Abstract
Background. Current evidence on the relationship between human papillomavirus (HPV) DNA detection and menstrual cycle has been inconsistent.
Methods. We included 21 nonoral contraceptive pill (non-OCP) users who self-collected vaginal samples twice per week for 16 weeks. We explored whether variable detection of HPV DNA exhibited cyclic or other structured temporal patterns. We also evaluated relationships between serial HPV prevalence, sexual behavior, and suspected bacterial vaginosis (BV) as defined by Nugent Gram stain score ≥7.
Results. During follow-up, any-type HPV prevalence varied between 61.1% and 85.0%. Although not statistically significant, we observed a maximum autocorrelation in serial HPV prevalence lagging 14 days (correlation coefficient [ρ], −0.24). Any-type HPV detection had a periodic behavior, generally repeating every 28.0 days (bootstrapped interquartile range, 22.4–28.0) and peaking around the ovulation time (adjusted odds ratio, 1.96; 95% confidence interval [CI], 1.06–3.62) as compared to menstruation. We also showed that an increase in any-type HPV prevalence preceded the beginning of a menstrual cycle by 9–12 days. There was no evidence of relationships between HPV prevalence and sexual activity or Nugent score.
Conclusions. Serially detected any-type HPV DNA showed a periodic behavior and was likely to peak in the periovulatory phase among non-OCP users.
Keywords: Auto-correlation, Bacterial Vaginosis, Human Papillomavirus, Menstrual Cycle, Nugent Score, Periodicity, Spectral Analysis, Time Series Analysis
Accumulating evidence has suggested a menstrual cycle effect on the detection of human papillomavirus (HPV) DNA, although current evidence is inconsistent and circumstantial. Studies that compared HPV prevalence in pregnant versus nonpregnant women showed that HPV detection could be affected by a woman's hormonal status [1, 2]. For example, while HPV prevalence was generally lower among women in the postpartum than in the prepartum period, the temporal pattern was not consistent across the 3 gestational terms [1, 2]. Reeves et al [3] and de Villiers et al [4] also noticed substantial intraperson variability in HPV detection in cohorts of nonpregnant women, and suggested that this could be due to temporal variation.
To date, reported effects of hormonal fluctuation within a menstrual cycle on the performance of HPV DNA testing in nonpregnant women have been inconsistent (Table 1) [5–12]. Van Ham et al [9] followed 20 women of regular menstrual cycles who visited a fertility clinic in the Netherlands for 4 weeks and obtained weekly cervical swabs for HPV DNA testing. The investigators found short-term fluctuations in HPV prevalence within a menstrual cycle, with the follicular phase being associated with a peak in HPV detection. Later, some studies [12, 13] presented similar findings, while others [7, 8, 10] did not. In a large cohort of women (n = 5060) with a history of abnormal cervical cytology over the previous decade, Sherman et al [11] found that, during a 2-year follow-up period, higher HPV viral load and more low-grade squamous intraepithelial lesion cytology were reported in midcycle (11–20 days since last menstrual period) specimens taken from women with HPV infection. In contrast, Schmeink et al [12] showed a higher HPV prevalence of high-risk types in the follicular phase and a decreasing trend toward the later phase of a cycle among nonoral contraceptive pill (non-OCP) users.
Table 1.
Summary of Previous Studies and Findings on the Association Between HPV Prevalence and Menstrual Cycle
| Year of Report | Authors | No. Participants/Mean No. Samples per Participant | Characterization of Menstrual Cycle | Data Exhibit Potential Autocorrelation | Statistical Method to Account for Autocorrelation | Statistical Method for Hypothesis Testing | Comments on Statistical Method | Study Findings |
|---|---|---|---|---|---|---|---|---|
| 1990 | McNicol et al [5] | 427/1 | Weekly (q7d since LMP) | No | … | χ2 test | Inference on association between HPV typing results and menstrual stage was made by single specimen from each woman at different menstrual stages. Assumption: women were exchangeable in the underlying HPV status. | Not related |
| McNicol et al [5] | 58/2 | Weekly (q7d since LMP) | Yes | Not mentioned | χ2 test | Correlation between two sequential specimens from the same women was not addressed. Assumption: repeated measures from the same women were independent. Small sample size in this subpopulation could also result in lack of power. | Not related | |
| 1992 | Schneider et al [6] | 7a/10b | Biweekly (1–14/15–28): follicular/luteal | Yes | GEE | Not mentioned | Each woman provided one specimen every 5 wks for 1 y. Assumption: women's underlying HPV status remained unchanged during the course of follow-up; unclear working correlation structure. | Luteal phase |
| 1994 | Fairley et al [7] | 298/1 | Individual standardized quartiles of days of cycle | No | … | χ2 or Fisher's exact test | Inference on association between HPV typing results and menstrual stage was made by single specimen from each woman at different menstrual stages. Assumption: women were exchangeable in the underlying HPV status. | Not related |
| 1996 | Wheeler et al [8] | 72/10 | Biweekly (1–14/15–32/33+): follicular/luteal/late | Yes | GEE (exchangeable working correlation) | Logistic regression | Each woman provided 1 specimen every wk for 10 wks. Assumption: women's underlying HPV status remained constant during the course of follow-up; constant correlation between any two HPV testing results from the same subject. | Not related |
| 2002 | van Ham et al [9] | 20/4 | 2–3/7–11/12–15/20–24c | Yes | None | χ2 test | Each woman provided 4 specimens on a weekly basis for 1 menstrual cycle at a fertility clinic. Assumption: all measurements of HPV status were independent. | Follicular phase |
| 2003 | Harper et al [10] | 203/4 | Weekly (q7d since LMP) | Yes | GEE (exchangeable working correlation) | Logistic regression | Each woman provided vaginal swabs at 3 sampling times during a period of 4–6 wks. Assumptions: women's underlying HPV status remained constant during the course of follow-up; constant correlation between any 2 HPV testing results from the same subject. | Not related |
| 2006 | Sherman et al [11] | 5060/3.4 | 0–10/11–21/22–28 | Yes | GEE (AR1, probably) | Each woman provided 1 cervical sample for Hybrid Capture 2 every 6 mo for 2 y. Assumptions: women's underlying HPV status remained constant during the course of follow-up; autocorrelation between any 2 HPV measurements from the same women. | Mid-cycle (11-21d) | |
| 2010 | Schmeink et al [12] | 1812/3 | Weekly (q7d since LMP) | Yes | Not mentioned | Logistic regression | Each woman provided a self-collected cervicovaginal swab every 6 mo for 1 y. Assumptions: women's underlying HPV status remained constant during the course of follow-up; all measurements of HPV status were independent. | High-risk HPV detection increased among OCP users but decreased among non-OCP users in the 2nd half of menstrual cycle. |
Abbreviations: GEE, generalized equation estimation; HPV, human papillomavirus; LMP, last menstrual period; OCP, oral contraceptive pill; q7d, every 7 days.
a Non-OCP users.
b Seven specimens from 1 woman during pregnancy were excluded from the analysis.
c Ovulation confirmed by pelvic ultrasound.
