Abstract
Background. Daily suppressive therapy with valacyclovir reduces risk of sexual transmission of herpes simplex virus type 2 (HSV-2) in HSV-2–serodiscordant heterosexual couples by 48%. Whether suppressive therapy reduces HSV-2 transmission from persons coinfected with HSV-2 and human immunodeficiency virus type 1 (HIV-1) is unknown.
Methods. Within a randomized trial of daily acyclovir 400 mg twice daily in African HIV-1 serodiscordant couples, in which the HIV-1–infected partner was HSV-2 seropositive, we identified partnerships in which HIV-1–susceptible partners were HSV-2 seronegative to estimate the effect of acyclovir on risk of HSV-2 transmission.
Results. We randomly assigned 911 HSV-2/HIV-1–serodiscordant couples to daily receipt of acyclovir or placebo. We observed 68 HSV-2 seroconversions, 40 and 28 in acyclovir and placebo groups, respectively (HSV-2 incidence, 5.1 cases per 100 person-years; hazard ratio [HR], 1.35 [95% confidence interval, .83–2.20]; P = .22). Among HSV-2–susceptible women, vaginal drying practices (adjusted HR, 44.35; P = .004) and unprotected sex (adjusted HR, 9.91; P = .002) were significant risk factors for HSV-2 acquisition; having more children was protective (adjusted HR, 0.47 per additional child; P = .012). Among HSV-2–susceptible men, only age ≤30 years was associated with increased risk of HSV-2 acquisition (P = .016).
Conclusions. Treatment of African HSV-2/HIV-1–infected persons with daily suppressive acyclovir did not decrease risk of HSV-2 transmission to susceptible partners. More-effective prevention strategies to reduce HSV-2 transmission from HIV-1–infected persons are needed.
Keywords: HSV-2, HIV-1, acyclovir, transmission, serodiscordant couples, Africa
Herpes simplex virus type 2 (HSV-2) is highly prevalent among human immunodeficiency virus type 1 (HIV-1)–infected persons in sub-Saharan Africa [1–4]. Epidemiologic studies suggest synergy between HSV-2 and HIV-1 facilitates the spread of both viruses; HSV-2 reactivation increases HIV-1 concentrations in plasma and genital secretions, increasing the risk of HIV-1 transmission and disease progression, while HIV-1 infection increases HSV-2 shedding and reactivation frequency [5–8]. Thus, coinfection with HIV-1 likely increases the risk of HSV-2 transmission.
Daily therapy with valacyclovir reduces HSV-2 transmission in HSV-2–serodiscordant, HIV-1–seronegative-concordant healthy, immunocompetent, heterosexual couples by 48%, likely by decreasing the frequency and amount of HSV-2 shed in the genital tract by the HSV-2–infected partner [9, 10]. HIV-1–infected persons tend to reactivate HSV-2 both more frequently and at higher copy number than their immunocompetent counterparts [7, 8]. It is unknown whether treatment of HSV-2/HIV-1–infected persons with acyclovir reduces transmission of HSV-2 to their HSV-2/HIV-1–susceptible partner.
The Partners in Prevention Trial was a double-blind, randomized, controlled trial among HIV-1–serodiscordant couples to determine whether treatment of HSV-2 infection would reduce the transmission of HIV-1. As previously reported, HSV-2 suppression provided to the HIV-1–infected partners did not reduce the risk of HIV-1 transmission to their initially HIV-1–uninfected partners despite a sustained 0.25 log10 copies/mL reduction in the plasma HIV-1 RNA load, a 73% reduction in genital ulcer disease (GUD) incidence, and a 16%–19% decrease in HIV-1 disease progression [3]. We conducted an analysis of the effect of daily acyclovir on the risk of HSV-2 transmission in the subset of participants randomly assigned to receive acyclovir or placebo whose partners who were susceptible to HSV-2 and HIV-1.
METHODS
Population and Procedures
We identified 911 HSV-2 serodiscordant couples from the 3408 heterosexual HIV-1 serodiscordant couples enrolled into the Partners in Prevention HSV/HIV Transmission Study (clinical trials registration: NCT00194519). Couples were enrolled at 14 sites in East Africa (Kenya, Rwanda, Tanzania, and Uganda) and Southern Africa (Botswana, South Africa, and Zambia) from 2004 through 2006. For the present study, we assessed whether HSV-2 suppression provided to the HSV-2–infected partner reduced the risk of HSV-2 transmission to the susceptible partner.
