Abstract
Background. Based on a hollow-fiber system model of tuberculosis, we hypothesize that microbiologic failure and acquired drug resistance are primarily driven by low drug concentrations that result from pharmacokinetic variability.
Methods. Clinical and pharmacokinetic data were prospectively collected from 142 tuberculosis patients in Western Cape, South Africa. Compartmental pharmacokinetic parameters of isoniazid, rifampin, and pyrazinamide were identified for each patient. Patients were then followed for up to 2 years. Classification and regression tree analysis was used to identify and rank clinical predictors of poor long-term outcome such as microbiologic failure or death, or relapse.
Results. Drug concentrations and pharmacokinetics varied widely between patients. Poor long-term outcomes were encountered in 35 (25%) patients. The 3 top predictors of poor long-term outcome, by rank of importance, were a pyrazinamide 24-hour area under the concentration–time curve (AUC) ≤ 363 mg·h/L, rifampin AUC ≤ 13 mg·h/L, and isoniazid AUC ≤ 52 mg·h/L. Poor outcomes were encountered in 32/78 patients with the AUC of at least 1 drug below the identified threshold vs 3/64 without (odds ratio = 14.14; 95% confidence interval, 4.08–49.08). Low rifampin and isoniazid peak and AUC concentrations preceded all cases of acquired drug resistance.
Conclusions. Low drug AUCs are predictive of clinical outcomes in tuberculosis patients.
Keywords: tuberculosis, nonlinear systems, classification and regression tree analysis, pharmacokinetic variability, drug concentrations, hollow-fiber system, outcomes
In African countries with a high tuberculosis burden, the 2-month sputum culture conversion rate is only 50%–70%, and acquired drug resistance (ADR) continues to be a major problem [1–4]. In the laboratory, the hollow-fiber model of tuberculosis has demonstrated that the microbial kill and ADR prevention of first-line anti-tuberculosis agents are driven by such drug concentration measures as the 0–24 hour area under the concentration–time curve (AUC) to minimum inhibitory concentration (MIC) ratio and the peak concentration to MIC ratio [5–7]. These data have been used in computer-aided clinical trial simulations in the face of 100% adherence, which led us to hypothesize that between-patient pharmacokinetic variability could explain a large proportion of therapy failure and that adherence plays a relatively minor role [8, 9]. Here, we investigated whether such pharmacokinetic variability would indeed lead to a large proportion of patients failing to attain adequate concentrations and then failing therapy. We also sought to identify the drug concentrations that are predictive of clinical outcome.
Several attempts to relate drug concentrations to tuberculosis outcomes have been made with conflicting results [10–16]. The reasons are unclear but could be one of several. First, in some studies, a single measure such as the 2-hour drug concentration (peak) was used to dichotomize patients into those with poor vs good outcomes. However, since drug AUCs are strongly associated with efficacy of first-line anti-tuberculosis agents in preclinical models [5–7, 17], a more intensive multisample schedule that allows AUC identification may be more informative.
Second, several studies utilized predetermined peak concentration drug cutoff values to classify patients as having either low or high drug concentrations. These are peak concentrations of 3–5 mg/L for isoniazid, 8–24 mg/L for rifampin, and 20–50 mg/L for pyrazinamide [18]. These concentrations need further validation with regard to clinical outcomes.
Third, noncompartmental pharmacokinetic analysis was utilized in some studies; however, pharmacokinetics of some anti-tuberculosis drugs may be best described using multiple compartments. A fourth possible reason may be the type of statistical analysis used. Biological systems such as anti-tuberculosis drug pharmacokinetics and the tuberculosis disease are best analyzed using nonlinear statistical approaches since they and most natural phenomena are nonlinear systems [19–23]. In linear analysis, complex problems are broken into smaller components that are then solved, after which the solutions are put together (superimposed) and added up to a solution of the whole problem. Nonlinear systems are characterized by discontinuities and relationships of higher-order complexity between components; the total function of the whole system is often more than the linear sum of its components. Therefore, components need to be analyzed in the context of all parameters interacting within the whole system. Here, we utilized classification and regression tree analysis (CART) to examine the role of several clinical factors, including drug concentrations, in toto, as predictors of clinical outcome in our cohort [24–29]. CART uses nonparametric techniques that examine both linear and nonlinear interactions simultaneously in the whole dataset and creates a hierarchy of predictors, starting with the most predictive to the least predictive.
