Res. 2009;104(6):712–714. doi: 10.1161/CIRCRESAHA.109.195503. [DOI] [PubMed] [Google Scholar]
- 36.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J. Clin. Invest. 2010;120(10):3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267(5194):108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 38.Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20(44):6339–6347. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- 39.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. CaV1.2 β-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):4996. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2011;50(3):460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Khoo MSC, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Thiel W, Guatimosim S, Song L-S, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11(43):409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 42.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, Allaf El D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA: The Journal of the American Medical Association. 2000;283(10):1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 43.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003;348(14):1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J-L, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N. Engl. J. Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira JCB, Moreira JBN, Campos JC, Pereira MG, Mattos KC, Coelho MA, Brum PC. Angiotensin receptor blockade improves the net balance of cardiac Ca(2+) handling-related proteins in sympathetic hyperactivity-induced heart failure. Life Sci. 2011;88(13–14):578–585. doi: 10.1016/j.lfs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proceedings of the National Academy of Sciences. 2009;106(7):2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. The Journal of Biological Chemistry. 2007;282(48):35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 48.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Invest. 2006;116(7):1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J. Physiol. Heart Circ. Physiol. 2005;289(1):H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 50.Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol. Ther. 2005;106(1):39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhang T, Maier LS, Dalton ND, Miyamoto S, John Ross J, Bers DM, Brown JH. The {delta}C Isoform of CaMKII Is Activated in Cardiac Hypertrophy and Induces Dilated Cardiomyopathy and Heart Failure. Circ. Res. 2003;92(8):912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 52.Currie S, Smith GL. Calcium/calmodulin-dependent protein kinase II activity is increased in sarcoplasmic reticulum from coronary artery ligated rabbit hearts. FEBS Lett. 1999;459(2):244–248. doi: 10.1016/s0014-5793(99)01254-5. [DOI] [PubMed] [Google Scholar]
- 53.Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized Calmodulin Kinase II Regulates Conduction Following Myocardial Infarction: A Computational Analysis. Plos Comput Biol. 2009;5(12) doi: 10.1371/journal.pcbi.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittköpper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 2010;107(9):1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 55.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117(11):1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 56.Lim HW, De Windt LJ, Steinberg L, Taigen T, Witt SA, Kimball TR, Molkentin JD. Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation. 2000;101(20):2431–2437. doi: 10.1161/01.cir.101.20.2431. [DOI] [PubMed] [Google Scholar]
- 57.Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5(3):246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- 58.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103(5):670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 59.Khoo MSC, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114(13):1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 60.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-Related Signaling and Protein Phosphorylation Abnormalities Play Central Roles in a New Experimental Model of Electrical Storm. Circulation. 2011;123(20):2192–2203. doi: 10.1161/CIRCULATIONAHA.110.016683. [DOI] [PubMed] [Google Scholar]
- 62.Hoch B, Meyer R, Hetzer R, Krause E. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999 Apr 2;84(6):713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 63.Kirchhefer U. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42(1):254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 64.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009;54(3):180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ. Res. 1994;75(5):854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 66.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am. J. Physiol. 1994;267(3 Pt 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- 67.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(20):9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nature Cell Biology. 2000;2(3):173–177. doi: 10.1038/35004052. Available at: http://www.nature.com/ncb/journal/v2/n3/abs/ncb0300_173.html. [DOI] [PubMed] [Google Scholar]
- 69.Hashambhoy YL, Winslow RL, Greenstein JL. CaMKII-induced shift in modal gating explains L-type Ca(2+) current facilitation: a modeling study. Biophys J. 2009;96(5):1770–1785. doi: 10.1016/j.bpj.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 71.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharmacol. Exp. Ther. 1998;287(3):996–1006. [PubMed] [Google Scholar]