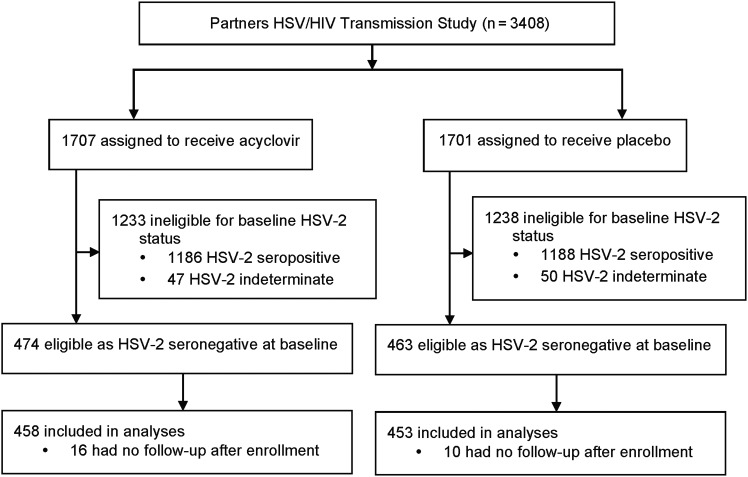
The clinical trial protocol required HIV-1–infected partners to be seropositive for both HSV-2 and HIV-1; a history of GUD was not required. At study entry, HSV-2/HIV-1–seropositive partners (henceforth referred to as “HSV-2–infected” individuals) had CD4+ T-cell counts of ≥250 cells/µL and were not receiving antiretroviral therapy (ART) according to national ART guidelines, which, at the time, involved initiation of ART at CD4+ T-cell counts of <200–250 cells/µL. Those who became pregnant or were eligible for initiation of ART during follow-up were referred to local antenatal and HIV-1 clinics. Open-label acyclovir was offered for recurrent genital herpes [3]. HIV-1–susceptible partners enrolled in the clinical trial could be either HSV-2 seropositive or seronegative. Only HSV-2/HIV-1–seronegative partners (henceforth referred to as “HSV-2–susceptible” individuals) were included in the present analyses (Figure 1).
Figure 1.
Trial profile. Abbreviation: HSV-2, herpes simplex virus type 2.
Fixed-block randomization stratified by site was used to randomly assign HSV-2–infected partners in a 1:1 ratio to receive acyclovir 400 mg twice daily or matching placebo (Ranbaxy Laboratories, Haryana, India), using a pseudo-random number generator. Only the study statistician and 2 data managers were aware of the treatment assignments. Participants were provided with a monthly resupply of study medication and adherence counseling for 12–24 months. Adherence was assessed by pill counts and self-reports as previously described [3, 11]. The serostatus of susceptible partners was unknown at the time of randomization; as such, random assignment of the HSV-2–infected partner to receive acyclovir or placebo was not stratified by their uninfected partner's HSV-2 serostatus. A secondary objective of the Partners in Prevention HSV/HIV Transmission Study was an analysis of HSV-2 transmission among the subset of HSV-2–susceptible partners, representing a randomized trial within the larger Partners in Prevention HSV/HIV Transmission Study.
Laboratory Methods
At all study sites, HSV-2–infected and –susceptible participants were tested for HSV-2 and HIV-1 at enrollment, and HSV-2–susceptible partners were tested quarterly for HIV-1 seroconversion. HSV-2 serostatus was determined using the Focus HerpeSelect-2 enzyme immunoassay (EIA; Focus Technologies, Cypress, CA), with a cutoff value of ≥3.5 to improve test specificity [12], and confirmed at the University of Washington Central Virology Laboratory by an HSV type–specific Western blot [13]. Participants with baseline indeterminate Western blot results (ie, those with an HSV-2–indistinct unapparent gG band after preabsorption against HSV-1 antigens) were excluded from the analysis [14]. For HIV-1–susceptible partners, archived enrollment sera were tested for HSV-2, and those whose serum specimens were negative at enrollment had serum specimens obtained at the exit visit tested by Western blot to ascertain their end-of-study HSV-2 serostatus. For participants who seroconverted during the study, samples collected at intervening quarterly visits were used to identify the time of HSV-2 seroconversion. HIV-1 testing was done at study sites, using 2 rapid assays in parallel, and were confirmed by HIV-1 EIAs, as previously reported [15].
Statistical Analysis
HSV-2 seroconversion (the primary end point) was defined as a negative HSV Western blot at enrollment followed by a positive HSV Western blot during follow-up. Kaplan–Meier survival curves for time to HSV-2 detection among susceptible partners were constructed, and a Cox proportional hazards regression model was used to assess risk factors related to HSV-2 transmission. Covariates included baseline demographic characteristics and time-dependent covariates, including sexual behavior, contraceptive methods, and CD4+ T-cell count and plasma HIV-1 load (for HSV-2–infected participants). A multivariate regression model was built, starting with measures that had a P value of <.1 in univariate analysis and performing backward elimination until a parsimonious model was produced, including only significant variables (P < .05) or those confounding other relationships. Vaginal drying practices were defined as the use of products that constrict or dry the vagina to enhance sexual pleasure. Factors associated with the incidence of GUD were assessed using Poisson regression, to permit analysis of multiple GUD episodes, while accounting for potential extra-Poisson variation, using an overdispersion parameter. Post hoc power calculations based on 68 HSV-2 transmissions indicated the study had 80% power to detect a 55%–75% reduction in the incidence of HSV-2 infection in the sex-specific acyclovir groups, compared with the placebo groups. Analyses of study data were performed using SAS, version 9.1 (SAS Institute, Cary, NC).