METHODS
Clinical Study
The study protocol was approved by the University of Cape Town Research Ethics Committee and regional health authorities. Study goals were to identify the pharmacokinetic parameters of first-line anti-tuberculosis drugs and to identify outcomes in patients. All patients who participated gave informed written consent. Patients who were admitted to Brewelskloof Hospital, Western Cape Province, South Africa, between August 1999 and February 2002, were enrolled. Patients were admitted to the hospital for poor socioeconomic circumstances, severe disease, suspicions of poor adherence, and poor response to treatment. Inclusion criteria were positive sputum microscopy or culture, aged ≥16 years, and no evidence of drug resistance based on direct sensitivity testing using BACTEC.
All patients were hospitalized for 2 months and received daily therapy as inpatients under supervision. During the first 2 months, patients received the following daily doses: 300 mg of isoniazid, 20–35 mg/kg pyrazinamide, 15 mg/kg ethambutol, and 600 mg of rifampicin if they weighed >50 kg or 450 mg if they weighed less. Retreatment patients received 1 g intramuscular streptomycin if they weighed >55 kg, 0.75 g if weight was 38–54 kg, and 0.5 g if ≤37 kg. After 2 months of treatment, sputum was sent for microscopy and liquid culture using the BACTEC 460 instrument, and blood was drawn immediately before and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, and 8 hours after a dose of drugs was given under fasting conditions. After 2 months, sputum-negative patients were treated with rifampin and isoniazid (plus ethambutol in retreatment patients) daily for 5 days each week for 4 more months. Patients were followed up for 2 years after hospital discharge. On completion of treatment at the clinics, the outcome category was recorded in the tuberculosis registers and categorized according to World Health Organization definitions [29]. Patients were asked to return to the clinic where they received their ambulatory treatment at 6, 9, 12, 18, and 24 months after admission. This was done so that a sputum sample could be sent for microscopy and culture. Sputum was collected for microscopy and culture if patients had continuing tuberculosis symptoms or had not been cured and could still provide sputum. In addition, the clinics were visited by the investigator (H. M.) or a designated assistant or they were contacted by telephone to ascertain the fate of study patients.
Pharmacokinetic Analysis
The blood samples were processed and drug assays performed as described previously [30, 31]. Noncompartmental pharmacokinetic analyses for the drugs have been published elsewhere [30, 31]. We characterized the compartmental pharmacokinetics of rifampin, isoniazid, and pyrazinamide using the concentration–time data in multicompartmental analyses using ADAPT 5 software [32]. First, we determined if the pharmacokinetic sampling schedule had been robust enough for parameter estimation without bias by applying optimal sampling theory utilizing parameters from prior pharmacokinetic studies, using D-optimality design [33–36]. We found that the sampling times that had been used adequately minimized bias (ie, maximized differential Shannon information) in estimation of pharmacokinetic parameter estimates of rifampin, isoniazid, and pyrazinamide, but not ethambutol. Thus, ethambutol concentrations were not further analyzed. Next, an initial estimate of the pharmacokinetic parameters was made using the standard 2-stage estimation method [19, 24]. One-, two-, and three- compartment models were examined for each drug. The parameter estimates from these models were then used as initial estimates in the POPINIT subroutine of ADAPT. Thereafter, pharmacokinetic parameter estimates were identified for each patient using the maximum-likelihood solution via the expectation-maximization algorithm. The optimal number of compartments for each drug was then chosen using Akaike and Bayesian information criteria, negative-log likelihoods, and parsimony. The results were used to calculate the 24-hour AUCs and trough concentrations just prior to the next dose for each patient and for each drug.
