Abstract
Elite controllers suppress human immunodeficiency virus (HIV) viremia to below the limit of detection in the absence of antiretroviral therapy (ART). However, precise frequencies of CD4+ T cells carrying replication-competent HIV and/or the dynamics of the infectious viral reservoirs in response to initiation and discontinuation of ART in elite controllers are unknown. We show that the size of the pool of CD4+ T cells harboring infectious HIV diminished significantly after initiation of ART and rebounded to baseline upon cessation of therapy. Our data provide compelling evidence that persistent viral replication occurs in untreated elite controllers even in the absence of detectable plasma viremia.
Keywords: human immunodeficiency virus, viral reservoirs, antiretroviral therapy, elite controllers
Antiretroviral therapy (ART) suppresses plasma viremia to below the limit of detection in the vast majority of human immunodeficiency virus (HIV)–infected individuals and reduces the rate of viral transmission, morbidity, and mortality where antiretroviral drugs are available [1]. However, standard ART alone cannot eradicate HIV in infected individuals, in part because of the persistence of viral reservoirs in the peripheral blood, lymphoid tissues, and other sequestered sites [2]. Consequently, there is a growing interest in developing therapeutic strategies to eliminate persistent HIV reservoirs and/or to enhance host immunity against the virus to control viral replication in the absence of ART [2]. In this regard, it has been shown that a small proportion of HIV-infected individuals spontaneously control plasma viremia in the absence of ART (controllers) [3]. Moreover, a subset of such individuals (elite controllers) are capable of maintaining relatively normal CD4+ T-cell counts and undetectable levels (<50 copies of HIV RNA/mL) of plasma viremia for years to decades without the need for ART [3]. Although previous studies have suggested that ongoing viral replication may occur in elite controllers based on persistence of residual plasma viremia and immune activation [4, 5] as well as evidence for viral evolution in plasma HIV [6, 7], precise frequencies of infected CD4+ T cells carrying replication-competent virus and/or the impact of ART on immunologic and virologic parameters have not been fully delineated in this patient population. Given recent interest in developing therapeutic strategies aimed at achieving containment of HIV replication in the absence of ART, as observed in elite controllers, and considering that a certain percentage of these individuals may eventually experience disease progression [8], we conducted this study to address these questions.
METHODS
Isolation of CD4+ T cells in Peripheral Blood
Peripheral blood mononuclear cells (PBMCs) were obtained from blood draw and leukapheresis from the study participants (Table 1) in accordance with protocols approved by the institutional review boards of the University of Toronto, Toronto, Canada, and by the Office of Human Subjects Research at the National Institutes of Health. CD4+ T cells were isolated from PBMCs of HIV-infected individuals using a cell separation technique (StemCell Technologies).
Table 1.
Profiles of HIV-Infected Study Participants
| Subject | Duration of Infection (years) | Number of Episodes of Detectable Plasma Viremia (highest viral load)a | Duration of Undetectable Plasma Viremiaa (years) | HLA B5701 Present | CD4 Count at the Time of Study | CD8 Count at the Time of Study | Plasma Viremia at the Time of Studyb | Half-Life of Infected CD4+ T Cells (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 0 | 5 | Yes | 670 | 490 | <20 | 16.91 |
| 2 | 23 | 0 | 14 | Yes | 650 | 550 | <20 | 2.06 |
| 3 | 14 | 1 (51) | 9 | Yes | 980 | 710 | <20 | 2.12 |
| 4 | 23 | 8 (372) | NA | No | 560 | 660 | 88 | 1.01 |
Abbreviations: HIV, human immunodeficiency virus; NA, not applicable.
a Measured by a branched DNA assay with a detection limit of 50 copies/mL plasma.
b Measured by Cobas Ampliprep/Cobas Taqman HIV-1 Test Version 2.0 with a detection limit of 20 copies/mL plasma.
Determination of Plasma Viremia in Infected Individuals Receiving Antiretroviral Therapy
During the study period, plasma viral loads were determined using Cobas Ampliprep/Cobas Taqman HIV-1 Test Version 2.0 (Roche Diagnostics) in quadruplicate. The published limit of detection for this system is 20 copies of HIV RNA/mL plasma. The copy number of HIV RNA <20 copies was determined as described in the Supplementary Methods.