Ethical Approval
The institutional review board of the University of Washington and 17 African and US institutional and ethical review boards approved study procedures. Both partners provided independent written, informed consent.
RESULTS
Population Characteristics
The serostatus of HSV-2–susceptible partners was ascertained after completion of study accrual, using stored samples collected at baseline, and equal distribution was confirmed to have been achieved during randomization; within each treatment arm, 27% of couples had an HSV-2–uninfected partner and were thus at risk for HSV-2 transmission (Figure 1). A total of 911 HSV-2–serodiscordant couples were included in this analysis. For 799 couples (88%), the HSV-2–susceptible partner was a man. At enrollment, HSV-2–susceptible partners had a median age of 31 years (interquartile range [IQR], 27–38 years), 99.5% were HSV-1 seropositive, and 60% were from East Africa (Table 1). Most (71%) couples were married, with a median duration of partnership of 4 years (IQR, 2–7 years). Sixty-one percent of HSV-2–susceptible men (484/799) and 33% of HSV-2–infected men (37/112) were circumcised. The median baseline CD4+ T-cell count and HIV-1 plasma RNA load were 470 cells/µL (IQR, 350–670 cells/µL) and 3.9 log10 copies/mL (IQR, 3.3–4.5 log10 copies/mL), respectively. Characteristics of participants were similar between treatment arms (Table 1).
Table 1.
Enrollment Characteristics of Herpes Simplex Virus Type 2 (HSV-2) Serodiscordant Couples
| Characteristic | Placebo Arm |
Acyclovir Arm |
||
|---|---|---|---|---|
| HSV-2–Infected Subjects | ||||
| Women (n = 400) | Men (n = 53) | Women (n = 399) | Men (n = 59) | |
| Age, y | ||||
| Overall (median, IQR) | 28 (24, 33) | 34 (29, 40) | 29 (25, 33) | 34 (28, 41) |
| ≥30 | 169 (42%) | 36 (68%) | 171 (43%) | 37 (63%) |
| HSV-1 positive | 385 (97%) | 48 (94%) | 387 (98%) | 53 (93%) |
| Circumcised at baseline | NA | 15 (28%) | NA | 22 (37%) |
| Contraception use | ||||
| Oral contraceptive pills | 12 (3%) | NA | 19 (5%) | NA |
| DMPA or implants | 58 (14%) | NA | 60 (15%) | NA |
| Vaginal drying used | 70 (18%) | NA | 73 (18%) | NA |
| Genital ulcers at baseline | ||||
| On physical examination | 15 (4%) | 2 (4%) | 17 (4%) | 2 (3%) |
| By self report, in prior 3 mo | 98 (25%) | 9 (17%) | 101 (25%) | 16 (27%) |
| CD4+ T-cell count, cells/µL | ||||
| >350 | 300 (75%) | 37 (70%) | 312 (78%) | 40 (68%) |
| 200–350 | 100 (25%) | 16 (30%) | 87 (22%) | 19 (32%) |
| Plasma viral load, copies/mLa | (n = 356) | (n = 50) | (n = 369) | (n = 53) |
| <10 000 | 198 (56%) | 23 (46%) | 195 (53%) | 21 (40%) |
| 10 000–49 999 | 92 (26%) | 10 (20%) | 102 (28%) | 23 (43%) |
| 50 000–99 999 | 29 (8%) | 8 (16%) | 28 (8%) | 1 (2%) |
| >100 000 | 37 (10%) | 9 (18%) | 44 (12%) | 8 (15%) |
| ARVs used (excluding PMCTC%) | 27 (9%) | 3 (7%) | 23 (7%) | 5 (10%) |
| HSV-2–Susceptible Subjects | ||||
| Men (n = 400) | Women (n = 53) | Men (n = 399) | Women (n = 59) | |
| Age, y | ||||
| Overall (median, IQR) | 33 (28, 39) | 26 (23, 30) | 32 (27, 39) | 26 (21, 30) |
| ≥30 | 253 (63%) | 13 (24%) | 237 (60%) | 14 (24%) |
| HSV-1 positive | 397 (99%) | 53 (100%) | 397 (99%) | 59 (100%) |
| East African | 238 (60%) | 33 (62%) | 245 (61%) | 35 (59%) |
| Any monthly income | 239 (60%) | 14 (26%) | 231 (58%) | 13 (22%) |
| Education duration, y (median, IQR) | 9 (7, 12) | 8 (7, 12) | 9 (7, 12) | 9 (7, 11) |
| Lives with study partner | 347 (87%) | 48 (91%) | 340 (85%) | 52 (88%) |
| Married to study partner | 282 (71%) | 41 (77%) | 279 (70%) | 42 (71%) |
| Relationship duration, y (median, IQR) | 4 (2, 7) | 4 (2, 9) | 4 (1, 7) | 3 (1, 7) |
| Children, no (median, IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 1 (1, 3) |