CART Analyses
CART analyses [26–28, 37, 38] were performed using the Salford Predictive Miner System software (San Diego, CA). First, we defined the clinically important categorical primary outcome as the composite long-term outcome of treatment failure, or relapse, or death, up to 2 years. Treatment failure was defined as inability to attain sputum smear conversion during the initial 6 months of treatment. The secondary outcome was 2-month sputum culture conversion. Second, a set of predictors was specified, all were clinical features, including observed peak and predose concentrations, calculated trough concentration, and 0–24 hour AUC for each drug, age, gender, weight, human immunodeficiency virus (HIV) status, smear positivity, and treatment with streptomycin. Third, we assumed prior probabilities (PRIORS EQUAL procedure in CART) that were uniformly distributed in the population from which the study sample was drawn and employed Gini splitting rules [25–27, 37]. Fourth, CART is a binary recursive partitioning technique, which means that it splits predictors at nodes into 2 groups of maximum homogeneity, followed by repetition of the same process to generate daughter nodes et sequens. This is accomplished by an automated search through all possible predictors and values in order to identify the most significant variables. CART identifies the optimal predictor cutoff value for continuous variables (eg, either concentration or patient age) at each node. In this way, it builds a tree, with the root node as the most significant. Fifth, CART generates a variable importance score, which is based on how much each of the subsequent predictors identified for daughter nodes improves the primary predictor (highest-ranked node). The score of the root node is 100%, with the improvement by the predictors identified in daughter nodes scored relative to the root node. We considered a relative contribution in improvement of predictive score of <20% to be clinically nonsignificant. In the end, CART constructs maximum trees. Sixth, goodness-of-fit for each tree was assessed by 10-fold cross-validation and receiver operating curves. In the cross-validation, the data are randomly split by the program into 2 datasets, with one used for training and the other as the test data, and CART analysis performed. This was repeated 10 times, so that 10 randomly split datasets were tested, for 10 CART analyses. True predictive power is defined as performance of the training set–derived tree on the test dataset. Pruning was used to select optimal trees, based on relative misclassification costs, complexity, and parsimony. The optimal tree was chosen based on the lowest cross-validated relative error.
CART has the advantage that it is specifically designed to handle “missing” data by identifying and using surrogate variables, thus minimizing ascertainment bias. However, while the main output of CART is predictive accuracy, clinicians are more familiar with association statistics and effect sizes. Therefore, we identified the odds ratios (ORs) for poor outcome in patients with CART-derived variables with ≥20% score using SPSS version 12.
RESULTS
In total, 142 patients were enrolled. The patients' demographic, clinical, and laboratory features are shown in Table 1. Notable clinical features are that 64% of patients had prior tuberculosis disease and HIV-infected patients comprised only 10% of the total. The doses administered to the patients were adequate based on South African guidelines and, in the case of pyrazinamide, were actually higher (Table 1). Patients received the following drug formulations of rifampin and isoniazid: 29 (20%) fixed-dose combination and 109 (77%) single drug products; in 4 (3%) the formulations were unclear.
Table 1.