CI, confidence interval; LL, lower limit; UL, upper limit.
In the current study, we sought to investigate whether a periodic pattern in detectable HPV DNA exists, and whether there was a relationship between detectable HPV DNA and the menstrual cycle. To more fully understand the complexity of the dynamic changes in HPV detection, we attempted to evaluate the interrelationships among HPV prevalence, sexual activity, and bacterial morphology of the vagina. Using time series analysis, we were able to fully utilize information repeatedly collected from a small number of subjects over multiple menstrual cycles. Time series analysis enabled the comparison of multiple sets of serially measured data, while adequately controlling for serial correlation [14, 15].
METHODS
Study Design and Population
We analyzed HPV testing results from archived swabs from a previous study on vaginal douching cessation. The study design and characteristics of the study population were published elsewhere [16]. Briefly, the study began with a 4-week (phase I) observation followed by a 12-week (phase II) cessation intervention. Eligible women enrolled between December 2005 and March 2007 included those who reported having regular menstrual cycles. Ethical approval for the primary study was obtained from the Institutional Review Boards of the Johns Hopkins University School of Medicine. All participants provided written informed consent before enrollment. As vaginal douching has not been shown to affect HPV DNA detection ability [17] and we did not find empirical evidence for an effect of douching on HPV detection, the current analysis included swabs taken by women who completed both phases I and II follow-up, who were positive for 1 or more HPV types during the study period, and who reported no OCP use at the baseline interview (Figure 1).
Figure 1.
Flow chart of participant selection. Among 33 participants who completed both phases I and II follow-up, 28 (84.8%) were ever positive for at least 1 type of HPV and provided 812 vaginal swabs over the study period. After excluding 6 OCP users, 1 woman with an incomplete cycle, and 8 redundant swabs, 21 women (595 swabs) were included for the current analysis. Abbreviations: HPV, human papillomavirus; OCP, oral contraceptive pill.
Data Collection
Experienced research nurses interviewed women at the baseline visit and at the end of phase II to collect demographic information such as age, race, educational background, and contraceptive use history as well as recent sexual activity (at baseline and at the end of phases I and II). None of the participants reported a change in contraceptive use during follow-up. Over the 16-week study period, women also kept daily diaries on menstrual bleeding, sexual activities, personal hygiene practices, and the use of medications, including topical antimycotics. All women entered the study on the first day of a menstrual cycle and self-collected midvaginal samples twice per week at prespecified days in the cycle (day 2, 6, 9, 13, 16, 20, 23, 27, etc.) consecutively for 16 weeks. One experienced microbiologist read and scored all sample slides to assign a Gram stain score of 0–10 according to the standardized method described by Nugent et al [18]. A Nugent score of 0–3 represents normal, 4–6 represents an intermediate state, and 7 or higher is indicative of bacterial vaginosis (BV). To differentiate from clinical BV, the latter was termed Nugent-score BV by Martin [19].
Extracted DNA from vaginal swabs [20] was genotyped using the Roche HPV Linear Array (LA) according to the manufacturer's instructions. The detection limit was 10–100 viral copies per polymerase chain reaction (PCR) test. Each PCR test included 81 specimens, 4 positive controls (25 and 100 copies of HPV 16 and 18 plasmid DNA in a background of roughly 30 000 human cellular DNA equivalents); the LA kit, positive and negative controls; 3 HPV- DNA negative (containing 1.0 × 106 K562 cells/mL); high HPV DNA positive (1.25 × 105 SiHa cells and 1.0 × 106 K562 cells/mL) and low HPV DNA negative extraction controls (5.0 × 103 SiHa cells and 1.0 × 106 K562 cells/mL); and 6 no-DNA (negative) controls [21]. When all positive controls were positive for human β-globin and the intended PCR genotype (and no others) as well as when the negative controls were completely negative, a PCR batch was considered valid. Otherwise, the procedure was repeated for the same batch. The current analysis included only valid PCR results.
Statistical Analysis
We employed descriptive analyses in initial characterizations of HPV positivity and other measures. We constructed time series data from serially measured point prevalence of HPV (of any type, of high-risk [HR] types, and of multiple types), vaginal sex activity, condom use, and Nugent score ≥7. Specifically, for HPV data, a series of point prevalence estimates was calculated at the prespecified sampling times since study entry. We allowed for temporally misplaced HPV testing results when women missed the sampling schedule by 1 or 2 days. We excluded measurements when women missed the scheduled sampling time by 3 or more days. These measurements, as well as those skipped by women entirely, were treated as missing. We similarly derived other univariate time series for covariates of interest. To better reflect the temporal variability in HPV detection at the genotype level, we constructed a time series of average HPV percent-type positivity. For each woman, we calculated percent-type positivity by dividing the number of positive HPV types by 37 genotypes tested. We used the average percent-type positivity across individuals at each sampling time in further analysis.
We first estimated the degree of serial correlation in each time series data using sample autocorrelation function (ACF) and partial autocorrelation function (PACF) [22, 23]. Next, we employed spectral analysis to explore whether a time series data had a periodic pattern. We applied the fast Fourier transform algorithm when calculating sample spectrum, which distributed variation of the data over a possible range of frequency. In doing so, we identified a dominant frequency at which each time series data repeated itself [23]. We further performed bootstrapping to quantify the uncertainty surrounding the serial prevalence estimates by resampling individuals with replacement at each sampling time.
