Abstract
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) antigens mediate parasite sequestration and host immune evasion. Reactivity to 21 PfEMP1 fragments on a protein microarray was measured in serum samples from Malian children aged 1–6 years and adults. Seroreactivity to PfEMP1 fragments was higher in adults than in children; intracellular conserved fragments were more widely recognized than were extracellular hypervariable fragments. Over a malaria season, children maintained this differential seroreactivity and recognized additional intracellular PfEMP1 fragments. This approach has the potential to identify conserved, seroreactive extracellular PfEMP1 domains critical for protective immunity to malaria.
Keywords: malaria, var genes, PfEMP1, immunity, seroreactivity, microarray
Older individuals in malaria-endemic regions develop protective natural immunity to clinical manifestations of Plasmodium falciparum malaria. The basis of this immunity appears to be, at least in part, acquisition of antibodies to parasite antigens expressed on the surface of infected erythrocytes. A previous epidemiologic study examining the association between antibodies to different P. falciparum antigens and the risk of clinical malaria episodes found that antibodies to these parasite-produced erythrocyte surface antigens was the only factor consistently associated with protection against clinical malaria [1].
The var family of genes encodes P. falciparum erythrocyte membrane protein 1 (PfEMP1) antigens, large molecules expressed on the surface of the infected erythrocyte that bind to endothelial receptors [2]. Each parasite genome carries 40–60 var genes but expresses only 1 PfEMP1 at a time, providing a large repertoire of surface molecules that are probably important for immune evasion and pathogenesis [3]. The relatively rapid development of immunity to cerebral malaria during the first 5 years of life suggests the possibility that a smaller subset of var genes is responsible for this severe manifestation of falciparum malaria.
All var genes have an upstream promoter sequence followed by 2 exons. Each var can be placed into 1 of 5 groups based on upstream promoter sequence, chromosomal location, and direction of transcription: A, B/A, B, B/C, or C [4]. Using this classification, several studies have found links between group A and B var group expression and symptomatic disease [5, 6]. Exon 1 encodes the extracellular binding domains and is hypervariable, which may help the parasite elude the immune system. Exon 2 encodes an acidic cytoplasmic tail that anchors PfEMP1 within the erythrocyte membrane. Compared with exon 1, exon 2 is well conserved, with group A exon 2 sequences forming a distinct clade [7].
Serologic testing with a microarray populated with P. falciparum proteins is an effective way to measure seroreactivity on a large scale, in terms of both the number of serum samples tested and the number of target proteins [8]. Other studies using this approach have shown that seroreactivity to group A PfEMP1 variants is associated with protection from clinical malaria [8] and that the breadth of PfEMP1 seroreactivity increases with the age of the subject [9]. With serum samples from children and adults living in an area with highly seasonal but intense malaria transmission in Mali, we used a microarray to characterize reactivity to protein fragments derived from PfEMP1 sequences found in the P. falciparum reference genome 3D7, including both intracellular and extracellular protein fragments, to determine differential seroreactivity and how this seroreactivity changes with age and over the course of a malaria season.
METHODS
A microarray was populated with P. falciparum protein fragments encoded by either exon 1 or exon 2 of var genes in the reference genome 3D7 (Supplementary Data; Supplementary Figure 1). Array construction [10, 11] included (1) polymerase chain reaction amplification of complete or partial P. falciparum open reading frames, (2) in vivo recombination cloning in Escherichia coli, (3) in vitro transcription or translation, and (4) chip printing. Each microarray contained 3 standard controls, as described elsewhere [9]. The PfEMP1 fragments were selected based on their successful amplification and cloning.
Antibody Profiling
Arrays were probed with serum from 25 children aged 1–6 years and 18 adults, all residing in Bandiagara, Mali, where malaria transmission is intense and sharply seasonal. Serum from 32 presumably malaria-naive US blood donors served as controls. Malian serum samples included samples collected from the same individuals before and after the malaria transmission season. Serum sample and slide preparations were performed as described elsewhere [10, 11].
Serum samples were obtained from adults (2005 malaria season) and children (2007 malaria season) enrolled as control volunteers in vaccine trials conducted in compliance with the International Conference on Harmonisation Good Clinical Practices, the Declaration of Helsinki, and Malian regulatory requirements (ClinicalTrials.gov NCT00308061 and NCT00358332) [12, 13]. The protocols were approved by institutional review boards of the University of Bamako Faculty of Medicine, the University of Maryland, Baltimore, and the US Army Surgeon General. Written informed consent was obtained for screening and enrollment in the trials. Verbal consent of illiterate parents or guardians was provided and documented using thumbprints and verified by independent witnesses.