| Sex with partner reported at last visit | ||||
| Any unprotected | 32 (8%) | 7 (13%) | 35 (9%) | 6 (10%) |
| Any protected | 264 (66%) | 39 (74%) | 277 (69%) | 43 (73%) |
| Any sex outside partnership reported at last visit | 54 (14%) | 3 (6%) | 76 (19%) | 3 (5%) |
| Circumcised at baseline | 247 (62%) | NA | 237 (59%) | NA |
| Contraception use at baseline | ||||
| Oral contraceptive pills | NA | 3 (6%) | NA | 3 (5%) |
| DMPA or implants | NA | 4 (8%) | NA | 8 (14%) |
| Vaginal drying used at any time | NA | 12 (23%) | NA | 7 (12%) |
| Genital ulcers at baseline | ||||
| On physical examination | 1 (<1%) | 0 (0%) | 6 (2%) | 1 (2%) |
| By self report, in prior 3 mo | 13 (3%) | 4 (8%) | 29 (7%) | 4 (7%) |
Each column refers to a couple in which one partner was seropositive for both human immunodeficiency virus type (HIV-1) and HSV-2 (the HSV-2–infected subjects) and the other partner was seronegative for both HIV-1 and HSV-2 (the HSV-2–susceptible subjects).
Abbreviations: DMPA, depot medroxyprogesterone acetate; NA, not applicable (item is sex specific); PMTCT, prevention of mother-to-child transmission of HIV.
a Data are for 356 coinfected women and 50 coinfected men in the placebo arm and 369 coinfected women and 53 coinfected men in the acyclovir arm.
Couples were followed for a median of 18 months (IQR, 3–24 months). Retention at 12 months was 95% for HSV-2–infected partners and 90% for HSV-2–susceptible partners, and retention at 24 months was 90% for HSV-2–infected partners and 82% for HSV-2–susceptible partners. HSV-2–susceptible participants contributed 1335 person-years of follow-up to the assessment of HSV-2 incidence. Median adherence to study drug based on pill count of unused study drug was 86% in both the acyclovir and placebo groups; 69% of visits involved at least 90% adherence. At their last visit, 16% of HSV-2–susceptible men reported sex with an outside partner, compared with 5% of women; 9% of susceptible partners reported unprotected sex with their study partner. HSV-2–susceptible women reported vaginal drying practices more frequently in East Africa (26%) than Southern Africa (2%). The number of children in the partnership was strongly associated with the duration of relationship (Spearman rho, 0.65; P < .0001).
Effect of Acyclovir on Transmission of HSV-2
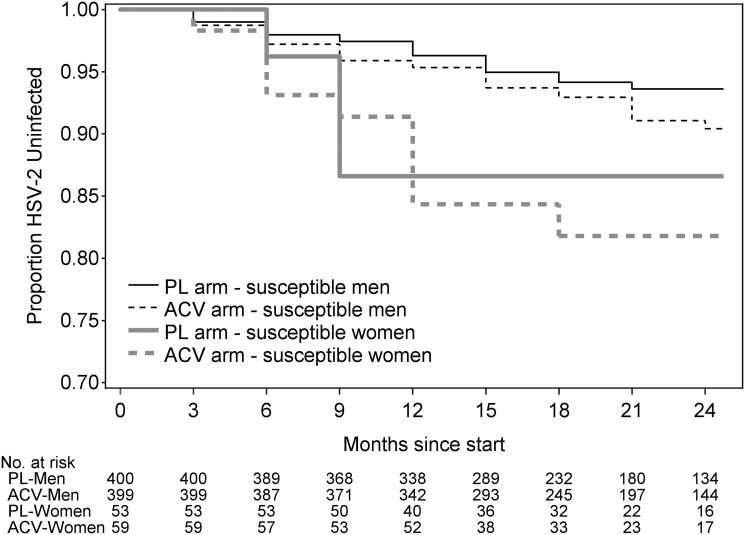
HSV-2 seroconversion was observed in 68 persons (51 men and 17 women), for an overall incidence of 5.1 cases per 100 person-years. Forty seroconversions were in the acyclovir group, and 28 were in the placebo group (incidence, 5.9 and 4.3 cases per 100 person-years, respectively; hazard ratio [HR], 1.35 [95% confidence interval {CI}, .83–2.20]; P = .22). The HSV-2 incidence was 10.7 cases per 100 person-years for male-to-female transmission and 4.3 cases per 100 person-years for female-to-male transmission (HR for male-to-female vs female-to-male transmission, 2.57 [95% CI, 1.47–4.49]; P = .001; Figure 2). The risk of HSV-2 transmission did not differ by GUD incidence, CD4+ T-cell count, or plasma HIV-1 RNA concentrations of the HSV-2–infected partner (Table 2). The lack of efficacy of acyclovir to prevent HSV-2 transmission did not differ by adherence group, defined as <90% versus ≥90% adherence per each quarterly period (P = .89 for susceptible women, and P = .99 for susceptible men). Open-label acyclovir to treat GUD was provided on 1.6% and 2.9% of visits in the acyclovir and placebo arms, respectively. Of the 911 HSV-2–susceptible persons, 13 also seroconverted to HIV-1, with 5 in the placebo arm and 8 in the acyclovir arm. Overall, 5 persons acquired both viruses; 1 seroconverted to both at the same visit, and 4 seroconverted first to HIV-1 then to HSV-2.