Determination of Infectious HIV Burden Using Quantitative Coculture Assays
To determine the frequency of CD4+ T cells carrying replication-competent HIV, standard and high input quantitative coculture assays were carried out as described in the Supplementary Methods. The half-life of CD4+ T cells carrying replication-competent HIV was determined by dividing −log102 by the slope of the log10-transformed infectious units per million cells obtained at baseline month 6 and month 9.
Quantitative Real-Time Polymerase Chain Reaction for Measurements of HIV DNA
The frequency of CD4+ T cells carrying HIV DNA was determined as described in the Supplementary Methods.
Levels of Immune Activation in Blood and Sigmoid Colon
The level of cellular activation was determined as described in the Supplementary Methods.
Measurements of HIV-specific CD8+ T cells
The frequency of HIV-specific CD8+ T cells was determined as described in the Supplementary Methods.
RESULTS
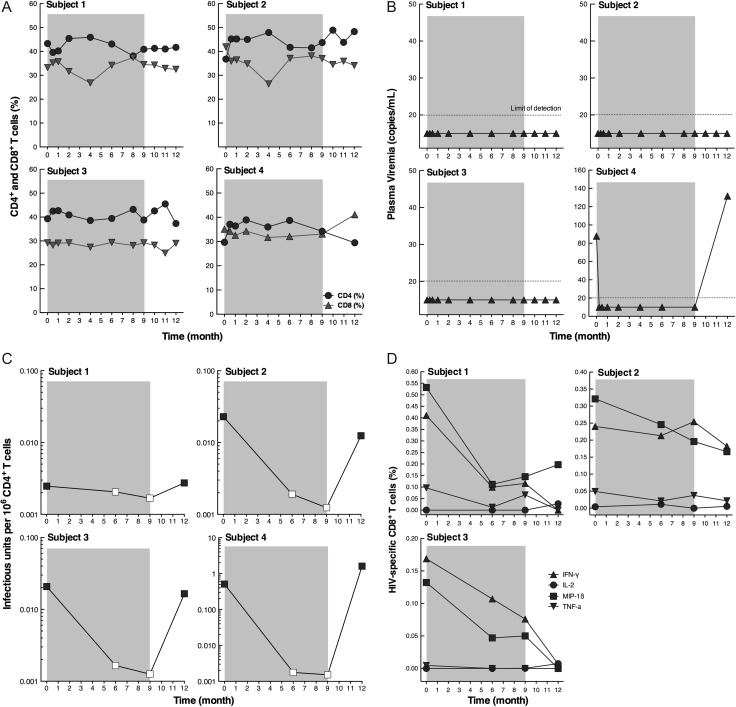
Three elite controllers (subjects 1, 2, and 3) and 1 controller (subject 4) (Table 1) received standard ART consisting of tenofovir/emtricitabine and raltegravir for 9 months followed by discontinuation of ART. For this study, controllers and elite controllers are defined by the ability to suppress HIV plasma viremia to <500 and <50 copies of HIV RNA/mL, respectively, in the absence of ART with no more than 1 viral blip above these cutoff values (Table 1). Blood draws and leukapheresis were conducted at month 0 (before the initiation of ART), 6, and 9 months after the initiation of ART, and 3 months after discontinuation of ART. Gut biopsies (sigmoid colon) were also obtained at months 0 and 6 and 3 months after cessation of ART. CD4+ and CD8+ T-cell percentages in the blood of all study subjects remained stable throughout the course of the study (Figure 1A). All elite controllers maintained <20 copies of HIV RNA/mL plasma throughout the entire study period (Figure 1B). There was no significant difference in residual plasma viremia (1–19 copies of HIV RNA/mL) before and after initiation and after discontinuation of ART in the elite controllers (data not shown). In subject 4, the baseline plasma viremia (mean ± SD, 88.0 ± 25.9) became undetectable shortly after initiation of ART and returned to a detectable level (mean ± SD, 131.5 ± 24.6) after discontinuation of therapy (Figure 1B).
Figure 1.