Clinical and Laboratory Characteristics of 142 Patients
| Characteristic | Estimate or Median | Percentage or Range |
|---|---|---|
| Sex: female (%) | 78 | 55 |
| Age, y | 36.00 | 16.00–72.00 |
| Self-identified “race” or ethnic group | ||
| Mixed race South African | 127 | 89% |
| Black South African | 15 | 11% |
| Weight, kg | ||
| Weight | 46.00 | 28.00–85.50 |
| Weight change with 2 months of therapy | 8.37 | −11.55 to 36.84 |
| Human immunodeficiency virus infection, % | 15 | 10 |
| Dose and range, mg/kg | ||
| Rifampin | 10.90 | 7.02–15.79 |
| Isoniazid | 6.52 | 3.51–10.53 |
| Pyrazinamide | 35.71 | 19.69–52.63 |
| Ethambutol | 24.62 | 12.88–34.12 |
| Received streptomycin, % | 68 | 47.89% |
| Prior tuberculosis, % | 91 | 64 |
| Symptoms at time of pharmacokinetic sampling | ||
| Resolved | 50 | 35.21% |
| Improved | 81 | 57.04% |
| Chest x-ray changes | ||
| Improved/resolved | 120 | 84.51% |
| No improvement/worse | 22 | 15.49% |
| Slow acetylators, %a | 17 | 18.30% |
| Hemoglobin, g/dL (range) | 11.90 | 7.4–14.90 |
| White cell count, ×109 cells/mL | 8.45 | 3.40–25.70 |
| Platelet count, ×109 cells/mL | 408.0 | 66.0–849.0 |
| Erythrocyte sedimentation rate, mm/h | 51.00 | 1.0–138.0 |
| Creatinine clearance rate, mL/min (range) | 72.65 | 20.12–128.90 |
| Total protein, g/L | 78.00 | 62–114 |
| Albumin, g/L | 34.00 | 19–47 |
| Elevated liver function testb (% patients) | 8 | 6 |
Creatinine clearance rate was calculated using the Cockcroft–Gault equation.
a Acetylation phenotype and genotype tests were performed on the first 93 patients.
b At least >1.5 normal for either alanine aminotransferase (1 patient) or aspartate aminotransferase (2 patients) or alkaline phosphatase (5 patients); no patient had >2 times upper limit of normal of any of the 3 enzymes.
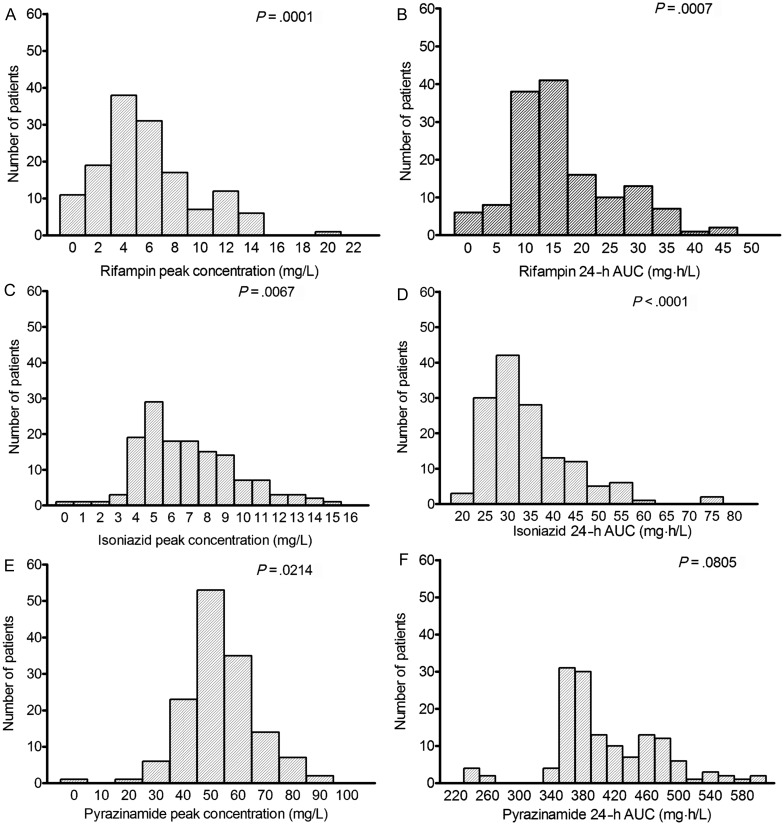
Rifampin and pyrazinamide pharmacokinetics were each best explained by a 1-compartment pharmacokinetic model, while isoniazid was best explained by a 2-compartment model. The summary (or “population”) pharmacokinetic parameter estimates are shown in Supplementary Table 1. Each of the 142 patients had a unique concentration–time profile for each of the 3 drugs, and each patient's drug concentrations were compared with the patient's outcome. The concentration distributions were wide (Figure 1). As an example, while the highest ratio of the highest-to-lowest dose (mg/kg) was 2.7, the ratios of the highest-to-lowest peak concentrations were >102-fold for rifampin, 31-fold for isoniazid, and 63-fold for pyrazinamide. Moreover, when the peak and AUCs were examined for departure from normality using the D'Agostino–Pearson omnibus normality test (K2), the pharmacokinetic parameters in the 142 patients were not normally distributed, with the exception of pyrazinamide AUC (Figure 1).
