Abstract
Background. Persistent infection with oncogenic human papillomavirus (HPV) is associated with an increased risk of cervical malignancy. Redetection of type-specific HPV after a period of nondetection may be caused by reactivation of a low-level persistent infection. Little is known about factors associated with type-specific HPV redetection.
Methods. For a longitudinal cohort of adolescent women with frequent behavioral and sexually transmitted infection (STI) information (every 3 months), Cox proportional hazard models were used to assess the influence of sexual behaviors and STIs on the redetection of oncogenic or high-risk HPV infections.
Results. A total of 210 type-specific high-risk HPV detection episode periods were identified in this longitudinal cohort; 71 (33.8%) were characterized by a period of nondetection followed by redetection. Chlamydia trachomatis (hazard ratio [HR], 3.14; 95% confidence interval [CI], 1.44–6.86) was associated with redetection; redetection was >2 times more likely with each additional self-reported sex partner in the past 3 months (HR, 2.26; 95% CI, 1.35–3.78).
Conclusions. This study demonstrates the role of C. trachomatis and number of recent sexual partners in type-specific HPV redetection. Given that persistent oncogenic HPV infections are associated with cancer-related outcomes, understanding the potential role of such factors in the pathogenesis of HPV-related outcomes is important.
Keywords: human papillomavirus, chlamydia, HPV redetection
Infections with human papillomaviruses (HPV) are very common in young, sexually active women [1, 2] and many studies have investigated the association of factors, including sexual behaviors and sexually transmitted infections (STIs), that influence HPV-related outcomes. An association of Chlamydia trachomatis genital infections and genital HPV is observed at several points in the development of HPV-associated cervical cancer, including the acquisition of HPV infection [3], duration of HPV persistence [4, 5], and development of cervical cancer [6–8]. An association between C. trachomatis infections and HPV redetection after an interval of viral clearance has not been described.
Ninety percent of type-specific HPV becomes undetectable after 12–24 months using standard polymerase chain reaction assays of genital samples and is deemed “cleared” if the HPV type is not detected in 2 or 3 subsequent, consecutive samples [9, 10]. Redetection refers to subsequent identification of a specific HPV type after it has apparently cleared. Such redetection may have occurred due to repeat infection with the same HPV (or variant) type or reemergence of an existing infection that had become undetectable [11]. Given that persistent oncogenic HPV infections are associated with HPV-related cancers, understanding of a role of C. trachomatis infections and other factors in HPV redetection would add to understanding of the pathogenesis of HPV-associated neoplasia as well as support clinical and public health chlamydia control efforts.
In this study, a cohort of adolescent women were followed closely for almost 6 years with quarterly testing for HPV and other STIs [12]. HPV infections were common in the cohort, and most HPV types became nondetectable after variable time periods. However, redetection of a specific HPV type after a period of nondetection (approximately >6 months) was observed in a subset of these study participants. There is no consensus whether the loss of HPV reflects HPV clearance or the establishment of latency [13]. There is human data to support that redetection of the same HPV type is explained, in part, by new sexual exposures [14, 15]. However, not all infections were accounted for by a new sex partner in these studies, and alternative explanations need to be assessed. Human data has suggested reactivation of HPV when new HPV types were found in immunosuppressed women who had remained sexually abstinent [16, 17]. Animal models with rabbits have demonstrated HPV viral latency and that these latent infections are virally capable of producing clinical lesions after wounding [18]. It has been suggested that the basal stem cells can be infected by and contain HPV in very low cell numbers in humans and that processes such as wound repair after an inflammatory event or trauma, hormonal regulation, or loss of immunological surveillance lead to basal cell differentiation and redetection [18, 19]. Therefore, we hypothesized that factors associated with states of inflammation (ie, STIs), immunogenicity, recurrent HPV exposure (lack of condom use, coital activity, number of sexual partners), or hormonal factors (birth control) could possibly be related redetection.
This study describes the occurrence and frequency of redetection after periods of nondetection and examines potential factors, biologic and behavioral, associated with type-specific redetection.