We used linear regression to identify a potential trend or periodicity in HPV prevalence. We added “days since the first day of the first menstrual cycle” in a linear and/or a quadratic form as well as an indicator for menstrual cycles (range 1–4) separately and in possible combinations. In addition, we attempted the following categories for menstrual phases: weekly (1–7th, 8–14th, 15–21st, 22–28th day); 10-day category (1–9th, 10–20th, 21–28th day) [11]; van Ham's category (2–3rd, 7–11th, 12–15th, 20–24th) [9]; modified van Ham's category (2–3rd, 4–6th, 7–11th, 12–15th, 16–19th, 20–24th, >24th day; with no gaps between levels). We relied on Akaike information criteria to select among competing models. Assuming weak temporal correlation in repeated HPV DNA detection, we further applied logistic regression with generalized estimation equation (GEE) methods to examine whether HPV detection varied by menstrual phases. While adjusting for a potential cycle-specific effect, we specified a working correlation of independence for the serially collected swabs results [24].
Lastly, we evaluated pair-wise interrelationships among detectable HPV DNA and covariate time series before and after removing the trend or periodicity (cyclic) component. We then employed the Ljung–Box test to ensure that, after removing the potential trend and/or cyclic component, the model residuals were close to independently and identically distributed random variables [25]. Finally, for each pair of trend- and periodicity-removed time series data, we estimated the intercorrelation using a cross-correlation function (CCF). We performed all analysis in R [26] with a 2-tailed significance level of 0.05, except for descriptive analysis, using Stata 11 [27].
RESULTS
Overall, among 33 participants who completed both phases I and II follow-up of the original study, 28 (84.8%) tested positive for at least 1 type of HPV and contributed 812 self-collected vaginal swabs over the 16-week study period. After excluding 6 individuals who reported OCP use at baseline, 1 woman with only 1 documented menstrual cycle over the full course and 8 swabs that were redundantly collected, we included 21 women (595 swabs) for the current analysis (Figure 1). Throughout the study, each of the 21 women collected a median number of 29 vaginal samples (interquartile range [IQR], 28–31) for HPV testing and recorded 4 menstrual cycles (IQR, 4–5), with a median length of each cycle being 29 days (IQR, 25–42). On average, a woman provided 7 vaginal swabs (IQR, 5–8) per menstrual cycle.
Table 2 shows demographic characteristics and sexual exposure risks for 21 women included in the current analysis as well as for 28 women ever shown positive over the study period. In general, women included in the study were of middle age (median, 39; IQR, 37–43) and predominantly non-white. Most women were sexually active at baseline (90.5%) or at follow-up (76.2%). At baseline, the majority reported ever having 5 or more lifetime sex partners (70.0%) or monogamy with a current partner over the past 12 months (85.7%). Few women reported condom use at baseline (33.3%) or at any time during the study (28.6%).
Table 2.
Selected Characteristics at Baseline and Follow-up of Women Included in the Current Study
| Baseline | All (N = 28) |
Included (N = 21) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Age, median (IQR) | 39 (34–42.5) | 39 (37–43) | ||
| Age ≥45 | 6 | 21.4 | 5 | 23.8 |
| Years with current partner, median (IQR)a | 7 (2–13) | 7 (3–13) | ||
| Race: white vs non-white | 11 | 39.3 | 8 | 38.1 |
| No. lifetime partners, median (IQR)b | 6 (4–10) | 6 (4–10) | ||
| 5+ lifetime partners | 20 | 74.1 | 14 | 70.0 |
| Ever had casual partner(s) | 3 | 10.7 | 3 | 14.3 |
| Current user of condom | 9 | 32.1 | 7 | 33.3 |
| Use of oral contraceptive or equivalent | 6 | 21.4 | 0 | 0.0 |
| Monogamous with current partner | 23 | 82.1 | 18 | 85.7 |
| Sexually active with a male partner | 25 | 89.3 | 19 | 90.5 |
| History of herpes simplex virus–related ulcerb | 1 | 3.7 | 1 | 5.0 |
| Follow-up over a 16-week period | ||||
| Total no. swabs collected | 813 | 595 | ||
| Mean no. swabs collected, per person | 29.0 (2.6) | 28.8 (2.9) | ||
| Median no. swabs collected, per person | 29.5 (28.5–31) | 29 (28–31) | ||
| Mean no. swabs collected/cycle, person | 7.2 (3.9) | 6.9 (3.3) | ||
| Median no. swabs collected/cycle, person | 7 (5–8) | 7 (5–8) | ||
| Total no. cycles recorded | 113 | 87 | ||
| Mean no. cycles recorded/person | 4.0 (1.0) | 4.1 (0.9) | ||
| Median no. cycles recorded/person | 4 (4–5) | 4 (4–5) | ||
| Mean duration of a cycle/person | 35.7 (18.2) | 34.6 (14.6) | ||
| Median duration of a cycle/person | 29 (26–42) | 29 (25–42) | ||
| Ever had vaginal intercourse, yes vs no | 22 | 78.6 | 16 | 76.2 |
| No vaginal sex | 6 | 21.4 | 5 | 23.8 |
| 1–4 times/16 wks | 7 | 25.0 | 5 | 23.8 |
| 5–8 times/16 wks | 6 | 21.4 | 4 | 19.0 |
| 9+ times/16 wks | 9 | 32.1 | 7 | 33.3 |
| Ever used tampon, yes vs no | 21 | 75.0 | 17 | 81.0 |
| Ever had anal sex, yes vs no | 2 | 7.1 | 1 | 4.8 |
| Ever used condom, yes vs no | 10 | 35.7 | 6 | 28.6 |
| No | 18 | 64.3 | 15 | 71.4 |
| 1–4 times/16 wks | 8 | 28.6 | 4 | 19.0 |
| 5+ times/16 wks | 2 | 7.1 | 2 | 9.5 |
Abbreviation: IQR, interquartile range.
a Five women did not answer the question.
b Self-reported, 1 woman who was included in the analysis did not answer the question.