Figure 1.
Pharmacokinetic variability in 142 patients. In most instances, except for pyrazinamide 24-hour area under the concentration–time curve (AUC), the pharmacokinetic parameters were not normally distributed, as demonstrated by P < .05. The figures demonstrate the wide variability in the peak concentration and AUC. No concentrations of one drug covaried with that of another.
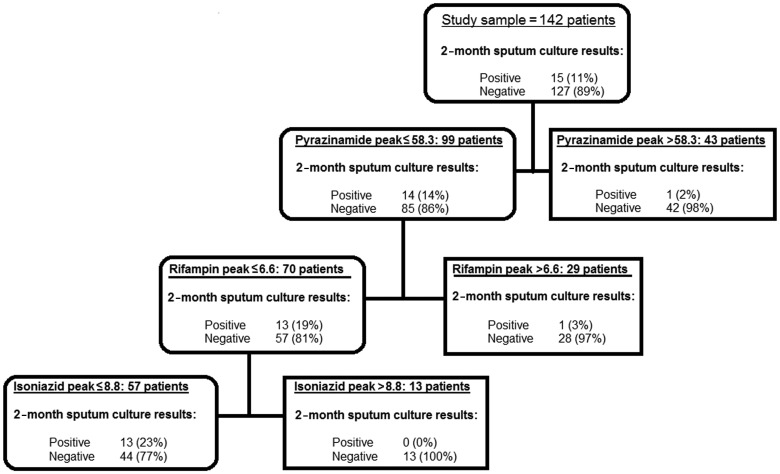
Of 142 patients, 15 (11%) did not convert their sputum cultures to negative after 2 months of treatment based on liquid cultures. CART identified the predictors of 2-month sputum culture shown in Figure 2. The highest predictor of 2-month sputum conversion among all clinical factors examined was pyrazinamide peak concentration. The peak concentration thresholds identified by CART are shown in Figure 2. Among patients who failed to sputum convert, 93% (confidence interval [CI], 70–99) had a low pyrazinamide peak concentration. Conversely, when pyrazinamide peak concentration was above threshold concentrations, only 2% of patients still had positive sputum at 2 months. If the drug concentrations were used as biomarkers to predict 2-month sputum conversion, the measures of association and test characteristics would be as shown in Table 2.
Figure 2.
Variables predictive of 2-month sputum conversion in 142 patients. Pharmacokinetic parameters as well as patient demographic factors were examined in the initial models and the decision trees. Peak concentrations (mg/L) of pyrazinamide, rifampin, and isoniazid were the best predictors of 2-month sputum conversion. Only 2% of patients with a pyrazinamide peak above threshold were still sputum positive at 2 months. In those who had a lower pyrazinamide peak (more likely to fail), a high rifampin peak was associated with a positive 2-month sputum in only 3% of patients.
Table 2.