- 72.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97(12):1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Roden DM, Anderson ME. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ. Res. 1999;84(8):906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- 74.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J. Mol. Cell. Cardiol. 2010;49(1):25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashambhoy YL, Greenstein JL, Winslow RL. Role of CaMKII in RyR leak, EC coupling and action potential duration: a computational model. J. Mol. Cell. Cardiol. 2010;49(4):617–624. doi: 10.1016/j.yjmcc.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erickson JR, Anderson ME. CaMKII and its role in cardiac arrhythmia. J. Cardiovasc. Electrophysiol. 2008;19(12):1332–1336. doi: 10.1111/j.1540-8167.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 78.Mazur A, Roden DM, Anderson ME. Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation. 1999;100(24):2437–2442. doi: 10.1161/01.cir.100.24.2437. [DOI] [PubMed] [Google Scholar]
- 79.Fang K, Colecraft HM. Mechanism of auxiliary β-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J. Physiol. (Lond.) 2011. 589(Pt 18):4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(18):7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hullin R, Matthes J, Vietinghoff von S, Bodi I, Rubio M, D'Souza K, Khan IF, Rottländer D, Hoppe UC, Mohacsi P, Schmitteckert E, Gilsbach R, Bünemann M, Hein L, Schwartz A, Herzig S. Increased Expression of the Auxiliary β2-subunit of Ventricular L-type Ca2+ Channels Leads to Single-Channel Activity Characteristic of Heart Failure. PLoS ONE. 2007;2(3):e292. doi: 10.1371/journal.pone.0000292. Available at: http://dx.plos.org/10.1371/journal.pone.0000292.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 2005;97(10):1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 83.Piacentino V. Cellular Basis of Abnormal Calcium Transients of Failing Human Ventricular Myocytes. Circ. Res. 2003;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 84.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ. Res. 1998;82(11):1206–1223. doi: 10.1161/01.res.82.11.1206. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9633920&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 85.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the Failing Human Heart. N. Engl. J. Med. 1997;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. Available at: http://www.nejm.org/doi/abs/10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 86.Bennett PB, Yazawa K, Makita N, George AL. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376(6542):683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 87.Tomaselli GF. What Causes Sudden Death in Heart Failure? Circ. Res. 2004;95(8):754–763. doi: 10.1161/01.RES.0000145047.14691.db. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 88.Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116(12):3127–3138. doi: 10.1172/JCI26620. Available at: http://www.jci.org/cgi/doi/10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. AJP: Cell Physiology. 2011;301(3):C577–C586. doi: 10.1152/ajpcell.00125.2011. Available at: http://ajpcell.physiology.org/cgi/doi/10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 90.Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O'Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85(3):454–463. doi: 10.1093/cvr/cvp324. Available at: http://cardiovascres.oxfordjournals.org/cgi/doi/10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AA, Balser JR. Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ. Res. 2000;86(9):E91–E97. doi: 10.1161/01.res.86.9.e91. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10807877&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 92.Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ. Res. 1999;85(9):810–819. doi: 10.1161/01.res.85.9.810. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10532949&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 93.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/Calmodulin Kinase II Differentially Modulates Potassium Currents. Circ Arrhythm Electrophysiol. 2009;2(3):285–294. doi: 10.1161/CIRCEP.108.842799. Available at: http://circep.ahajournals.org/cgi/doi/10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colinas O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006;291(4):H1978–H1987. doi: 10.1152/ajpheart.01373.2005. Available at: http://ajpheart.physiology.org/cgi/doi/10.1152/ajpheart.01373.2005. [DOI] [PubMed] [Google Scholar]
- 95.Sergeant GP. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. AJP: Cell Physiology. 2005;288(2):C304–C313. doi: 10.1152/ajpcell.00293.2004. Available at: http://ajpcell.physiology.org/cgi/doi/10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- 96.Li J, Marionneau C, Zhang R, Shah V, Hell JW, Nerbonne JM, Anderson ME. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ. Res. 2006;99(10):1092–1099. doi: 10.1161/01.RES.0000249369.71709.5c. [DOI] [PubMed] [Google Scholar]