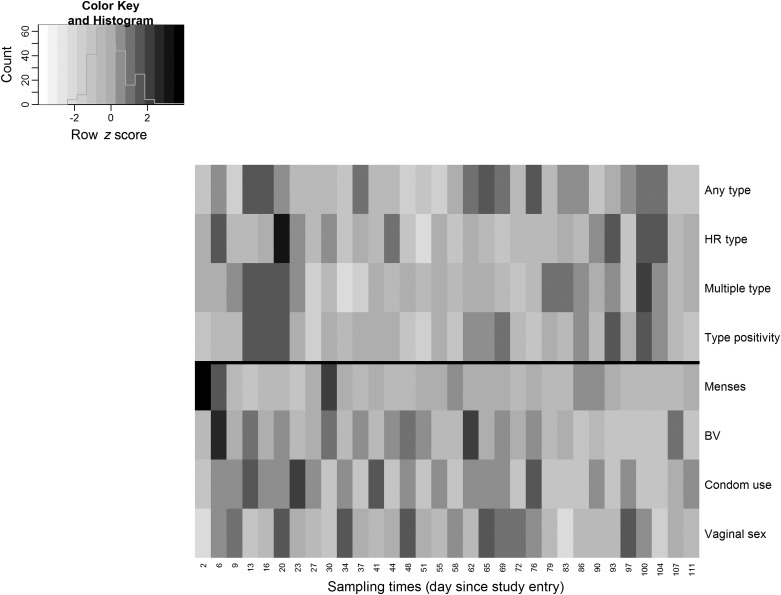
Altogether, 73.5% of the 595 vaginal swabs were positive for 1 or more types of HPV DNA (range, 61.1%–85.0%; Table 3). The point prevalence for HR types was low and showed a large variation (range, 15.0%–50.0%), whereas the prevalence of multiple types of HPV detected on a single swab at any given time fluctuated around a median of 30.8% (IQR, 26.3%–36.8%). The average percent-type positivity remained low at follow-up (range, 2.9%–5.6%). Prevalence of women with a Nugent score ≥7 also varied substantially during the course of follow-up, ranging from 5.3% to 43.8% (Table 3). Table 3 provides additional descriptions of HPV prevalence and other covariates over time. Of note, Figure 2 suggests a periodic pattern of menstruation by clearly marking the beginning of each menstrual cycle for the first 2–3 cycles, against which the temporal pattern of other covariates were less remarkable.
Table 3.
Summary of Prevalence Time Series for HPV, Bacterial Vaginosis, Menstrual Bleeding, and Sex Activities Among 21 Women (595 Swabs) Included in the Study Over the 16-Week Period (32 Sampling Time Points)
| Mean | 95% CI |
Min. | Percentiles |
Max. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | p10 | p25 | p50 | p75 | p90 | ||||
| Number of vaginal swabs available at any given sampling time | 18.7 | 18.6 | 18.8 | 15 | 17 | 15 | 19 | 20 | 20 | 21 |
| HPV prevalence | ||||||||||
| Any type | 73.5% | 70.8% | 76.3% | 61.1% | 64.7% | 66.7% | 71.4% | 80.0% | 84.2% | 85.0% |
| High-risk type | 28.6% | 26.0% | 31.2% | 15.0% | 21.1% | 23.7% | 27.0% | 31.4% | 38.9% | 50.0% |
| Multiple type | 31.5% | 28.5% | 34.6% | 15.0% | 22.2% | 26.3% | 30.8% | 36.8% | 44.4% | 50.0% |
| HPV type positivitya | 4.2% | 3.9% | 4.4% | 2.9% | 3.5% | 3.6% | 4.1% | 4.7% | 5.4% | 5.6% |
| Nugent Gram stain score | 2.1 | 1.9 | 2.3 | 1.4 | 1.5 | 1.7 | 2.1 | 2.6 | 2.8 | 3.5 |
| 7–10 (suspected bacterial vaginosis) | 17.1% | 13.7% | 20.5% | 5.3% | 5.6% | 10.5% | 16.7% | 22.6% | 27.8% | 43.8% |
| Menstrual bleeding | 20.2% | 13.0% | 27.4% | 0% | 5.0% | 7.9% | 15.8% | 24.3% | 33.3% | 100% |
| Use of condom | 4.0% | 2.6% | 5.3% | 0% | 0.0% | 0.0% | 5.1% | 5.9% | 10.0% | 12.5% |
| Vaginal sex | 18.5% | 15.1% | 21.8% | 0% | 10.5% | 11.8% | 17.7% | 25.1% | 31.6% | 35.0% |
Abbreviations: HPV, human papillomavirus; CI, confidence interval; LL, lower limit; UL, upper limit.
a Percent-positive types among 37 genotypes tested at each sampling time.
Figure 2.
Heat maps of time series data for HPV prevalence and covariates with adjustment for row mean. Serially measured point prevalence estimates for HPV data and covariates of interest were plotted against the sampling times in the x-axis. Each row represented deviations of the point prevalence at each sampling time from its own overall mean. The shades reflected the magnitude of the deviation, with a lighter (darker) shade indicating a larger negative (positive) difference from its overall mean. As shown in the color key and histogram on the top left corner, most time series fluctuated around its overall mean, except for the menstruation data whose cyclic pattern was quite evident with a period of 28 days (or, equivalently, every 8 swabs) in the first 2–3 cycles. Abbreviations: BV, bacterial vaginosis HPV, human papillomavirus.
Autocorrelation and Periodicity
According to the Ljung–Box test, none of the time series examined showed statistically significant autocorrelation in sample ACF or PACF (data not shown). We observed a maximum autocorrelation in any-type HPV prevalence at lag 14 days (ACF, −0.24), which was not statistically significant (P = .282). In contrast, we noticed a statistically significant autocorrelation at lag 28 days in menstruation data (ACF, 0.43; P = .003) but the strength of correlation diminished after removing the linear dependence among the in-between time lags (PACF, 0.21), suggesting that the observed periodicity was confounded by autocorrelation.
In spectral analysis, we recovered 28.0 days (bootstrapped median, 28.0; IQR, 28.0–28.0) as a dominant periodic frequency of the menstruation time series (Table 4). Both any-type HPV prevalence and HPV percent-type positivity also showed a periodicity of 28.0 days (bootstrapped medians, 28.0; IQRs, 22.4–28.0) whereas HR- (bootstrapped median, 22.4; IQR, 12.4–112 days) or multiple-type HPV prevalence (bootstrapped median, 56.0; IQR, 28.0–112 days) did not show any particular regularity (Table 4). In contrast, prevalence of BV changed every 8 days (bootstrapped median, 8.6; IQR, 8.0, 56), whereas vaginal sex activity (empirical period, 12.4 days) or condom use (empirical period, 7 days) varied on a nearly biweekly or weekly basis. Bootstrapping results provided nearly the same degree of uncertainty for the mean frequency estimate of all the time series considered, except for the menstruation and sexual behavior data (Table 4).