METHODS
Study Participants
Subjects for this analysis were a cohort of adolescent women recruited for a longitudinal study that assessed risk and protective behaviors associated with STIs. This project was known as the Young Women Project [20]. Recruitment began in the fall of 1999, and the last observation occurred in 2008. Participants were enrolled under the main study protocol, which was approved by the local institutional review board at the Indiana University School of Medicine. Adolescent women attending 1 of 3 primary care clinics in Indianapolis were eligible for enrollment in this study. Inclusion criteria for the study and this analysis were as follows: aged 14–17 years, able to understand English and provide written consent, not have any serious psychiatric problems or mental deficiencies, and have 1 parent who was able to give permission for participation in the study. Both sexually active (ever reported vaginal intercourse) and non–sexually active adolescent women were enrolled. Adolescents who were pregnant at the time of enrollment were excluded; however, those who became pregnant were followed per protocol. Participant informed consent and parental consent were obtained at enrollment. All participants received financial compensation for their time and effort.
At the time of enrollment and at every study visit (approximately every 3 months apart), participants completed face-to-face interviews that assessed specifically for contraceptive methods used (condoms, oral contraceptive pills, and depo-medroxyprogesterone), number of sexual partners, frequency of coital events, and number of condom-protected and -unprotected coital events during the previous quarter (3 months). Participants provided self-obtained vaginal swabs at these visits that were used to test for HPV, C. trachomatis, Neisseria gonorrhea, and Trichomonas vaginalis. No assessment of human immunodeficiency virus was conducted in this study because of low prevalence within the study clinics from which participants were recruited.
Testing for HPV and Other STIs
DNA was extracted from self-obtained vaginal swabs as previously described [21]. The Linear Array HPV Genotyping Test was used for HPV detection and genotyping [21–23]. This assay detects 37 HPV types using nondegenerate, 5′ biotin–labeled primer pools for polymerase chain reaction amplification within the L1 region of the HPV genome. Reactions were amplified in a PerkinElmer TC9600 Thermal Cycler as previously described [24]. A positive control reaction (sample provided by Roche Molecular Diagnostics) and a negative control reaction (no DNA) were performed with each assay. The GH20/PC04 human β-globin target was coamplified to determine sample adequacy.
Detection of specific HPV types was performed as previously described [4, 21]. There are 37 individual HPV types detected in the Linear Array assay, but for this analysis only 7 oncogenic (HPV-16, -18, -31, -33, -45, -52, and -58) type-specific infections were examined. These types were chosen because they were detected frequently in the cohort and because currently available vaccines protect against oncogenic HPV types 16 and 18, and second-generation HPV vaccines, currently in phase 3 trials, will include HPV types 31, 33, 45, 52, and 58. Swabs were also tested by polymerase chain reaction assays for C. trachomatis, N. gonorrhea, and T. vaginalis, as previously described [4].
Statistical Analysis
Descriptive statistics were used to characterize the frequency of HPV detection and the distributions of high-risk HPV types detected. A participant was considered to have a type-specific HPV infection if >2 quarterly samples tested positive (consecutive or nonconsecutive) for that HPV type during the study. If a specific HPV type was detected in only a single swab (n = 83) from a participant during the study, this HPV type was not considered in this analysis (given that it could also represent temporary deposition from sexual intercourse). Conversely, there were 68 single intermittent negative episodes found within the type-specific episodes defined for this analysis. Each participant could contribute up to 7 HPV type-specific infections to the analysis, 1 for each of the 7 oncogenic types examined.