Raw signal intensity was reduced by 2 standard deviations above the mean for the no-DNA negative control [9] to define significant fluorescence intensity [8]. Positive seroreactivity for a protein fragment was defined as antibody binding producing a fluorescence intensity of ≥5000.
Seroprevalence refers to the proportion of serum samples that were reactive to a protein fragment or group of protein fragments, reported as means. Unmatched seroprevalence comparisons were made with the χ2 test. Paired comparisons of seroprevalence for extracellular versus intracellular protein fragments were performed with a 2-tailed McNemar test.
The magnitude of seroreactivity (fluorescence intensity) was compared between serum samples only for the same protein fragment, not across protein fragments. Paired comparisons of pre- and postseason seroreactivity magnitude were performed with the Wilcoxon signed rank test. Unmatched comparisons of seasonal differences in seroreactivity between children and adults were performed with a 2-sample Kolmogorov-Smirnov test. For all tests, significance was set at P < .05, without correction for multiple comparisons. Statistical analyses were performed using MYSTAT 12 software (Systat Software, Inc., version 12.02.00).
RESULTS
Serum samples from malaria-exposed children recognized significantly more extracellular and intracellular-derived PfEMP1 protein fragments than did samples from malaria-naive controls (Figure 1), for both samples obtained before (extracellular, P = .004; intracellular, P < .001) and those obtained after (extracellular, P = .04; intracellular, P < .001) the malaria season. Likewise, serum samples from malaria-exposed adults recognized significantly more PfEMP1 extracellular and intracellular protein fragments than did samples from malaria-naive controls, for both pre- and postseason samples (all 4 comparisons, P < .001). Serum samples from malaria-exposed adults recognized more PfEMP1 fragments (both extracellular and intracellular) than did samples from malaria-exposed children, again for both pre- and postseason samples (all 4 comparisons, P < .001). Seroreactivity was significantly greater in adults than in children in both pre- and postseason samples for a majority of intracellular PfEMP1 fragments (Figure 2A) but for only a minority of extracellular protein fragments (Figure 2B).
Figure 1.
A peptide microarray containing 21 protein fragments from Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) variants encoded by the reference genome 3D7 was probed with serum samples from 25 children and 18 adults from Bandiagara, Mali, and from 32 healthy North American blood donors with no known exposure to malaria. Signal intensities were quantified and are shown on the heat map (green indicates weakest intensity; red, strongest; black, intermediate). Each column displays the seroreactivity profile of 1 serum sample, labeled by participant at the bottom, and each row displays the seroreactivity profile of an individual protein fragment. Protein fragments from intracellular and extracellular PfEMP1 variants are labeled on the right, segregated by PfEMP1 groups. Samples from malaria-endemic individuals included 2 time points, before and after malaria season. Pediatric serum samples from children are ordered by increasing age, ranging from 1 to 6 years at enrollment.
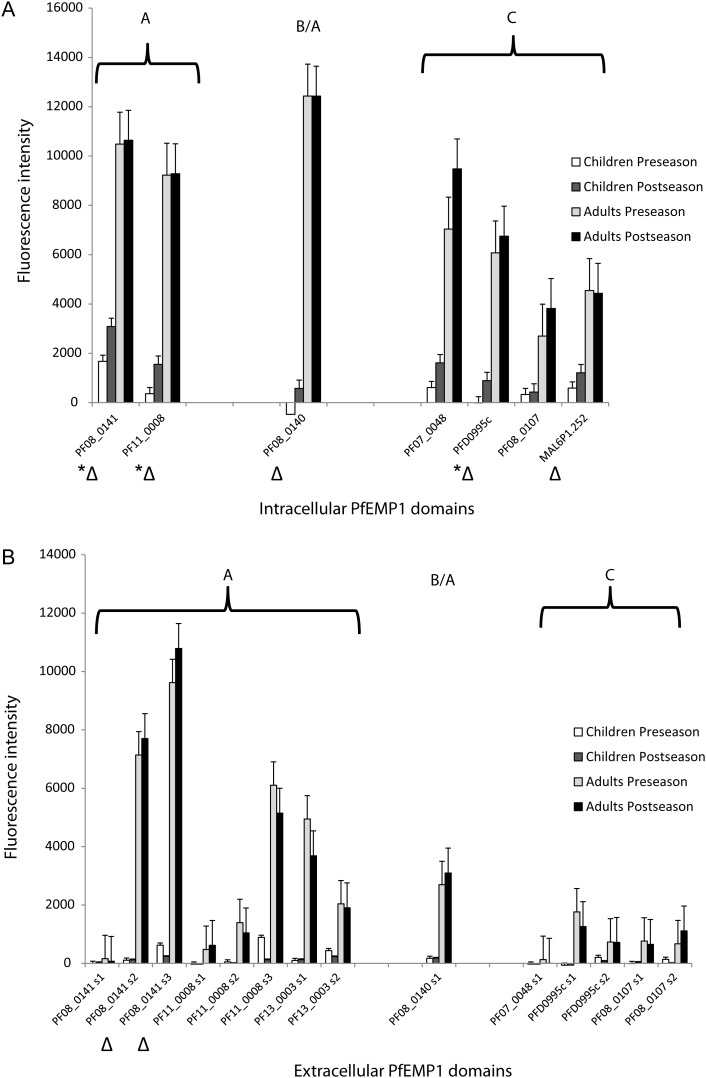
Figure 2.