Table 4.
Periodicitya of Prevalence Time Series for HPV, Bacterial Vaginosis, and Sex Behavior in 21 Women (595 Swabs)
| Time Series | Period of Dominant Frequency |
Bootstrapping Results (days)b |
||||
|---|---|---|---|---|---|---|
| (swabs) | (days)c | Mean | (95% CI) | Median | (IQR) | |
| HPV point prevalence | ||||||
| Any type | 8.0 | 28.0 | 31.6 | (8.0, 112) | 28.0 | (22.4, 28.0) |
| High-risk type | 32.1 | 112.4 | 56.5 | (8.0, 112) | 22.4 | (12.4, 112) |
| Multiple type | 32.1 | 112.4 | 69.4 | (14, 112) | 56.0 | (28.0, 112) |
| HPV type positivity | 8.0 | 28.0 | 36.3 | (8.0, 112) | 28.0 | (22.4, 28.0) |
| HPV signal strength | ||||||
| All type | 8.0 | 28.0 | 37.6 | (7.0, 112) | 28.0 | (28.0, 28.0) |
| High-risk type | 32.1 | 112.4 | 74.0 | (7.5, 112) | 112 | (22.4, 112) |
| Covariate point prevalence | ||||||
| Menstruation | 8.0 | 28.0 | 29.6 | (22.4, 56) | 28.0 | (28.0, 28.0) |
| Suspected Bacterial vaginosisd | 2.3 | 8.0 | 28.6 | (7.0, 112) | 8.6 | (8.0, 56) |
| Condom use | 2.0 | 7.0 | 16.4 | (7.0, 112) | 7.0 | (7.0, 10.2) |
| Vaginal sex | 3.6 | 12.4 | 16.0 | (7.0, 28) | 12.4 | (11.2, 22.4) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; IQR, interquartile range.
a Estimated by mean- and trend-adjusted periodogram with a mixture of smoothing spans of 2 and 3 observations, or equivalently, of 7 or 11.5 days.
b 1000 bootstrapping with replacement.
c Assuming a constant sampling interval of 3.5 days.
d Nugent score was missing in 27 occasions among 15 women; this affected the bootstrapping results only.
Variability Within a Menstrual Cycle
In a linear model, there was a linear decreasing trend from early to late menstrual phases for HR-HPV prevalence (β for the linear term, −0.0034; P = .031). However, neither any-type prevalence nor HPV percent-type positivity was statistically associated with sampling times in a menstrual cycle (data not shown). Table 5 summarizes results of fitting GEE models to the (8-time point) time series data. The implicit assumption that the periodicity in HPV prevalence was synchronous to a menstrual cycle had support from the earlier spectral analysis. In all categorization systems employed, the periovulatory phase was consistently associated with a higher yield of HPV detection. Specifically, swabs collected in the periovulatory phase (modified van Ham's category, 12–15th day of cycle) had a nearly 2-fold likelihood of detecting any-type HPV (adjusted odds ratio [aOR], 1.96 [95% CI, 1.06–3.62]) as compared to that of the menstruation period. The odds of HR-HPV detection also increased by 63% (aOR, 1.63 [95% CI, 1.03–2.58]) when swabs were collected around the time of ovulation (Table 5). Results for multiple-type HPV detection and for HPV percent-type positivity were essentially similar (Table 5).
Table 5.
Associations of HPV Prevalence Data and Percent Type Positivity With Sampling Times in a Menstrual Cycle by Different Phase Categorization, Results of Regression with GEEa Method in 21 Women (595 Swabs) from the Baltimore-Washington Area, 2005–2007
| Phase Categories | Any Type |
High-Risk Type |
Multiple Type |
Percent-Type Positivity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORb | LL | UL | ORb | LL | UL | ORb | LL | UL | Betab | LL | UL | |
| Model 1: weekly | ||||||||||||
| 1–7 d | Ref | Ref | Ref | Ref | ||||||||
| 8–14 d | 1.35 | 0.89 | 2.03 | 1.25 | 0.89 | 1.75 | 1.91** | 1.45 | 2.51 | 0.013* | 0.003 | 0.024 |
| 15–21 d | 1.35 | 0.98 | 1.85 | 1.61* | 1.04 | 2.50 | 2.31** | 1.60 | 3.35 | 0.017* | 0.004 | 0.031 |
| 22+ day | 0.81 | 0.47 | 1.41 | 0.65 | 0.42 | 1.02 | 1.19 | 0.67 | 2.11 | −0.001 | −0.008 | 0.007 |
| Model 2: every 10 d | ||||||||||||
| 1–10 d | Ref | Ref | Ref | Ref | ||||||||
| 11–21 d | 1.46** | 1.10 | 1.95 | 1.56* | 1.08 | 2.25 | 2.01** | 1.36 | 2.96 | 0.015* | 0.002 | 0.029 |
| 21+ day | 0.80 | 0.46 | 1.39 | 0.65* | 0.43 | 0.96 | 1.02 | 0.57 | 1.85 | −0.003 | −0.011 | 0.005 |
| Model 3: van Ham's categoryc | ||||||||||||
| 2–3 d | Ref | Ref | Ref | Ref | ||||||||
| 7–11 d | 1.05 | 0.68 | 1.63 | 1.03 | 0.76 | 1.40 | 1.55** | 1.17 | 2.06 | 0.008* | 0.001 | 0.015 |
| 12–15 d | 1.84* | 1.02 | 3.29 | 1.50 | 0.93 | 2.43 | 2.35** | 1.47 | 3.77 | 0.019* | 0.002 | 0.036 |
| 20+ day | 0.91 | 0.59 | 1.41 | 0.94 | 0.62 | 1.41 | 1.48 | 0.93 | 2.34 | 0.004 | −0.002 | 0.011 |
| Model 4: modified van Ham's category | ||||||||||||
| 2–3 d | Ref | Ref | Ref | Ref | ||||||||
| 4–6 d | 1.17 | 0.68 | 2.02 | 1.19 | 0.73 | 1.94 | 0.67 | 0.38 | 1.17 | −0.006 | −0.019 | 0.006 |
| 7–11 d | 1.13 | 0.68 | 1.87 | 1.12 | 0.81 | 1.57 | 1.29 | 0.82 | 2.02 | 0.005 | −0.002 | 0.012 |
| 12–15 d | 1.96* | 1.06 | 3.62 | 1.63* | 1.03 | 2.58 | 1.95** | 1.25 | 3.04 | 0.016** | 0.004 | 0.028 |
| 16–19 d | 1.57* | 1.06 | 2.33 | 1.41 | 0.87 | 2.31 | 1.83** | 1.18 | 2.83 | 0.016** | 0.004 | 0.027 |
| 20–24 d | 1.36 | 0.87 | 2.13 | 1.51 | 0.97 | 2.34 | 1.57 | 0.96 | 2.55 | 0.008* | 0.001 | 0.014 |
| 25+ day | 0.71 | 0.33 | 1.54 | 0.60 | 0.32 | 1.12 | 0.91 | 0.43 | 1.93 | −0.006 | −0.018 | 0.006 |
Abbreviations: HPV, human papillomavirus; LL, lower limit of 95% confidence interval; OR, odds ratio; Ref, reference; UL, upper limit of 95% confidence interval.