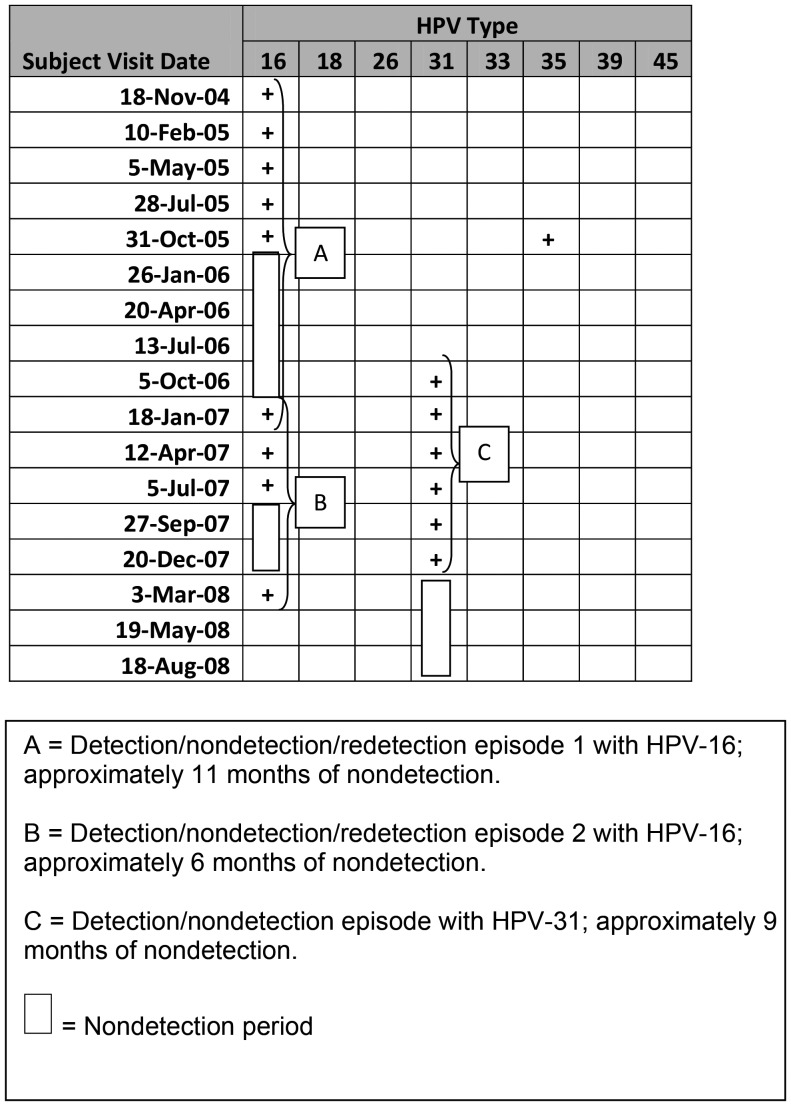
Type-specific infections were further broken down into “detection episodes” and are the focus of this analysis. Two types of patterns (Figure 1) were seen within type-specific infections: detection followed by a period of nondetection followed by redetection (a detection/nondetection/redetection [D/N/R] episode) or detection without subsequent redetection (a detection/nondetection [D/N] episode). A period of nondetection was defined as >2 consecutive negative swabs (approximately >6 months). The duration of nondetection within a D/N/R was defined as the length of time between the date of the first negative test and the date of redetection. Duration of nondetection was also calculated for a D/N episode and defined as the length of time between the date of first negative test and the date of last negative test (ie, last date of the study observation). Some type-specific infections would contain >1 episode for any given individual.
Figure 1.
Longitudinal human papillomavirus detection and episode definition (example subject). Abbreviation: HPV, human papillomavirus.
Independent variables assessed included STIs (N. gonorrhea, C. trachomatis, and T. vaginalis), which were assessed at the time of HPV redetection (or last negative swab for a D/N episode). Use of oral contraceptive pills, use of medroxyprogesterone, condom use (calculated as numbers of unprotected coital events), coital frequency, and partner numbers were assessed over the 3 months just before the redetection date for a D/N/R episode; whereas these variables were assessed at the time period 3 months before the last sample collected for a D/N episode. Frailty models were used to assess effect of each independent variable on time of redetection individually in univariable models, as well as all independent variables together in a multivariable model. Given a more stringent definition of 6 months to define a period of nondetection, sensitivity analysis was performed using 3 months as the minimum time for nondetection. Correlations between multiple type-specific infections and episodes from an individual participant were taken into account by the frailty model. Kaplan–Meier curve of time to redetection and C. trachomatis was plotted. All analyses were performed using SAS version 9.1.3 [25]
RESULTS
Participant Summary
The entire cohort consisted of 146 adolescent women with a mean age of 15.4 years (14–17 years) at enrollment; 94.5% were black. At the time of enrollment, 124 (84.9%) had reported vaginal–penile intercourse, and these women reported a mean of 2.9 (standard deviation [SD], 3.7) sexual partners. By the end of enrollment, all but 1 participant had reported vaginal intercourse. The cohort was followed for a mean of 5.8 years (range, 3.9–9.2 years) and provided a total of 3038 samples; specimen adequacy was >95%. The mean number of self-collected vaginal samples per participant was 21.7 (range, 9–36 swabs).