Levels of CD4+ and CD8+ T cells (A), plasma viremia (B), CD4+ T cells carrying replication-competent human immunodeficiency virus (HIV) (C), and HIV-specific CD8+ T cells (D) in infected study subjects after initiation and discontinuation of antiretroviral therapy (ART). B, Plasma viremia was determined using Cobas Ampliprep/Cobas Taqman HIV-1 Test Version 2.0 (Roche Diagnostics) with a detection limit of 20 copies/mL plasma. C, The open squares represent values below the limit of detection. When cocultures were negative, the frequency was estimated to be lower than the number calculated based on the assumption that 1 well containing 10 million cells would be culture-positive by p24 enzyme-linked immunosorbent assay. D, Peripheral blood mononuclear cells (PBMCs) were incubated with overlapping 15-mer HIV Gag peptides for 6 hours, and the frequency of CD8+CD45RO+CD27+ T cells expressing intracellular interferon γ (INF-γ), interleukin 2 (IL-2), macrophage inflammatory protein 1ß (MIP-1ß), and tumor necrosis factor α (TNF-α) was determined by flow cytometry. The shaded area represents the period of ART.
Given that the vast majority of HIV-infected CD4+ T cells carry defective virus, we conducted quantitative coculture assays to determine the frequency of cells carrying replication-competent HIV. When standard quantitative coculture assays (up to 1 × 106 cells) were used, no replication-competent HIV could be detected from the CD4+ T cells of the elite controllers. Subsequently, large numbers of CD4+ T cells (37–92 replicates of 10 × 106 cells per well, Supplementary Table 1) of the elite controllers were subjected to high-input quantitative coculture assays to determine the frequency of cells that could give rise to replication-competent virus. A dramatic decrease (>1 log) in the frequency of CD4+ T cells carrying replication-competent HIV was seen in 2 elite controllers (subjects 2 and 3) and 1 controller (subject 4) after initiation of ART (Figure 1C and Supplementary Table 1). In subject 1, the reduction in the frequency of CD4+ T cells carrying replication-competent HIV at baseline vs month 6 or month 9 was modest, in part because the baseline infectious HIV burden was extremely low in this subject and detecting a larger difference after ART would have required a prohibitive number of cells. The level of replication-competent HIV was below the limit of detection in all study subjects during ART despite culturing extraordinarily large numbers of cells in replicate (average of 62 wells containing 10 × 106 cells per well). In contrast with subject 1, the cultures from subjects 2, 3, and 4 had readily detectable levels of replication-competent HIV at baseline and 3 months after cessation of ART (Supplementary Table 1). Of note, the infectious HIV burden returned to the pretherapy level in all study subjects 3 months after discontinuation of ART (Figure 1C and Supplementary Table 1).
Although testing for statistical significance was not performed because of the small sample size, the mean log10 decrease (month 0 vs month 9) and increase (month 9 vs month 12) of the infectious HIV burden were −1.2938 and +1.3379, respectively, clearly demonstrating that ART had an impact on the size of the pool of infected CD4+ T cells carrying replication-competent HIV in the study subjects. The half-lives of the HIV reservoir during the treatment period (months 0, 6, and 9) in subjects 1, 2, 3, and 4 were 16.93, 2.06, 2.12, and 1.01 months, respectively. Of note, short half-lives (<4 months) of the viral reservoir had been reported in previous studies involving HIV-infected individuals who initiated ART during the acute/early phase of infection [9].
Frequencies of CD4+ T cells in the blood carrying HIV DNA were measured but no significant changes were observed in the elite controllers (Supplementary Figure 1), possibly because the vast majority of infected CD4+ T cells carry replication-defective HIV. In addition, levels of immune activation (T cells expressing CD38 and HLA-DR) were evaluated in the blood and sigmoid colon and found to be decreased during ART and returning to pre-ART levels after discontinuation of therapy in 3 of the 4 subjects studied (Supplementary Figure 2).
Finally, we investigated the impact of ART on the frequency of HIV-specific CD8+ T cells in the blood of the 3 elite controllers. As shown in Figure 1D, the frequency of HIV-specific CD8+ T cells expressing intracellular interferon γ and macrophage inflammatory protein 1ß decreased during the treatment period, and levels remained low to undetectable after cessation of ART in all 3 individuals.