- 97.Hagiwara K, Nunoki K, Ishii K, Abe T, Yanagisawa T. Differential inhibition of transient outward currents of Kv1.4 and Kv4.3 by endothelin. Biochemical and biophysical research communications. 2003;310(2):634–640. doi: 10.1016/j.bbrc.2003.09.062. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=14521958&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 98.Maier LS, Zhang T, Chen L, Desantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 2003;92(8):904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 99.Wehrens XHT, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004;94(6):e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 100.Kranias EG, Gupta RC, Jakab G, Kim HW, Steenaart NA, Rapundalo ST. The role of protein kinases and protein phosphatases in the regulation of cardiac sarcoplasmic reticulum function. Mol. Cell. Biochem. 1988;82(1–2):37–44. doi: 10.1007/BF00242513. [DOI] [PubMed] [Google Scholar]
- 101.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. Available at: http://www.nature.com/nature/journal/v415/n6868/full/415198a.html. [DOI] [PubMed] [Google Scholar]
- 102.Bers DM. Sarcoplasmic Reticulum Ca2+ and Heart Failure: Roles of Diastolic Leak and Ca2+ Transport. Circ. Res. 2003;93(487):490–205. doi: 10.1161/01.RES.0000091871.54907.6B. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 103.Lindner M. Calcium Content of the Sarcoplasmic Reticulum in Isolated Ventricular Myocytes from Patients with Terminal Heart Failure. J. Mol. Cell. Cardiol. 1998;30(4):743–749. doi: 10.1006/jmcc.1997.0626. Available at: http://linkinghub.elsevier.com/retrieve/pii/S002228289790626X. [DOI] [PubMed] [Google Scholar]
- 104.Wehrens XHT. CaMKII regulation of the cardiac ryanodine receptor and sarcoplasmic reticulum calcium release. Heart Rhythm. 2011;8(2):323–325. doi: 10.1016/j.hrthm.2010.09.079. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1547527110010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245(1):C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 106.Song L-S, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez P. Stoichiometric Phosphorylation of Cardiac Ryanodine Receptor on Serine 2809 by Calmodulin-dependent Kinase II and Protein Kinase A. Journal of Biological Chemistry. 2003;278(40):38593–38600. doi: 10.1074/jbc.C301180200. Available at: http://www.jbc.org/cgi/doi/10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 108.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca(2+)-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J. Physiol. (Lond.) 1997;501(Pt 1):17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9174990&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ. Res. 2006;99(4):398–406. doi: 10.1161/01.RES.0000236756.06252.13. Available at: http://circres.ahajournals.org/cgi/content/full/99/4/398. [DOI] [PubMed] [Google Scholar]
- 110.Yang D, Zhu WZ, Xiao B, Brochet DXP, Chen SRW, Lakatta EG, Xiao RP, Cheng H. Ca2+/Calmodulin Kinase II-Dependent Phosphorylation of Ryanodine Receptors Suppresses Ca2+ Sparks and Ca2+ Waves in Cardiac Myocytes. Circ. Res. 2007;100(3):399–407. doi: 10.1161/01.RES.0000258022.13090.55. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- 111.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and Phospholipase Cε Regulate Ca2+ Release in theHeart by Activation of Protein Kinase Cε and Calcium-Calmodulin KinaseII. The Journal of Biological Chemistry. 2009;284(3):1514. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ. Res. 2007;100(3):391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 113.Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Bénitah J-P, Lezoualc'h F, Gómez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J. Physiol. (Lond.) 2007;583(Pt 2):685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XHT. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J. Clin. Invest. 2009;119(7):1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XHT. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122(25):2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Y, Zhu W-Z, Joiner M-L, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao R-P, Anderson ME. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am. J. Physiol. Heart Circ. Physiol. 2006;291(6):H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 117.Wu Y, Shintani A, Grueter C, Zhang R, Hou Y, Yang J, Kranias EG, Colbran RJ, Anderson ME. Suppression of dynamic Ca(2+) transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J. Mol. Cell. Cardiol. 2006;40(2):213–223. doi: 10.1016/j.yjmcc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Mattiazzi A, Kranias EG. CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm. 2011;8(5):784–787. doi: 10.1016/j.hrthm.2010.11.035. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1547527110012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ. Res. 2010;106(2):354–362. doi: 10.1161/CIRCRESAHA.109.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toyofuku T, Curotto Kurzydlowski K, Narayanan N, MacLennan DH. Identification of Ser38 as the site in cardiac sarcoplasmic reticulum Ca(2+)-ATPase that is phosphorylated by Ca2+/calmodulin-dependent protein kinase. The Journal of Biological Chemistry. 1994;269(42):26492–26496. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=7929371&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 121.Odermatt A, Kurzydlowski K, MacLennan DH. The vmax of the Ca2+-ATPase of cardiac sarcoplasmic reticulum (SERCA2a) is not altered by Ca2+/calmodulin-dependent phosphorylation or by interaction with phospholamban. The Journal of Biological Chemistry. 