Figure 2.
Kaplan–Meier plots of time to herpes simplex virus type 2 (HSV-2) seroconversion, by treatment group and sex. Abbreviations: ACV, acyclovir; PL, placebo.
Table 2.
Univariate and Multivariate Cox Regressions for Herpes Simplex Virus Type 2 (HSV-2) Transmission
| Characteristic | HSV-2–Susceptible Men (n = 799) |
HSV-2–Susceptible Women (n = 112) |
||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | AHR (95% CI) | P | HR (95% CI) | P | AHR (95% CI) | P | |
| Age <30 y | 2.00 (1.14–3.52) | .016 | 2.00 (1.14–3.52) | .016 | 2.59 (.54–12.41) | .23 | … | |
| East Africana | 0.66 (.38–1.16) | .15 | … | 0.82 (.31–2.17) | .69 | … | ||
| Relationship duration, yb | 0.97 (.91–1.03) | .30 | … | 0.93 (.84–1.04) | .22 | … | ||
| Children, no.b | 0.90 (.76–1.06) | .19 | … | 0.65 (.42–1.01) | .056 | 0.47 (.26– .85) | .012 | |
| Sex with partner reported at last visit | ||||||||
| Any unprotected | 1.08 (.41–2.79) | .88 | … | 6.15 (1.57–24.02) | .009 | 9.91 (2.28–43.17) | .002 | |
| Any protected | 0.59 (.32–1.06) | .078 | … | 2.15 (.47–9.95) | .33 | … | ||
| Any sex outside partnership reported at last visit | 1.77 (.87–3.61) | .12 | … | 2.74 (.44–17.26) | .28 | … | ||
| Uncircumcised | 0.95 (.46–1.96) | .90 | … | NA | NA | … | ||
| Any hormonal contraceptive use | NA | NA | … | 1.66 (.33–8.29) | .54 | … | ||
| Vaginal drying used | NA | NA | … | 15.29 (1.44–162.2) | .024 | 44.35 (3.38–581.1) | .004 | |
| Currently pregnant | NA | NA | … | 2.58 (.73–9.09) | .14 | … | ||
| Acyclovir (vs placebo) | 1.37 (.78–2.41) | .27 | … | 1.31 (.48–3.58) | .56 | … | ||
| Uncircumciseda | NA | NA | … | 8.28 (1.10–62.44) | .040 | … | ||
| Any hormonal contraceptive use | 1.46 (.73–2.91) | .28 | … | NA | NA | … | ||
| Vaginal drying used | 1.33 (.46–3.83) | .60 | … | NA | NA | … | ||
| Currently pregnant | 1.01 (.36–2.89) | .97 | … | NA | NA | … | ||
| Genital ulcers at baseline | ||||||||
| On physical examination | 1.81 (.63–5.18) | .27 | … | NC | NC | … | ||
| By self report, in prior 3 mo | 1.31 (.45–3.80) | .62 | … | 0.48 (.05–4.23) | .51 | … | ||
| CD4+ T-cell count, cells/μL | ||||||||
| >350 | Ref | … | Ref | … | ||||
| 200–350 | 0.65 (.30–1.42) | .28 | … | 0.46 (.14–1.52) | .20 | … | ||
| <200 | 0.94 (.12–7.66) | .95 | … | NC | NC | … | ||
| Plasma viral load, copies/mL | ||||||||
| <10 000 | Ref | … | Ref | … | ||||
| 10 000–49 999 | 1.19 (.60–2.35) | .62 | … | 1.60 (.38–6.88) | .53 | … | ||
| 50 000–99 999 | 1.40 (.58–3.33) | .46 | … | 0.77 (.13–4.61) | .78 | … | ||
| >100 000 | 0.74 (.29–1.87) | .52 | … | 2.06 (.46–9.11) | .34 | … | ||
| ART taken (excluding PMTCT) | 2.89 (.97–8.60) | .057 | … | NC | NC | … | ||
Abbreviations: AHR, adjusted hazard ratio from multivariate model; ART, antiretroviral therapy (initiated after enrollment); CI, confidence interval; HR, hazard ratio; NA, not applicable (item is sex specific); NC, no convergence because there was not enough events in all categories of the covariate, even when site stratification was removed; PMTCT, prevention of mother-to-child transmission of HIV; Ref, reference.