a Assuming independence working correlation (see justification in text).
b As there was no significant cycle-specific difference, we used a linear rather than categorical form for cycle adjustment in the final models.
c 67 swabs were excluded from the analysis due to gaps in this particular category for menstrual phases.
*P < .05; **P < .01.
Cross-Correlation Analysis
According to sample CCF, there was no significant cross-correlation among HPV detection and covariate time series considered, with the exception of a single pair: any-type HPV prevalence and menstruation data. Before removing the temporal correlation in each of the pair, the 2 series were significantly cross-correlated at 2 lags (CCF−10.5 days,0.41;CCF−35 days, 0.43), suggesting that a peak in HPV prevalence was followed by (the beginning of) menstruation 9–12 days later (or 35–40 days later, representing 9–12th days of the next cycle). This finding was consistent with the GEE results, which showed that HPV detection was at its peak around the periovulatory stage, declining afterward (Table 3). This HPV-menstruation pair of time series was negatively correlated contemporaneously (ρ, −0.24), although was not statistically significant (β, −0.09; P = .187). After removing the serial correlation using an AR (1) model (first-degree correlation coefficient, φ1, 0.62), the contemporaneous association diminished (β, −0.04; P = .641) whereas a temporally lagged (9–12 days) linear association between any-type HPV and menstruation became significant (β, 0.19; P = .016). In particular, fluctuations in any-type HPV prevalence were positively associated with changes in menstruation data 9–12 days later (ρ, 0.36). In sensitivity analysis, we excluded 1 woman who reported the use of an intrauterine device at baseline and did not find differences in any of the aforementioned results.
DISCUSSION
Using HPV testing results from 595 vaginal swabs intensively collected by 21 women over a 16-week period, we found that point prevalence of HPV fluctuated greatly over a short time period, which was consistent with our and others' previous work [28–30]. We identified a cyclic pattern in which any-type HPV detection and HPV percent-type positivity altered approximately every 28 days, corresponding to the median length of menstrual cycles documented by the study participants. In addition, we showed a higher detection rate of HPV DNA of any type or of HR types around the time of ovulation than that in menstruation, confirming what Sherman et al [11] have observed in a much larger cohort with fewer follow-up swabs from a single participant. Given the high negative predictive value of HPV DNA testing [30], our findings are unlikely to have any impact on the performance of HPV testing in routine cervical cancer screening. However, results of the current study contribute to a better understanding of host factors that may or may not affect HPV DNA detection as well as to natural history inferences regarding HPV infection status based on single versus multiple swab collection.
Several factors may have contributed to the conflicting findings regarding the menstrual cycle effect on HPV prevalence in previous studies (Table 1). First, studies with few swabs from the same individual made inferences based on testing results from random samples across 2 or fewer menstrual cycles of the same woman or from different individuals at various menstrual phases. In the former case, the traditional approach may fail to distinguish between the within-person correlation and a possible longer-term periodic effect; in the latter case, conventional regression techniques can fail to incorporate the within-person variation while addressing between-person variability. Considering the sample size of our study population and the stringent assumptions required by stochastic models (such as the autoregressive moving average model), we believe that the GEE model provides the most robust results because of its flexible working correlation assumption.
Second, various categorizations of menstrual phases and participants' varying cycle lengths have further complicated comparisons across studies (Table 1). Last, while assessing potential interrelationships between concomitantly measured variables, 2 series of repeated observations can appear correlated simply because of similar temporal scales of autocorrelation or of sharing the same temporal trend. When the serial correlation in each time series data is not removed, spurious associations between HPV prevalence and other repeatedly observed host factors will result [22, 23].
Researchers have proposed several potential mechanisms to explain the epidemiologic link between the menstrual cycle and detectable HPV DNA, including estrogen-facilitated proliferation and maturation of cervical epithelium making more shedding of virus-infected cells, hormone-enhanced viral replication, and a generally suppressed mucosal immunity at midcycle [11]. In-vitro evidence also suggests anti-inflammatory effects of exogenous sex hormones on peripheral blood mononuclear cells [31–37]. The resulting shift in immune and cytokine responses to viruses in the presence of reproductive hormones reportedly raises the risk of viral persistence, suggesting that sex hormone swings during a menstrual cycle could also affect viral detectability. Gajer and Brotman et al [20] also found that bacterial communities are more stable in the middle of the menstrual cycle, suggesting an interplay between the vaginal microbiota and HPV. Future studies utilizing molecular techniques will provide a refined estimate of the role of the bacterial communities in HPV detection studies.