1996;271(24):14206–14213. doi: 10.1074/jbc.271.24.14206. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8662932&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 122.Kaneko H, Anzai T, Naito K, Kohno T, Maekawa Y, Takahashi T, Kawamura A, Yoshikawa T, Ogawa S. Role of Ischemic Preconditioning and Inflammatory Response in the Development of Malignant Ventricular Arrhythmias After Reperfused ST-Elevation Myocardial Infarction. Journal of Cardiac Failure. 2009;15(9):775–781. doi: 10.1016/j.cardfail.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Elmas E, Popp T, Lang S, Dempfle CE, Kälsch T, Borggrefe M. Sudden death: Do cytokines and prothrombotic peptides contribute to the occurrence of ventricular fibrillation during acute myocardial infarction? Int. J. Cardiol. 2010;145(1):118–119. doi: 10.1016/j.ijcard.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 124.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 2008;29(12):600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood. 2008;112(13):4961–4970. doi: 10.1182/blood-2008-03-144022. [DOI] [PubMed] [Google Scholar]
- 126.Herrmann TL, Agrawal RS, Connolly SF, McCaffrey RL, Schlomann J, Kusner DJ. MHC Class II levels and intracellular localization in human dendritic cells are regulated by calmodulin kinase II. Journal of Leukocyte Biology. 2007;82(3):686–699. doi: 10.1189/jlb.0107045. [DOI] [PubMed] [Google Scholar]
- 127.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J. Clin. Invest. 2009;119(4):986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 2003;6(10):1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 129.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012 May;52(5):1135–44. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kannel WB, Doyle JT, McNamara PM, Quickenton P, Gordon T. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation. 1975;51(4):606–613. doi: 10.1161/01.cir.51.4.606. [DOI] [PubMed] [Google Scholar]
- 131.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II Histone Deacetylases Act as Signal-Responsive Repressors of Cardiac Hypertrophy. Cell. 2002;110(4):479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin–dependent kinase II in the transition from pressure overload–induced cardiac hypertrophy to heart failure in mice. J. Clin. Invest. 2009;119(5):1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri A-K, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ. Res. 2009;105(4):316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ. Res. 2001;89(11):997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 135.Backs J, Worst BC, Lehmann LH, Patrick DM, Jebessa Z, Kreusser MM, Sun Q, Chen L, Heft C, Katus HA, Olson EN. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 2011;195(3):403–415. doi: 10.1083/jcb.201105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wright SC, Schellenberger U, Ji L, Wang H, Larrick JW. Calmodulin-dependent protein kinase II mediates signal transduction in apoptosis. FASEB J. 1997;11(11):843–849. doi: 10.1096/fasebj.11.11.9285482. [DOI] [PubMed] [Google Scholar]
- 137.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 2003;23(1):131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5(2):161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. The Journal of Clinical Investigation. 2009;119(10):2925. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salas MA, Valverde CA, Sánchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2010;48(6):1298. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toko H, Takahashi H, Kayama Y, Oka T, Minamino T, Okada S, Morimoto S, Zhan DY, Terasaki F, Anderson ME, Inoue M, Yao A, Nagai R, Kitaura Y, Sasaguri T, Komuro I. Ca2+/Calmodulin-Dependent Kinase IIδ Causes Heart Failure by Accumulation of p53 in Dilated Cardiomyopathy. Circulation. 2010;122(9):891. doi: 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73(4):689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Zhu W-Z, Wang S-Q, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao R-P. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A– independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 2003;111(5):617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta 1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009;297(4):H1377–H1386. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela M-K, Backs J, Olson EN, Brown JH, Neef S, Maier SKG, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2(6):664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- 147.Lakatta EG, Maltsev VA, Vinogradova TM. A Coupled SYSTEM of Intracellular Ca2+ Clocks and Surface Membrane Voltage Clocks Controls the Timekeeping Mechanism of the Heart's Pacemaker. Circ. Res. 2010;106(4):659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca(2+)/calmodulin-dependent protein kinase II activation. Circ. Res. 2000;87(9):760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 149.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003;91(6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 150.Gomes JA, Kang PS, Matheson M, Gough WB, El-Sherif N. Coexistence of sick sinus rhythm and atrial flutter-fibrillation. Circulation. 1981;63(1):80–86. doi: 10.1161/01.cir.63.1.80. [DOI] [PubMed] [Google Scholar]