a Regressions are not stratified by site. With regard to circumcision among men indexes, estimation would not converge when stratified by site, so stratification was removed. Small numbers forced this step: of the 17 HSV-2 transmissions that occurred from men to women, only 1 occurred from a circumcised man.
b Per unit increase.
Correlates of HSV-2 Transmission
We examined risk factors for HSV-2 transmission separately by the sex of the susceptible partner. In univariate analyses among the 17 susceptible women who acquired HSV-2, vaginal drying (HR, 15.29; P = .024), unprotected sex with the study partner (HR, 6.15; P = .009), and having an uncircumcised partner (HR, 8.28; P = .040) were significantly associated with an increased risk of HSV-2 acquisition. Notably, of the 17 male-to-female HSV-2 transmissions, only 1 occurred from a circumcised man, for an incidence of 1.7 cases per 100 person-years, compared with 16.2 cases per 100 person-years from uncircumcised male HSV-2–infected partners. Among the HSV-2–susceptible men, younger age was the only characteristic associated with increased risk of HSV-2 infection (HR, 2.00 for age <30 years, compared with age ≥30 years; P = .016).
In a multivariate analysis of HSV-2–susceptible women, vaginal drying practices (adjusted HR, 44.35 [95% CI, 3.38–581.1]; P = .004), any unprotected sex (adjusted HR, 9.91 [95% CI, 2.28–43.17]; P = .002), and number of children (adjusted HR, 0.47 for each additional child [95% CI, .26–.85]; P = .012) remained significant risk factors for HSV-2 acquisition. Notably, circumcision status of the male HSV-2–infected partner was not significantly associated with HSV-2 transmission in the final multivariate model, and this term was removed (adjusted HR before removal, 4.35 [95% CI, .56–34.04]; P = .16). This change was perhaps due to the association between circumcision and the practice of vaginal drying in East Africa. Among couples with circumcised male HSV-2–infected partners, 5% (2/37) practiced vaginal drying, compared with 23% (17/75) among couples with uncircumcised men (P = .03, by the Fisher exact test). Age of <30 years remained the only significant risk factor for HSV-2 acquisition among susceptible men (adjusted HR, 2.00 [95% CI, 1.14–3.52]; P = .02).
Effect of Acyclovir on GUD Incidence
At enrollment, 25% and 22% of HSV-2–infected women and men, respectively, reported symptoms of GUD in the previous 3 months. During follow-up, the overall incidence of GUD observed on genital examination was 6.8 cases per 100 person-years, with 7.2 cases per 100 person-years among women and 3.6 cases per 100 person-years among men. Acyclovir significantly reduced the overall frequency of GUD observed among HSV-2–infected partners on genital examination (risk ratio, 0.43 [95% CI, .26–.72]; P = .0013) and was protective among women (risk ratio, 0.40 [95% CI, .23–.67]; P = .0007) but not men (risk ratio, 1.32 [95% CI, .21–8.41]; P = .77), although only 5 incident GUD episodes were observed in men.
Among the HSV-2–infected partners, GUD incidence for self-reported genital ulcers was 31.9 cases per 100 person-years among men and 24.0 cases per 100 person-years among women, for an overall incidence of 24.9 cases per 100 person-years. Acyclovir significantly reduced the overall frequency of self-reported GUD (risk ratio, 0.50 [95% CI, .37–.66]; P < .0001), with a similar protective effect of acyclovir among women (risk ratio, 0.49 [95% CI, .37–.68]; P < .0001), compared with men (risk ratio, 0.50 [95% CI, .22–1.06]; P = .069), although the efficacy among men (the smaller group) was not statistically significant, because only 45 episodes were reported.
DISCUSSION
Our study shows that among HSV-2/HIV-1–coinfected persons, 400 mg of acyclovir twice daily did not reduce transmission of HSV-2 to the susceptible immunocompetent partners. In this randomized trial that included 911 HSV-2/HIV-1–serodiscordant couples, no reduction in HSV-2 transmission was seen in either male-to-female or female-to-male transmission, despite a significant influence on the incidence of GUD in the acyclovir arm. Although most HSV-2 seroconversions occurred among the much larger pool of susceptible men, the annual HSV-2 seroincidence among women was >2-fold higher than among men. The risk of HSV-2 transmission to susceptible women was increased by unprotected sex, vaginal drying practices, having fewer children, and lack of circumcision of the male partner. Younger age was the only significant risk factor for HSV-2 transmission to susceptible men.