One or more HPV type-specific infections (HPV-16, -18, -31, -33, -45, -52, or -58) occurred in each of the 146 participants (100%). A total of 210 episodes were identified within the type-specific infections; 139 type-specific episodes ended without redetection (D/N), and 71 ended with redetection (D/N/R).
The D/N/R pattern occurred for all 7 high-risk HPV types, as shown in Table 1. The D/N/R pattern was least frequently seen with HPV-31 (15.8%) and most frequently seen with HPV-16 (40%). Periods of nondetection for D/N/R episodes ranged from 5.7 months for HPV-58 to 18.3 months for HPV-18. The mean duration of a period of nondetection for any of the 7 HPV types exhibiting the D/N/R pattern was 15.2 months (SD, 13.1). In comparison, the average length of time between the date of first negative test (after detection of a specific HPV) type to the end of observation for D/N episodes was 23.6 months (SD, 15.3).
Table 1.
Description of Human Papillomavirus Type Specific Episodes
| HPV Type | Type-Specific Infections | Infections With D/N/R Episodes, | Subjects With D/N/R Episodes, | Median Duration of Nondetection, | Infections With D/N Episodes, | Subjects With D/Nb Episodes | Median Duration of Nondetection, |
|---|---|---|---|---|---|---|---|
| N | No. (%) | No. | Months | No. (%) | No. | Months | |
| 16 | 60 | 24 (40.0) | 21 | 13.2 | 36 (60.0) | 36 | 19.8 |
| 18 | 29 | 8 (27.6) | 7 | 18.3 | 21 (72.4) | 21 | 20.7 |
| 31 | 19 | 3 (15.8) | 3 | 10.7 | 16 (84.2) | 16 | 12.7 |
| 33 | 5 | 1 (20.0) | 1 | 8.2 | 4 (80.0) | 4 | 19.4 |
| 45 | 23 | 8 (34.8) | 7 | 7.0 | 15 (65.2) | 15 | 17.3 |
| 52 | 48 | 16 (33.3) | 14 | 15.1 | 32 (66.7) | 32 | 29.2 |
| 58 | 26 | 11 (42.3) | 9 | 5.7 | 15 (57.7) | 15 | 20.0 |
Abbreviations: D/N, detection/nondetection; D/N/R, detection/nondetection/redetection; HPV, human papillomavirus.
Effect of Concurrent STI on HPV Redetection
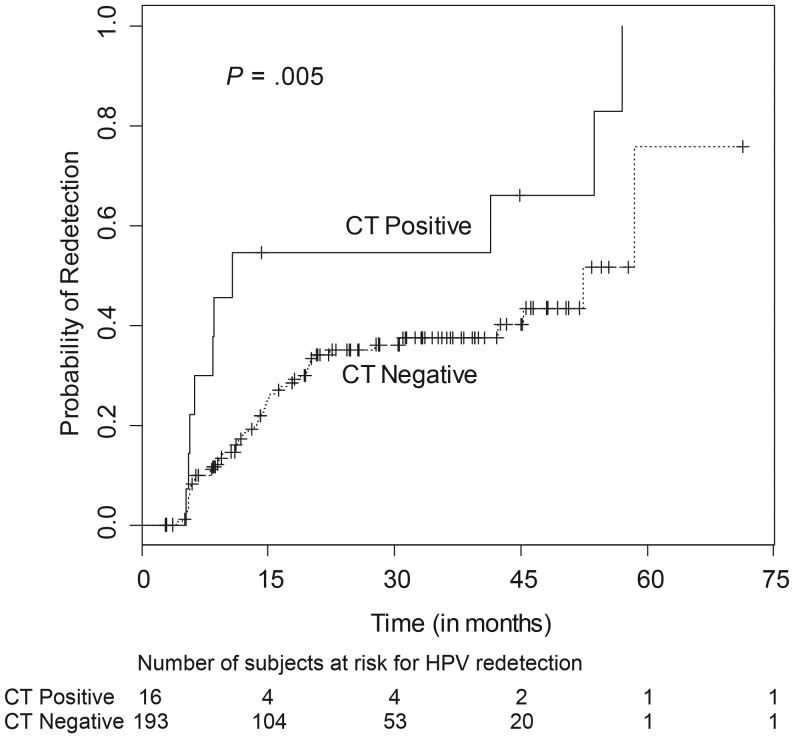
The effect of STIs on the likelihood of HPV redetection was determined (Table 2). Chlamydia was the only STI significantly associated with redetection in both univariable and multivariable models. Participants with chlamydia at the last sample collection were >3 times more likely to have redetection than those subjects without chlamydia (hazard ratio [HR], 3.14; 95% confidence interval [CI], 1.44–6.86; P < .001). The Kaplan–Meier curve (Figure 2) demonstrates the relationship of chlamydia with the high probability of HPV redetection. In other words, probability of continued nondetection of HPV was much lower in cases with concomitant chlamydia infection.