DISCUSSION
Despite the remarkable success of ART in controlling plasma viremia and recent advances in our understanding of the pathogenesis of HIV, the prospect for eradication of HIV by standard ART alone remains challenging [2]. Given the considerable difficulties of achieving a complete eradication (sterilizing cure) of HIV in a large proportion of infected individuals, immune-based strategies aimed at achieving containment of viral replication in infected individuals in the absence of ART represent more realistic and feasible avenues. In this regard, the unique ability of elite controllers to suppress plasma viremia and maintain normal CD4+ T cell counts for years to decades in the absence of ART has served as a model for achieving a “functional” cure. To this end, it is critical to understand the dynamics of HIV reservoirs and the interplay between the virus and the host immune system in elite controllers.
In this study, we used the modulation of virologic and immunologic parameters by ART to provide evidence that ongoing/residual HIV replication does, in fact, occur in individuals who had been controlling their plasma viremia in the absence of ART. We demonstrated that a short course of ART dramatically decreased the infectious HIV burden in elite controllers, providing compelling evidence that residual viral replication occurs in such individuals in the absence of therapy. Recent studies have suggested that ongoing/residual HIV replication may be absent in infected individuals receiving ART as long as undetectable levels of plasma viremia (<50 copies) are maintained [10], although other studies have suggested otherwise [11, 12]. In addition, it has been suggested that residual plasma viremia fluctuates over time in elite controllers [4, 5, 13] although the clinical significance of such events and residual plasma viremia in general have not been fully delineated to date. Of note, previous studies have shown that initiation of ART in elite controllers led to decreases in the level of immune activation [14] and increases in CD4+ T-cell counts [15], although the size of the pool of CD4+ T cells carrying replication-competent HIV was not determined in these studies. Our data suggest that ongoing/residual viral replication can occur in infected individuals in the absence of detectable plasma viremia and indicate that 1 virologic marker (plasma viremia) alone may not accurately reflect the dynamics of HIV replication in vivo. Although the mechanism by which ART reduced the frequency of CD4+ T cells carrying replication-competent HIV is unclear, it is conceivable that the presence of ART inhibited bystander CD4+ T cell infection and/or induced transient lymphocyte redistribution.
Interestingly, the infectious HIV burden in the CD4+ T cell compartment of the elite controllers rebounded back to their original baseline levels upon cessation of ART, an indication that a virologic “set point” exists even at this very low level of viral replication and that host immunity can efficiently control clinically relevant plasma viremia without completely blocking viral replication or completely eliminating the infected CD4+ T cells that are the source of this residual viral replication. We also demonstrated that the level of HIV-specific CD8+ T cells in elite controllers gradually declined upon initiation of ART, further supporting the virologic evidence that low levels of viral replication occur in these infected individuals. It is unclear why the levels of HIV-specific CD8+ T cells did not return to baseline within the observed time frame after discontinuation of therapy, although possible explanations include elimination of viral antigens during the period of ART and/or bystander cellular activation of CD8+ T cells after cessation of therapy. Long-term immunologic and virologic studies involving larger numbers of elite controllers, including non-HLA-B5701+ individuals should be conducted to strengthen and confirm our findings.
In summary, we demonstrated that ART results in a marked diminution of the number of infected CD4+ T cells carrying replication-competent HIV in elite controllers, suggesting that low levels of ongoing viral replication contribute to the maintenance of HIV reservoirs in the absence of detectable plasma viremia.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study volunteers for their participation in this study.
Financial support. This work was supported the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG, Autran B, Berkhout B, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–14. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington M, Walker BD. Immunogenetics of spontaneous control of HIV. Annu Rev Med. 2012;63:131–45. doi: 10.1146/annurev-med-062909-130018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–90. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. 2010;84:7018–28. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mens H, Kearney M, Wiegand A, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84:12971–81. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madec Y, Boufassa F, Porter K, Meyer L. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005;19:2001–7. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 9.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–4. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 12.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–60. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008;47:102–4. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedaghat AR, Rastegar DA, O'Connell KA, Dinoso JB, Wilke CO, Blankson JN. T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin Infect Dis. 2009;49:1763–6. doi: 10.1086/648081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okulicz JF, Grandits GA, Weintrob AC, et al. CD4 T cell count reconstitution in HIV controllers after highly active antiretroviral therapy. Clin Infect Dis. 2010;50:1187–91. doi: 10.1086/651421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.