Our results differ from the findings of the prior multicenter clinical trial that assessed whether antiviral therapy could reduce HSV-2 transmission within HSV-2–serodiscordant couples in North America, Europe, and Australia [9]. In that randomized trial, in which the HSV-2–infected partner was not infected with HIV-1, once-daily suppressive therapy with 500 mg of oral valacyclovir reduced HSV-2 transmission to sexual partners by 48% [9]. Although valacyclovir achieves higher plasma concentrations than acyclovir, the 2 drugs have shown comparable efficacy in reducing HSV-2 reactivation in HIV-1–infected persons [10], in suppressing the frequency and quantity of genital HSV-2 shedding in HIV-1–uninfected persons [16], and in treating genital herpes [17, 18]. However, antiviral activity against HSV-2 was demonstrated in the Partners in Prevention HSV/HIV Transmission Study; both GUD incidence and plasma HIV-1 RNA levels were significantly reduced in the acyclovir arm [3]. Thus, it is clear that acyclovir exerted an antiviral effect in HIV-1–infected participants. HIV-1 coinfection increases HSV-2 genital shedding [8], and persistent, frequent and short episodes of genital HSV-2 shedding occur even in the presence of higher doses of valacyclovir suppressive therapy [19], which may explain the lack of efficacy of acyclovir suppression in preventing HSV-2 transmission. Moreover, the clinical efficacy of acyclovir against HSV-2 in African women has appeared reduced in prior studies perhaps because of differential pharmacokinetics in African populations [20] or HSV-2 strain diversity [21]. Finally, the divergent results of the 2 trials are unlikely to be due to differences in medication adherence or participant retention [3, 9]. The HSV-2 incidence observed in our cohort was lower than that reported in other prospective studies in Africa [22–25], likely because of the lower biologic risk of transmission within stable partnerships and the monthly provision of risk reduction counseling, condoms, and other standard prevention services. It is clear more potent antiviral regimens are needed for effective HSV-2 control in African men and women.
Our findings suggest that women using vaginal drying practices were at significantly increased risk of HSV-2 infection. To our knowledge, no data have been reported regarding the relationship between vaginal drying and HSV-2 acquisition. This practice may increase HSV-2 susceptibility directly, by disrupting the vaginal epithelium, or indirectly, by facilitating the occurrence of bacterial vaginosis, which may increase the risk of HSV-2 acquisition [26]. Fertility intentions may explain unprotected sex that resulted in the high risk of HSV-2 transmission among couples with younger men or women with fewer children. As such, number of children may be a better proxy measure of unprotected sexual exposure, perhaps because of fertility intentions.
Our study strengths include the randomized design; the large sample size; the diversity inherent in a multinational cohort; the known exposure to HSV-2, since all HIV-1–infected partners were HSV-2 infected as part of eligibility for the trial; and confirmation of end points by using HSV Western blots to reduce misclassification of HSV-2 status. The parent study was not originally designed to assess HSV-2 transmission, and therefore we might have targeted a larger number of events than was obtained to optimize power. Nevertheless, the CI (.83–2.20) for the HR (1.35) is consistent with acyclovir's efficacy being <17%, invalidating the argument that more precision might reverse our conclusions. A limitation of our study was that few HSV-2–susceptible partners were women, limiting the precision of our point estimate. We relied on self-report for measures of sexual practices, including condom use and vaginal drying. Finally, we did not genotype the transmitted HSV-2 strains to document whether the transmission occurred within the study couple. However, HSV-2 strain typing to document transmission within couples has been done infrequently even in research protocols [27], partly reflecting the challenge of collecting viral samples from both partners, given the intermittent nature of HSV reactivation.
In conclusion, we found that acyclovir suppressive therapy did not decrease the risk of HSV-2 transmission within serodiscordant couples in which the HSV-2–infected partner also had HIV-1 infection. Unprotected sex, vaginal drying, having fewer children, and an uncircumcised HSV-2–infected partner were significant risk factors for HSV-2 transmission to women. More-potent antiviral therapy may be needed to reduce HSV-2 transmission in this population.
STUDY TEAM MEMBERS
Members of the Partners in Prevention HSV/HIV Transmission Study Team are as follows: University of Washington Coordinating Center and Central Laboratories, Seattle, Washington: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, and James I. Mullins. Study sites and site principal investigators are as follows: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife and Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex and Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira and Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group and Emory University): Susan Allen, Kayitesi Kayitenkore, and Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi and Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group and Emory University): Susan Allen and William Kanweka; Lusaka, Zambia (Rwanda–Zambia HIV Research Group and Emory University): Susan Allen and Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga and Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, and James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group and Emory University): Susan Allen and Mubiana Inambao; Orange Farm, South Africa (Wits Reproductive Health and HIV Institute, University of the Witwatersrand): Sinead Delany-Moretlwe and Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, and James McIntyre; and Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo.