Table 2.
Biological and Behavioral Association with Human Papillomavirus Redetection Frailty Models
| Variable | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratioa (95% CI) |
|---|---|---|
| Chlamydia trachomatis | 2.67 (1.34–5.31)* | 3.14 (1.44–6.86)** |
| Neisseria gonorrhea | 1.82 (.73–4.54) | 1.18 (.36–3.93) |
| Trichomonas vaginalis | 1.36 (.66–2.78) | 1.02 (.41–2.54) |
| Sex partners, last 3 mo, No. | 1.30 (.80–2.12) | 2.26 (1.35–3.78)** |
| New sex partner, last 3 mo, yes/no | 1.14 (.68–2.12) | 1.07 (.52–2.17) |
| Coital events, last 3 mo, No. | 1.01 (.99–1.00) | 1.00 (.97–1.03) |
| Condom-unprotected coital events, last 3 mo, No. | 1.01 (.99–1.02) | 1.01 (.98–1.04) |
| DMPA use in the last 3 mo, yes/no | .64 (.35–1.17) | .51 (.25–1.06) |
| OCPs use in the last 3 mo, yes/no | 1.29 (.59–2.84) | 1.03 (.41–2.60) |
Abbreviations: CI, confidence interval; DMPA, depo-medroxyprogesterone; OCPs, oral contraceptive pills
a All variables included in adjusted model.
*P = .005
**P < .005
Figure 2.
Kaplan–Meier curve for association of chlamydia with human papillomavirus redetection. Abbreviation: CT, Chlamydia trachomatis.
Partner Effect on HPV Infection Duration
The number of unique sex partners was recorded for each participant during the study period, which allowed for calculating cumulative number of unique partners and delineating newly acquired sexual partners. Redetection was not associated with acquiring a recently new sex partner. The median number of sex partners over the last 3 months (just before the time point of examination) for both groups was 2. No association was found between number of partners over the last 3 months and redetection (HR, 1.3; 95% CI, .80–2.12) in the univariable analysis; however, a significant association with number of partners within the last 3 months (HR, 2.26; 95% CI, 1.35–3.78; P < .005) was seen in the multivariable model, suggesting that redetection was >2 times more likely with each additional self-reported sex partner (Table 2).
Contraceptive/Hormonal Effects
Neither use of depo-medroxyprogesterone or oral contraceptive pills over the last 3 months was associated with type-specific HPV redetection in the univariable or multivariable models. More condom-unprotected coital events were associated with redetection; however, this association was not statistically significant.
Finally, regression models were repeated using ≥3 months to define a period of nondetection. No differences were seen in the univariable and multivariable models with regards to significant variables.