Mean fluorescence intensities of serum samples from children and adults before and after malaria season for each intracellular (A) and extracellular (B) Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) fragment. Triangles represent PfEMP1 fragments for which serum samples from adults were significantly more seroreactive than those from children, both before and after the malaria season. *P < .05 for difference in the seasonal change in mean fluorescence intensity between serum samples from children and those from adults (Kolmogorov-Smirnov test); this difference was not significant for any extracellular PfEMP1. Error bars indicate standard errors.
Seroprevalence of Exon Groups in Children
Serum samples from malaria-exposed children reacted strongly to a limited number of PfEMP1 protein fragments. This enhanced seroreactivity was demonstrated for a higher proportion of intracellular than extracellular protein fragments, both before (8.9% vs 1.5%; P < .001) and after (16.7% vs 1.2%; P < .001) the malaria season. The proportion of recognized intracellular protein fragments increased after the malaria season (P = .007), but the proportion did not change for extracellular protein fragments.
Seroprevalence of Exon Groups in Adults
Malaria-exposed adults had greater seroreactivity to intracellular than to extracellular PfEMP1 fragments, both before and after the malaria season (both P < .001). The proportions of recognized extracellular and intracellular protein fragments did not differ significantly between pre- and postseason serum samples.
Magnitude of Seroreactivity
Compared with matched preseason serum samples, children's serum samples obtained after the malaria season reacted intensely to intracellular PfEMP1 fragments overall (Figure 2A; n = 161; P < .001) and significantly for 3 of 7 individual intracellular protein fragments, but not to extracellular fragments (Figure 2B). This was true for intracellular protein fragments from both PfEMP1 groups A (n = 46; P = .009) and C (n = 92; P = .004). No such differences were seen with matched adult serum samples. The mean fluorescence intensity did not uniformly increase for extracellular protein fragments over the course of the malaria season for either children or adults.
Seroreactivity to Extracellular and Intracellular Protein Fragments From the Same PfEMP1 Variant
Before the malaria season, children's serum samples were as likely to recognize intracellular PfEMP1 fragments as corresponding extracellular fragments from the same variant. After the malaria season, in contrast, significant seroreactivity was demonstrated against the intracellular protein fragment but not against the corresponding extracellular fragment for 3 PfEMP1 variants, including the 2 group A– and 1 group B/A–derived protein fragments tested (all 3 comparisons, P = .03).
Before the malaria season, adult seroreactivity to intracellular protein fragments for 6 PfEMP1 variants was more common than seroreactivity to the corresponding extracellular protein fragments, including protein fragments derived from both group A (3 comparisons, P = .002, P = .001, P = .001) and group C (3 comparisons, P = .002, P = .039, P = .001). After the malaria season, adult seroreactivity to the same 6 intracellular protein fragments was more common than seroreactivity to the corresponding extracellular protein fragments from the same PfEMP1 variant (3 group A comparisons, P = .002, P = .0002, P = .002; 3 group C comparisons, P = .004, P = .012, P = .012). One of these variants, PF08_0141, encoded by a group A var gene, also had differential exon seroreactivity in serum samples from children after the malaria season (Figure 2A). No additional changes in differential exon seroreactivity for adult serum samples were detected. Extracellular PfEMP1 fragments were not recognized more commonly than corresponding intracellular fragments from the same PfEMP1 variant in children or adults at either time point.
DISCUSSION
Both children and adults living in an area of intense seasonal malaria transmission had measureable seroreactivity to PfEMP1 fragments derived from the conserved region exon 2, which encodes the intracellular cytoplasmic tail of PfEMP1. In contrast, protein fragments derived from exon 1, encoding the hypervariable extracellular region, were relatively unrecognized by serum samples from both children and adults. Over the course of a malaria season, children's seroreactivity to intracellular PfEMP1 fragments consistently increased, and they acquired seroreactivity to additional intracellular group A protein fragments, suggestive of acquired immunity. In contrast, the proportion of intracellular PfEMP1 fragments recognized by adults over the course of a malaria season remained the same.