Notes
Acknowledgments. We thank the study participants, for their participation and dedication, and the study team members at the research sites and at the University of Washington, for their contributions to data collection.
Data management was provided by DF/Net Research (Seattle, WA), and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
A. M., A. W., and C. C. designed the study. R. A. M. oversaw all HSV Western blot testing. A. M. wrote the first draft. A. S. M. performed the statistical analyses. All authors contributed to data collection, interpretation of the results, and the writing of the manuscript, and all approved the final draft.
Disclaimer. The authors designed and executed the study, had full access to the raw data, performed all analyses, wrote the manuscript, and had final responsibility for the decision to submit for publication. The funder had no role in design, data collection, analysis, interpretation, or writing of the report.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant 26469) and the National Institutes of Health (grant D43 TW000007, funded by the Fogarty International Center (to A. M.); K24 mentor award AI071113 [to A. W.]; and grant AI030731 [to A. W., L. C., R. A. M., and A. S. M.]).
Potential conflicts of interest. L. C. is on the scientific advisory board for, and holds stock (<1% of company) in, Immune Design Corp. and is a co-inventor on several patents involving potential HSV vaccine development. A. W. is a consultant for AiCuris GmbH and has received research funding from GSK, Agenus, Gilead Sciences, and Genocea Biosciences. R. A. M. has been a consultant for Roche Diagnostics and Biokit, SA. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 2.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24–35A. [PubMed] [Google Scholar]
- 3.Celum C, Wald A, Lingappa JR, et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–8. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 6.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8:490–7. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Zeh J, Hu HL, Hill J, Corey L. Frequency of symptomatic and asymptomatic HSV-2 reactivations among HIV-infected men. J Infect Dis. 1998;178:1616–22. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 8.Augenbraun M, Feldman J, Chirgwin K, et al. Increased genital shedding of herpes simplex virus type 2 in HIV-seropositive women. Ann Intern Med. 1995;123:845–7. doi: 10.7326/0003-4819-123-11-199512010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the isk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 10.Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV- infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 11.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mujugira A, Morrow RA, Celum C, et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect. 2011;87:238–41. doi: 10.1136/sti.2010.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (Immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley RL. Current concepts of laboratory diagnosis of herpes simplex infection. In: Sacks SL, Straub S, Whitley RJ, Griffiths PD, editors. Clinical management of herpes viruses. Washington DC: IOS Press; 1995. pp. 137–71. [Google Scholar]
- 15.Lingappa JR, Kahle E, Mugo N, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the partners study. PLoS One. 2009;4:e5272. doi: 10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 17.Fife KH, Barbarash RA, Rudolph T, Degregorio B, Roth R. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection: results of an international, multicenter, double-blind randomized clinical trial. Sex Transm Dis. 1997;24:481–6. doi: 10.1097/00007435-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Bodsworth NJ, Crooks RJ, Borelli S, et al. Valaciclovir versus acyclovir in patient-initiated treatment of recurrent genital herpes: a randomized, double-blind clinical trial. International Valaciclovir HSV Study Group. Genitourin Med. 1997;73:110–16. doi: 10.1136/sti.73.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012;379:641–7. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs J, Celum C, Wang J, et al. Clinical and virologic efficacy of herpes simplex virus type 2 suppression by acyclovir in a multicontinent clinical trial. J Infect Dis. 2010;201:1164–8. doi: 10.1086/651381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Celum C, Wald A, et al. Acyclovir achieves lower concentration in African HIV-seronegative, HSV-2 seropositive women compared to non-African populations. Antimicrob Agents Chemother. 2012;56:2777–9. doi: 10.1128/AAC.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramjee G, Williams B, Gouws E, Van Dyck E, De Deken B, Karim SA. The impact of incident and prevalent herpes simplex virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. J Acquir Immune Defic Syndr. 2005;39:333–9. doi: 10.1097/01.qai.0000144445.44518.ea. [DOI] [PubMed] [Google Scholar]
- 23.Chohan V, Baeten JM, Benki S, et al. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sex Transm Infect. 2009;85:489–92. doi: 10.1136/sti.2009.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bruyn G, Shiboski S, van der Straten A, et al. The effect of the vaginal diaphragm and lubricant gel on acquisition of HSV-2. Sex Transm Infect. 2011;87:301–5. doi: 10.1136/sti.2010.047142. [DOI] [PubMed] [Google Scholar]
- 25.Tobian AA, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;199:945–9. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319–25. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 27.Martin ET, Koelle DM, Byrd B, et al. Sequence-based methods for identifying epidemiologically linked herpes simplex virus type 2 strains. J Clin Microbiol. 2006;44:2541–6. doi: 10.1128/JCM.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]