DISCUSSION
In this study of adolescent women, periods of nondetection followed by redetection of HPV was seen in approximately one-third (33.8%) of all high-risk HPV episodes. These periods of nondetection would typically equate to “clearance” in studies of shorter duration. Redetection may represent intermittent detection of a persistent infection, as our previous work has demonstrated that low-level persistence does exist for HPV-16 in these periods of nondetection [26]. As stated earlier, these redetections may be also associated with reinfection and, if so, would suggest that a protective immune response did not develop. However, we have also demonstrated that the long control region regions of the HPV just before a period of nondetection and at the time of redetection (with the Linear Array assay) contain >98% DNA homology, suggesting that the detection of HPV-16 after a period of nondetection probably does not represent a new HPV-16 infection [27] . Reactivation of HPV has been suggested in women with human immunodeficiency virus [16, 17], and more recently others [10, 14] have reported redetections in longitudinal followed cohorts of college and older adult women. This would suggest that latency of HPV is plausible and that early infections during adolescence with high-risk HPV put young women at risk for future HPV-related cervical outcomes, including cancer.
More intriguing was the influence of chlamydia on redetection. Chlamydia has been associated with HPV persistence [28] and HPV-related outcomes [3–8]. Many studies have used detection of serum antibodies to demonstrate the association between the 2 organisms but have not fully elucidated the timing of each infection in relationship to the other. Possible alterations in cell-mediated immunity by chlamydia infections could stimulate viral replication. Others have suggested that periods of inflammation by an STI have been associated with increased oxidative stress proteins that may enhance HPV viral replication or DNA breaks that may facilitate viral integration [29]. It is also plausible that the inflammation results in sufficient desquamation of the epithelium to enhance HPV detection by traditional methodologies.
The association of type-specific HPV redetection with the number of sex partners in the 3 months before redetection was independent of a newly acquired STI or condom nonuse. It is potentially plausible that chlamydia may be in the causal pathway between number of recent partners and HPV redetection. However, additional analyses (not shown) did not allow us to declare chlamydia as the mediating factor. Mechanistically, the association with partner numbers could represent a new HPV infection of the same type by a different partner; more coital activity with subsequent inflammation, desquamation and/or coital “trauma” exposing more virus for detection; or the introduction of an alteration in the immunological environment from other antigens associated with a new partner, such as sperm or other microbes (eg, Mycoplasma genitalial) that are not traditionally evaluated.
As reported, the associations found in this cohort may not be generalizable to all populations, but results may be applicable to women at greatest risk for cervical cancer—those of ethnic minority and lower economic status—because the majority this cohort were black and all were recruited from Medicaid-related clinical sites. With regard to our definition of nondetection (at least ≥2 consecutive negative swabs), we forced a lower bound of duration (for nondetection) of 6 month (as opposed to 3 months) and may have introduced a bias if the underlying biology of redetection is more frequent. However, the stringency of our definition may have prevented bias if a single negative represented lab or sample collection error. The sensitivity analyses suggest robustness of our definition. Finally, measures about smoking and immune status were not available, and pregnancy-related events were too few to assess. Any or all of these factors could have altered the analysis.
In summary, the implications for understanding HPV redetection are significant for understanding the natural history of HPV infection and legitimize the need for screening and early treatment for chlamydia infections and counseling around sexual behaviors. Finally, these findings may clinically assist in the understanding of HPV appearance in times of no sexual activity and support the need for early HPV vaccination. Clearly more research is needed to understand the development of premalignant and malignant transformation following HPV infections.
Notes
Financial support. This work was supported in part by the National Institutes of Health, R01 A1072020–01A2 (to D. R. B.)