Association Between Number of Drugs With Peak Concentration Above Classification and Regression Tree Analysis–Derived Threshold and 2-month Sputum Conversion
| Drug | Odds Ratio of Success (95% confidence interval) | Sensitivity, % | Specificity, % |
|---|---|---|---|
| Pyrazinamide alone | 6.9 (.9–54.4) | 33.1 | 93.3 |
| Pyrazinamide OR rifampin | 10.3 (2.2–48.1) | 61.4 | 86.7 |
| Pyrazinamide AND rifampin | 12.4 (1.6–99.1) | 48.8 | 92.9 |
| Pyrazinamide AND rifampin AND isoniazid | 12.3 (2.7–56.8) | 65.4 | 86.7 |
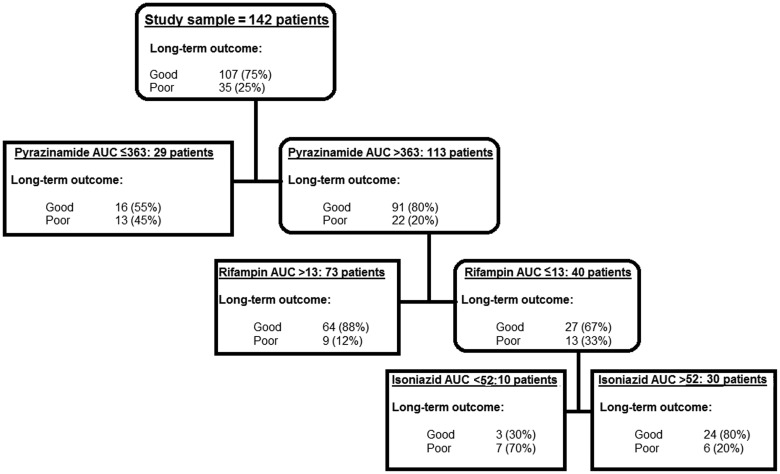
With regard to long-term outcomes after discharge, 6 (4%) patients became either nonadherent or absconded in the last 4 months of therapy. Further follow-up of these 6 patients found that 1 had confirmed relapse during the 2-year observation. The patient had drug-susceptible tuberculosis. A second developed hemoptysis after the 2-year period and was not further characterized. In total, 25% of 142 patients had poor long-term outcomes (19 relapsed, 15 died, and 2 had therapy failure). CART revealed that the 24-hour AUCs of pyrazinamide, rifampin, and isoniazid were the most predictive of long-term outcomes among all factors (Figure 3). The drug concentration thresholds predictive of this outcome were a 24-hour AUC of 363 mg·h/L for pyrazinamide, 13 mg·h/L for rifampin, and 52 mg·h/L for isoniazid. In total, ≥91% of patients with poor long-term outcomes received at least 1 drug with a low AUC. We used these CART-derived AUC thresholds to calculate the odds of poor long-term outcomes in patients. The OR for poor outcome in 32/78 (41%) patients with an AUC of at least 1 drug below threshold vs 3/64 (5%) patients without any low AUC was 14.14 (CI, 4.08–49.08). Indeed, 17/60 patients who had a low pyrazinamide or rifampin AUC (first and second decision nodes) relapsed compared with 0/64 of patients without (OR = 51.90; CI, 3.04–886). Table 3 further demonstrates that the lower the cumulative number of drugs above the cutoff AUC threshold, the higher the odds of poor long-term outcomes (P = .001). To put this into context, we also examined the association with failure of the drug concentrations currently used in the field for therapeutic drug monitoring [18]: the ORs for poor long-term outcome were as shown in Supplementary Table 2.
Figure 3.
Variables predictive of poor long-term outcome in 142 patients. Pharmacokinetic parameters as well as patient demographic factors were examined in the initial models and the decision trees. The decision nodes demonstrate the primary node was for pyrazinamide 24-hour area under the concentration–time curve (AUC), followed by rifampin AUC. The AUC cutoff values that were identified as important predictive factors are shown.
Table 3.
Association Between Cumulative Number of Drugs Below Classification and Regression Tree Analysis–Derived Threshold AUC and Long-term Outcome
| Drug AUCs | Long-Term Outcomes |
Odds Ratio for Poor Outcome (95% confidence interval) | |
|---|---|---|---|
| Poor, % | Good, % | ||
| No drug above threshold | 1 | 2 | (…)a |
| Any 1 drug above threshold | 13 (52) | 12 (48) | 7.57 (2.57–22.34) |
| Any 2 drugs above threshold | 14 (26) | 40 (74) | 2.65 (0.99–7.18) |
| All 3 drugs above threshold | 7 (12) | 53 (88) | Reference |
| Total | 35 (100) | 107 (100) | |
Abbreviation: AUC, 0–24 hour area under the concentration time curve.
a Combined with “one drug above threshold” due to small cell size.