Children had weaker seroreactivity to intracellular PfEMP1 fragments than adults but had increased seroreactivity to these fragments after a malaria season. The PfEMP1 intracellular domain is thought to be shielded from the immune system but may become exposed when infected erythrocytes are lysed. Individuals probably acquire seroreactivity to particular intracellular domain subgroups rapidly because of sequence conservation, whereas serorecognition of particular extracellular domains may be less likely owing to their hypervariability. Alternatively, technical issues may have contributed to the differential recognition of intracellular and extracellular PfEMP1 fragments in our study. The extracellular regions of PfEMP1 are cysteine rich and composed of domains with a complex Duffy binding–like fold [7]. Expression of PfEMP1 Duffy binding–like domains in E. coli is challenging [14], and reduced recognition of PfEMP1 fragments could result from incorrectly folded protein fragments. However, 2 findings mitigate this concern. First, the observed differential PfEMP1 serorecognition patterns confirmed what we predicted based on malaria exposure history, namely, that adults would have broader serorecognition of PfEMP1 protein fragments than children and that postseason serum samples would have broader serorecognition than preseason samples in both age groups. Second, a previous validation of this approach demonstrated a correlation in seroreactivity between well-characterized P. falciparum proteins used as vaccine candidates and the same proteins expressed through the rapid translation E. coli system that we used to generate protein fragments, both spotted on a microarray [8].
The hypervariable regions encoded by exon 1 probably present extreme antigenic diversity to the host immune system, hindering serorecognition and rapid clearance of parasitized erythrocytes. Such a role for antigenic diversity in pathogens is seen across taxa, including bacteria (eg, Neisseria meningitides), protozoans (eg, Trypanosoma brucei), and fungi (eg, Pneumocystis jiroveci). However, it remains possible that within these hypervariable regions, antigenically conserved epitopes are shared by subsets of PfEMP1 types that are the targets of strain-transcending antibodies [15]. The method described herein has the potential to identify broadly recognized conserved epitopes within hypervariable domains that could be important targets of protective immunity.
A protein microarray is a powerful tool to identify seroreactive domains of polymorphic malaria antigens, shedding light on the acquisition of natural immunity to malaria. With appropriate clinical data, this approach could be used to identify extracellular PfEMP1 domains critical for the development of protective immunity to malaria, which may serve as targets for a vaccine. Such studies would ideally include larger numbers of samples from diverse malaria-endemic settings, multiple time points, and a more exhaustive catalog of PfEMP1 fragments.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Danzele Coulibaly, Sekouba Mariko, and Moctar Traore for administrative support; Nicole Eddington and Carey Martin for technical and administrative support; Yukun Wu for statistical advice; the team of the Bandiagara Malaria Project in Bandiagara for their dedication; and the community of Bandiagara, Mali.
Financial support. This work was supported by the Howard Hughes Medical Institute; the National Institute of Allergy and Infectious Diseases (NIAID; cooperative agreement U19AI065683); the Fogarty International Center, National Institutes of Health (training grant D43TW001589); the Doris Duke Charitable Foundation (Distinguished Clinical Scientist Award to C. V. P. and Clinical Scientist Development Award to K. E. L.); a Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene postdoctoral fellowship (M. A. Travassos); the Wellcome Trust (grant 084226 to J. A. R.); and International Centers for Excellence in Malaria Research cooperative agreements from NIAID (grants U19AI089672 [Southeast Asia] and U19AI089686 [Southwest Pacific] to P. L. F.).
Potential conflicts of interest. P. L. F. holds patents related to technology applied in this study and has stock positions with Antigen Discovery. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–80. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyriacou HM, Stone GN, Challis RJ, et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. 2006;150:211–8. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warimwe GM, Keane TM, Fegan G, et al. Plasmodium falciparum var gene expression is modified by host immunity. Proc Natl Acad Sci U S A. 2009;106:21801–6. doi: 10.1073/pnas.0907590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rask TS, Hansen DA, Theander TG, Gorm PA, Lavstsen T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—divide and conquer. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000933. e1000933:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crompton PD, Kayala MA, Traore B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry AE, Trieu A, Fowkes FJ, et al. The stability and complexity of antibody responses to the major surface antigen of Plasmodium falciparum are associated with age in a malaria endemic area. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies DH, Liang X, Hernandez JE, et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–52. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doolan DL, Mu Y, Unal B, et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–94. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS One. 2008;3:e1465. doi: 10.1371/journal.pone.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS One. 2010;5:e9041. doi: 10.1371/journal.pone.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghumra A, Khunrae P, Ataide R, et al. Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum. PLoS One. 2011;6:e16414. doi: 10.1371/journal.pone.0016414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghumra A, Semblat JP, Ataide R, et al. Induction of strain-transcending antibodies against group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog. 2012;8:e1002665. doi: 10.1371/journal.ppat.1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.