Contrary to the current analysis, prior research has reported a protective effect of Lactobacillus spp. and an increase in susceptibility to viral infections when the normal microbiota is replaced by BV-associated bacteria [38–40]. A recent meta-analysis based on mostly cross-sectional studies has shown a positive association between BV and HPV [41]. However, the causal temporality remains inconclusive, as 2 prospective studies showed contradictory findings [42, 43]. Mao et al [42] performed a time-lag analysis and found that incident HPV infection predated incident BV in a cohort of young women aged 18–24, whereas Watts et al [43] showed that BV was associated with later-incident HPV infection in high-risk women enrolled in the Women's Interagency HIV Study (WIHS). This latter result was further supported by a recent prospective analysis of women enrolled in the HIV Epidemiology Research Study (HERS) [44]. In addition, prior work failed to adjust for possible serial correlation in the time series data such that previously reported associations might be misleading [15, 45].
There are several limitations in our approach. First, although this is one of the largest datasets measuring both HPV and multiple menstrual cycles, our statistics and inferences are limited by the small number of individuals in the study. Second, while many studies have confirmed the high concordance between self-collected samples and cervical-directed sampling on HPV detection [46], we are unable to confirm that the same temporal variability would be observed at the more clinically relevant cervical epithelium. Third, the validity of the phase dependence of HPV DNA detection relied on how well the time metric used in the model (ie, day of cycle) could characterize each menstrual cycle for every individual over time. Without a definitive indicator for ovulation, phases of the menstrual cycle could only be identified approximately. Future investigations that use more specific phase indicators (eg, serum levels of sex hormones or phase-specific cytokines such as interleukin-1β [47–50]) may help elucidate the phase dependence of HPV detection. Fourth, although we attempted to capture the type-to-type variability among women with multiple-type infection by the use of HPV percent-type positivity, it was inevitably a summary measure. Future research may consider standard measurements of viral loads to further explore the link between the menstrual cycle and viral DNA detection.
In conclusion, we demonstrate that any-type HPV DNA detection varies within a menstrual cycle, with a higher probability of detection around the time of ovulation than in menstruation. In the current analysis, serially measured HPV prevalence was not statistically associated with sexual activities or Nugent-score BV. Overall, our findings add to the growing literature on the menstrual cycle effect on HPV testing performance.
Notes
Acknowledgments. The authors thank Roslyn Howard for laboratory testing, and the women of the Douching Cessation Study for their participation and commitment. The authors also thank Dr Gary Rosner at The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, for his helpful comments on statistical methods. Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases grants K01 AI080974 (to R. M. B) and R03 AI061131 (to J. M. Z). D. A. T. C. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. D. A. T. C.'s work was supported by the US National Institutes of Health National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109.
Potential conflicts of interest. P. E. G. has served as a member of the Women's Health Scientific Advisory Board for Qiagen within the past 5 years. D. A. T. C. has acted as a consultant to Medimmune on influenza. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fife KH, Katz BP, Brizendine EJ, Brown DR. Cervical human papillomavirus deoxyribonucleic acid persists throughout pregnancy and decreases in the postpartum period. Am J Obs Gyn. 1999;180:1110–4. doi: 10.1016/s0002-9378(99)70602-2. [DOI] [PubMed] [Google Scholar]
- 2.Rando RF, Lindheim S, Hasty L, Sedlacek TV, Woodland M, Eder C. Increased frequency of detection of human papillomavirus deoxyribonucleic acid in exfoliated cervical cells during pregnancy. Am J Obs Gyn. 1989;161:50–5. doi: 10.1016/0002-9378(89)90231-7. [DOI] [PubMed] [Google Scholar]
- 3.Reeves WC, Arosemena JR, Garcia M, et al. Genital human papillomavirus infection in Panama City prostitutes. J Infect Dis. 1989;160:599–603. doi: 10.1093/infdis/160.4.599. [DOI] [PubMed] [Google Scholar]
- 4.de Villiers EM, Wagner D, Schneider A, et al. Human papillomavirus infections in women with and without abnormal cervical cytology. Lancet. 1987;2:703–6. doi: 10.1016/s0140-6736(87)91072-5. [DOI] [PubMed] [Google Scholar]
- 5.McNicol PJ, Guijon FB, Paraskevas M, Heywood E, Gray MJ, Brunham RC. Effect of the menstrual cycle on detection and typing of human papillomavirus in uterine cervical cells. Am J Obs Gyn. 1990;162:1037–41. doi: 10.1016/0002-9378(90)91311-y. [DOI] [PubMed] [Google Scholar]
- 6.Schneider A, Kirchhoff T, Meinhardt G, Gissmann L. Repeated evaluation of human papillomavirus 16 status in cervical swabs of young women with a history of normal Papanicolaou smears. Obs Gyn. 1992;79:683–8. [PubMed] [Google Scholar]
- 7.Fairley CK, Robinson PM, Chen S, Tabrizi SN, Garland SM. The detection of HPV DNA, the size of tampon specimens and the menstrual cycle. Genitourin Med. 1994;70:171–4. doi: 10.1136/sti.70.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler CM, Greer CE, Becker TM, Hunt WC, Anderson SM, Manos MM. Short-term fluctuations in the detection of cervical human papillomavirus DNA. Obstet Gynecol. 1996;88:262–8. doi: 10.1016/0029-7844(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 9.van Ham MAPC, Melchers WJG, Hanselaar AGJM, Bekkers RLM, Boonstra H, Massuger L. Fluctuations in prevalence of cervical human papillomavirus in women frequently sampled during a single menstrual cycle. Br J Cancer. 2002;16:130–3. doi: 10.1038/sj.bjc.6600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper DM, Longacre MR, Belloni DR, Cole BF. Factors affecting the detection rate of human papillomavirus. Ann Fam Med. 2003;1:221–7. doi: 10.1370/afm.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman ME, Carreon JD, Schiffman M. Performance of cytology and human papillomavirus testing in relation to the menstrual cycle. Br J Cancer. 2006;94:1690–6. doi: 10.1038/sj.bjc.6603151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeink C, Massuger L, Lenselink C, Quint W, Melchers W, Bekkers R. Effect of the menstrual cycle and hormonal contraceptives on human papillomavirus detection in young, unscreened women. Obs Gyn. 2010;116:67–75. doi: 10.1097/AOG.0b013e3181e238f0. [DOI] [PubMed] [Google Scholar]