All patients had confirmed drug-susceptible tuberculosis at the start of treatment. Of these patients, 2.11% (CI, .72-6.00) developed ADR and 0.7% (CI, .12-3.87) developed ADR prior to the third month of therapy; all 3 had been adherent to therapy. The drug concentrations and the timing of ADR emergence in these patients are shown in Table 4. The table demonstrates that these patients had suboptimal rifampin and isoniazid drug concentrations prior to developing ADR, so that despite adequate dosing, the pharmacokinetic variability-related suboptimal exposures preceded development of drug resistance.
Table 4.
Pharmacokinetic Parameters in Patients Who Developed Acquired Drug Resistance
| Patient | Rifampin |
Isoniazid |
Treatment Period When ADR Detected | Type of ADR | ||
|---|---|---|---|---|---|---|
| Peak, mg/L | AUC, mg·h/L | Peak, mg/L | AUC, mg·h/L | |||
| 1 | 4.62 | 10.83 | 0.88 | 25.36 | First 2 mo | Isoniazid |
| 2 | 0.81 | 7.31 | 8.6 | 30.37 | Continuation phase | Isoniazid |
| 3 | 1.64 | 7.24 | 8.6 | 32.56 | During relapse | Resistance to both rifampin and isoniazid |
Abbreviations: ADR, acquired drug resistance; AUC, 0–24 hours area under the concentration time curve.
DISCUSSION
First, the belief that “first-line drugs (INH [isoniazid], RIF [rifampin], PZA [pyrazinamide], and EMB [ethambutol]) have relatively predictable pharmacokinetics” is likely unjustified [39]. Indeed, wide between-patient pharmacokinetic variability for rifampin, isoniazid, and pyrazinamide has been a consistent finding in prior studies [34–36, 40–42]. The reasons for this variability are many but could include pharmacogenetic factors; drug formulation; quality of tablets; and patient weight, age, gender, adherence patterns, and comorbid conditions such as AIDS [19, 30, 31, 34–36, 40–42]. We show that this pharmacokinetic variability leads a proportion of patients who have suboptimal drug concentrations, which is associated with poor 2-month sputum conversion rates, to higher relapse and ADR. One could not predict a priori the concentrations achieved in a particular patient, suggesting that it may be necessary to ascertain the drug concentrations achieved in patients.
Second, we identified AUC concentration cutoff values that predict >91% of long-term clinical outcomes. These differ from the target concentrations of anti-tuberculosis drugs that have been most frequently cited in the past [18], which we found were not associated with long-term outcomes in our dataset. We derived the new concentration thresholds based on efficacy in a combination regimen in the context of all other potential clinical predictors in patients with tuberculosis. We were able to perform cross-validations in CART, which confirmed the threshold concentrations. However, the predictive accuracy of these new concentration thresholds will need to be externally validated in studies by others.
Third, pyrazinamide concentrations were the most important predictor of both sputum conversion and sterilizing activity. This is a surprise since it is generally believed that rifampin has the most important sterilizing effect in the regimen while isoniazid has the best bactericidal effect and role on sputum conversion [39, 43, 44]. Our findings on the predominant role of pyrazinamide for both sputum conversion and sterilizing effect demonstrate an important advantage of nonlinear system analysis. When examined as monotherapy, pyrazinamide has the lowest microbial kill rates compared with rifampin and isoniazid and is thus the “least” dominant by those criteria [5–7, 43–45]. However, our analysis shows that pyrazinamide is the dominant drug in combination therapy with these 2 drugs. This is because of the nonlinear nature of drug efficacy interactions; pyrazinamide does better when part of a combination therapy regimen as a whole in the context of interaction with the effects of other drugs than it does as an isolated component. In other words, the effect of the regimen is not the sum of its components combined. This suggests that as new multidrug regimens are fashioned to shorten therapy duration, pyrazinamide is likely to continue playing an important role.