Potential conflicts of interest. M. L. S. was an investigator for Merck and Co., Inc. related HPV vaccine trials. D. R. B. received lecture fees, advisory board fees, and intellectual property fees from Merck and Co., Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weaver B, Shew M, Qadadri B, et al. Natural history of multiple human papillomavirus infections in female adolescents with prolonged follow-up. J Adolesc Health. 2011;48:473–80. doi: 10.1016/j.jadohealth.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Widdice LE, Brown DR, Bernstein DI, et al. Prevalence of human papillomavirus infection in young women receiving the first quadrivalent vaccine dose. Arch Pediatr Adolesc Med. 2012;166:774–6. doi: 10.1001/archpediatrics.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safaeian M, Quint K, Schiffman M, et al. Chlamydia trachomatis and risk of prevalent and incident cervical premalignancy in a population-based cohort. J Natl Cancer Inst. 2010;102:1794–804. doi: 10.1093/jnci/djq436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shew ML, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160:151–6. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Wallin KL, Wiklund F, Luostarinen T, et al. A population-based prospective study of chlamydia trachomatis infection and cervical carcinoma. Int J Cancer. 2002;101:371–4. doi: 10.1002/ijc.10639. [DOI] [PubMed] [Google Scholar]
- 6.Anttila T, Saikku P, Koskela P, et al. Serotypes of chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Koskela P, Anttila T, Bjorge T, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer. 2000;85:35–9. doi: 10.1002/(sici)1097-0215(20000101)85:1<35::aid-ijc6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Lehtinen M, Ault KA, Lyytikainen E, et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex Transm Infect. 2011;87:372–6. doi: 10.1136/sti.2010.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Supplement 3):42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Winer RL, Hughes JP, Feng Q, et al. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2010;20:699–707. doi: 10.1158/1055-9965.EPI-10-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermel A, Qadadri B, Morishita A, et al. Human papillomavirus detection and typing in thin prep cervical cytologic specimens comparing the Digene Hybrid Capture II Assay, the Roche Linear Array HPV Genotyping Assay, and the Kurabo GeneSquare Microarray Assay. J Virol Methods. 2010;169:154–61. doi: 10.1016/j.jviromet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Gravitt PE, Rositch AF, Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis. 2013;207:272–80. doi: 10.1093/infdis/jis660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González P, Hildesheim A, Rodríguez AC, et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010;19:3044–54. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 17.Theiler RN, Farr SL, Karon JM, et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women risk factors for cervical viral shedding. Obstet Gynecol. 2010;115:1150–8. doi: 10.1097/AOG.0b013e3181e00927. [DOI] [PubMed] [Google Scholar]
- 18.Maglennon GA, Doorbar J. The biology of papillomavirus latency. Open Virol J. 2012;6:190–7. doi: 10.2174/1874357901206010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. Apmis. 2010;118:422–49. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 20.Katz BP, Fortenberry JD, Harezlak J. Sexual behavior of adolescent women at high risk for sexually transmitted infections. Sex Transm Dis. 2001;28:247–51. doi: 10.1097/00007435-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE, Gutierrez EC, Leitch SV, et al. Evaluation of a new DNA test for detection of carcinogenic human papillomavirus. J Clin Microbiol. 2011;49:3029–32. doi: 10.1128/JCM.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gage JC, Partridge EE, Rausa A, et al. Comparative performance of human papillomavirus DNA testing using novel sample collection methods. J Clin Microbiol. 2011;49:4185–9. doi: 10.1128/JCM.01254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fife KH, Wu JW, Squires KE, Watts DH, Andersen JW, Brown DR. Prevalence and persistence of cervical human papillomavirus infection in HIV-positive women initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:274–82. doi: 10.1097/QAI.0b013e3181a97be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SAS Institute, Inc. Base SAS 9.1.3 Procedures Guide. 2nd Edition. Cary, N.C.: 2006. [Google Scholar]
- 26.Weaver B, Shew M, Qadadri B, et al. Low-level persistence of human papillomavirus 16 DNA in a cohort of closely followed adolescent women. J Med Virol. 2011;83:1362–9. doi: 10.1002/jmv.22116. [DOI] [PubMed] [Google Scholar]
- 27.Ermel A, Weaver B, Shew M, Fortenberry JD, Brown D. Re-infection vs. re-detection: persistence of oncogenic hpv types in a cohort of adolescent women. 28th International Papillomavirus Conference & Clinical and Public Health Workshops; San Juan, Puerto Rico. 2012. HPV2012. [Google Scholar]
- 28.Samoff E, Koumans EH, Markowitz LE, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162:668–75. doi: 10.1093/aje/kwi262. [DOI] [PubMed] [Google Scholar]
- 29.Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol. 2011;6:45–57. doi: 10.2217/fvl.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]