- 13.Andrea L, Marie-Noelle DR, Nicolas N, et al. Cervical infection with human papillomavirus (HPV) 6 or 11 in high-risk women in Burkina Faso. Sex Transm Infect. 2010;86:342–4. doi: 10.1136/sti.2009.041053. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree BF, Ray SC, Schmidt PM, O'Connor PJ, Schmidt DD. The individual over time: time series applications in health care research. J Clin Epidemiol. 1990;43:241–60. doi: 10.1016/0895-4356(90)90005-a. [DOI] [PubMed] [Google Scholar]
- 15.Upshur RE, Knight K, Goel V. Time-series analysis of the relation between influenza virus and hospital admissions of the elderly in Ontario, Canada, for pneumonia, chronic lung disease, and congestive heart failure. Am J Epidemiol. 1999;149:85–92. doi: 10.1093/oxfordjournals.aje.a009731. [DOI] [PubMed] [Google Scholar]
- 16.Brotman RM, Ghanem KG, Klebanoff MA, Taha TE, Scharfstein DO, Zenilman JM. The effect of vaginal douching cessation on bacterial vaginosis: a pilot study. Am J Obs Gyn. 2008;198:628.e621–27. doi: 10.1016/j.ajog.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen FC, Shaw SW, Cheng PJ, Hsueh S, Lin CT. Diagnosis of human papillomavirus infection by abnormal cervical cytology is highly reproducible after vaginal douching. Taiwanese J Obs Gyn. 2008;47:412–6. doi: 10.1016/S1028-4559(09)60008-5. [DOI] [PubMed] [Google Scholar]
- 18.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DH. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci. 2012;343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003605. 132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trimble C, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLAP henotype. Clin Cancer Res. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Box GEP, Jenkins GM, Reinsel GC. Time series analysis: forecasting and control. 4th Ed. Wiley. Hoboken, New Jersey: 2008. [Google Scholar]
- 23.Cowpertwait PSP, Metcalfe AV. Introductory time series with R. New York: Springer; 2009. [Google Scholar]
- 24.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 25.Ljung GM, Box GEP. On a measure of lack of fit in time series models. Biometrika. 1978;65:297–303. [Google Scholar]
- 26.Ihaka R, Gentleman R. R: a Language for data analysis and graphics. J Comp Graph Stat. 1996;5:299–314. [Google Scholar]
- 27.StataCorp LP. Stata Statistical Software. College Station, TX: 2009. [Google Scholar]
- 28.Liu SH, Cummings DAT, Zenilman JM, Gravitt PE, Brotman RM. Temporal dynamics of HPV DNA detectability in sexually active women using short-interval sampling. doi: 10.1158/1055-9965.EPI-13-0666. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 30.Woodman C, Collins S, Young L. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 31.Mittal R, Tsutsumi K, Pater A, Pater MM. Human papillomavirus type 16 expression in cervical keratinocytes: role of progesterone and glucocorticoid hormones. Obstet Gynecol. 1993;81:5–12. [PubMed] [Google Scholar]
- 32.Brisson J, Bairati I, Morin C, et al. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis. 1996;173:794–9. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 33.Tibbetts TA, Conneely OM, O'Malley BW. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod. 1999;60:1158–65. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- 34.Kamada M, Irahara M, Maegawa M, et al. Transient increase in the levels of T-helper 1 cytokines in postmenopausal women and the effects of hormone replacement therapy. Gynecol Obstet Invest. 2001;52:82–8. doi: 10.1159/000052948. [DOI] [PubMed] [Google Scholar]
- 35.Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. JCEM. 2001;86:2403–8. doi: 10.1210/jcem.86.6.7528. [DOI] [PubMed] [Google Scholar]
- 36.Marks MA, Gravitt PE, Burk RD, Studentsov Y, Farzadegan H, Klein SL. Progesterone and 17beta-estradiol enhance regulatory responses to human papillomavirus type 16 virus-like particles in peripheral blood mononuclear cells from healthy women. Clin Vaccine Immunol. 2010;17:609–17. doi: 10.1128/CVI.00441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gravitt P. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis: a public health review. Br J Obs Gyn. 2001;108:439–50. doi: 10.1111/j.1471-0528.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect. 2001;77:402–8. doi: 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platz-Christensen JJ, Mattsby-Baltzer I, Thomsen P, Wiqvist N. Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am J Obs Gyn. 1993;169:1161–6. doi: 10.1016/0002-9378(93)90274-m. [DOI] [PubMed] [Google Scholar]
- 41.Gillet E, Meys JF, Verstraelen H, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao C, Hughes JP, Kiviat N, et al. Clinical findings among young women with genital human papillomavirus infection. Am J Obs Gyn. 2003;188:677–84. doi: 10.1067/mob.2003.164. [DOI] [PubMed] [Google Scholar]
- 43.Watts DH, Fazzari M, Minkoff H, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–39. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 44.King CC, Jamieson DJ, Wiener J, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obs Gyn. 2011;2011:319460. doi: 10.1155/2011/319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz J, Spix C, Touloumi G, et al. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J Epidemiol Community Health. 1996;50(Suppl 1):S3–11. doi: 10.1136/jech.50.suppl_1.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gravitt P, Belinson J, Salmeron J, Shah K. Looking ahead: a case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. International journal of cancer. J Int Cancer. 2011;129:517–27. doi: 10.1002/ijc.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abrahamsen B, Stilgren LS, Rettmer E, Bonnevie-Nielsen V, Beck-Nielsen H. Effects of the natural and artificial menstrual cycle on the production of osteoprotegerin and the bone resorptive cytokines IL-1beta and IL-6. Calcif Tissue Int. 2003;72:18–23. doi: 10.1007/s00223-002-2037-y. [DOI] [PubMed] [Google Scholar]
- 48.Bouman A, Moes H, Heineman MJ, de Leij LF, Faas MM. The immune response during the luteal phase of the ovarian cycle: increasing sensitivity of human monocytes to endotoxin. Fertil Steril. 2001;76:555–9. doi: 10.1016/s0015-0282(01)01971-9. [DOI] [PubMed] [Google Scholar]
- 49.Young JE, Friedman CI, Danforth DR. Interleukin-1 beta modulates prostaglandin and progesterone production by primate luteal cells in vitro. Biol Reprod. 1997;56:663–7. doi: 10.1095/biolreprod56.3.663. [DOI] [PubMed] [Google Scholar]
- 50.Simon C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. JCEM. 1993;77:549–55. doi: 10.1210/jcem.77.2.8345061. [DOI] [PubMed] [Google Scholar]