- 151.Grimm M, Brown JH. beta-Adrenergic receptor signaling in the heart: Role of CaMKII. J. Mol. Cell. Cardiol. 2010;48(2):322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jessup M, Brozena S. Heart Failure. N. Engl. J. Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 153.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–226. doi: 10.1038/415219a. Available at: http://www.nature.com/nature/journal/v415/n6868/full/415219a.html. [DOI] [PubMed] [Google Scholar]
- 154.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circ. Res. 2010;106(6):1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 155.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114(7):670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. Available at: http://circ.ahajournals.org/cgi/content/abstract/114/7/670. [DOI] [PubMed] [Google Scholar]
- 156.Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110(11):1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. Available at: http://circ.ahajournals.org/cgi/content/full/110/11/1358. [DOI] [PubMed] [Google Scholar]
- 157.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9725923&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 158.Lo L-W, Chen Y-C, Chen Y-J, Wongcharoen W, Lin C-I, Chen S-A. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. European journal of pharmacology. 2007;571(2–3):197–208. doi: 10.1016/j.ejphar.2007.05.066. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17612522&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 159.Müller FU, Lewin G, Baba HA, Boknik P, Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J, Riemann B, Schmitz W. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. The Journal of Biological Chemistry. 2005;280(8):6906–6914. doi: 10.1074/jbc.M407864200. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15569686&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 160.Li N, Wang T, Wang W, Culter MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XHT. Inhibition of CaMKII Phosphorylation of RyR2 Prevents Induction of Atrial Fibrillation in FKBP12.6 Knockout Mice. Circ. Res. 2011 doi: 10.1161/CIRCRESAHA.111.253229. Available at: http://circres.ahajournals.org/cgi/doi/10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed]
- 161.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. J. Mol. Cell. Cardiol. 2011;50(1):214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 162.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8089–8096. doi: 10.1073/pnas.0502506102. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15863612&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106(10):1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 164.Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song L-S, Mohler PJ, Anderson ME. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation. 2008;118(22):2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qi X, Yeh Y-H, Chartier D, Xiao L, Tsuji Y, Brundel BJJM, Kodama I, Nattel S. The calcium/calmodulin/kinase system and arrhythmogenic afterdepolarizations in bradycardia-related acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2(3):295–304. doi: 10.1161/CIRCEP.108.815654. [DOI] [PubMed] [Google Scholar]
- 166.Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and I(Na) remodeling after myocardial infarction: insights from mathematical modeling. J. Mol. Cell. Cardiol. 2008;45(3):420–428. doi: 10.1016/j.yjmcc.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chuang H-Y, Hofree M, Ideker T. A decade of systems biology. Annu. Rev. Cell Dev. Biol. 2010;26:721–744. doi: 10.1146/annurev-cellbio-100109-104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dosemeci A, Albers RW. A mechanism for synaptic frequency detection through autophosphorylation of CaM kinase II. Biophys J. 1996;70(6):2493–2501. doi: 10.1016/S0006-3495(96)79821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93(11):3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12(5):943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 171.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110(20):3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(9):3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lisman JE, Goldring MA. Feasibility of long-term storage of graded information by the Ca2+/calmodulin-dependent protein kinase molecules of the postsynaptic density. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(14):5320–5324. doi: 10.1073/pnas.85.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am. J. Physiol. Heart Circ. Physiol. 2007;292(6):H2854–H2866. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95(10):4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283(5400):381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 177.Zhabotinsky AM. Bistability in the Ca(2+)/calmodulin-dependent protein kinase-phosphatase system. Biophys J. 2000;79(5):2211–2221. doi: 10.1016/S0006-3495(00)76469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iribe G, Kohl P, Noble D. Modulatory effect of calmodulin-dependent kinase II (CaMKII) on sarcoplasmic reticulum Ca2+ handling and interval-force relations: a modelling study. Philos Transact A Math Phys Eng Sci. 2006;364(1842):1107–1133. doi: 10.1098/rsta.2006.1758. [DOI] [PubMed] [Google Scholar]
- 179.Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca(2+) handling. Biophys J. 2010;99(7):2038–2047. doi: 10.1016/j.bpj.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J. 2005;88(1):85–95. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]