Fourth, ADR was preceded in time by low rifampin and isoniazid concentrations. This was a hypothesis first generated based on hollow-fiber studies and clinical trial simulations [8]. We have proposed that wide pharmacokinetic variability leads to a proportion of patients with 1 (or more) drug concentrations below the effective threshold who are then effectively on monotherapy, despite 100% adherence, which would lead to ADR in about 0.68% of Western Cape patients between the second and third months. This compares well with 0.7% ADR that we encountered in the current Western Cape study. Thus, pharmacokinetic variability is the proximate cause of ADR; it is the “bow” initiating the “antibiotic resistance arrow of time” [46].
Finally, in our in vitro hollow-fiber system tuberculosis model, we found that AUC and peak concentration (indexed to MIC) are associated with both efficacy and suppression of ADR for first-line anti-tuberculosis compounds [5–7]. The pyrazinamide AUC/MIC derived as most optimal (ie, associated with ≥90% of maximal kill) at the site of infection in the hollow-fiber system tuberculosis model was ≥209 [7, 47]. In contrast, murine and Guinea pig studies have suggested that optimal pyrazinamide exposures would be 12-fold lower than identified in the hollow-fiber system tuberculosis model [48]. The pyrazinamide median MIC in clinical isolates is 37.5 mg/L [47, 49], so that the AUC/MIC cutoff ratio in serum calculates as 363 mg·h/L divided by 37.5 mg/L, or 9.68. Pyrazinamide penetrates into epithelial lining fluid where it is concentrated up to 22-fold [50], so that the optimal AUC/MIC ratio at the site of pulmonary infection calculates as ≥213. This value is reasonably close to the ratio of 209 identified in the hollow-fiber system tuberculosis model 4 years ago [7]. Similarly, given the highest MIC that indicates isoniazid susceptibility of 0.1 mg/L and an epithelial lining fluid to serum penetration ratio of 1, the optimal isoniazid AUC of 52 mg·h/L in patients calculates to an AUC/MIC of 520 in the lung, which is close to the 567 derived in the hollow-fiber system tuberculosis model [6, 47]. These similarities highlight the possibility that drug concentrations might play a powerful role in determining outcome in preclinical models and in patients.
Our study has several limitations. First, 64% of patients had a recurrent tuberculosis episode, which could limit the generalizability of results. “Retreatment” tuberculosis patients would be expected to have low response rates not generalizable to new patients. However, our retreatment patients had relatively good outcome, even with the more sensitive liquid media used for culture. A second limitation is that we had a low rate of HIV-infected patients, so that our results should not be generalized to patients with AIDS. A third potential limitation is that CART is prone to overfitting and biasing toward covariates with many possible splits. In addition, the exact cutoff value will be influenced by the distance between 2 independent variable data points, so that if data with less distance between AUCs were used, the exact threshold may shift somewhat. However, examination of the CART-derived thresholds using standard measures of association demonstrated statistically different outcomes in patients with drug concentrations above and below the CART-derived thresholds. Moreover, inherent in these machine learning–based methods is the fact that as the sizes of datasets increase in the field, further precision of the concentration thresholds will likely be introduced by those efforts. A fourth potential limitation is that our results were for pulmonary tuberculosis patients. For tuberculosis of other organs, different drug concentration cutoff values will need to be derived since drug penetration into different organs is likely to affect predictive serum concentrations. Fifth, sputum was not collected from all patients after discharge, for example, patients who could not provide sputum. Thus, ascertainment of this aspect of long-term outcomes was not assessed in a uniform manner. However, CART is specifically designed to minimize ascertainment bias in such situations. Finally, our study design did not capture the quantitative sputum bacillary burden, which is known to predict microbiologic outcomes. Such interactions of drug concentrations and bacterial burden are the subject of an ongoing study in another dataset by our group.
In summary, we show that with standard tuberculosis therapy, a considerable proportion of patients have low drug concentrations, which is associated with therapy failure and ADR. We derived AUCs that predict poor long-term outcome in such patients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge the patients who participated in the study and the nursing staff of Brewelskloof Hospital, Western Cape, who assisted in collection of data and taking care of patients.
Financial support. This work was supported by the Division of Pharmacology, University of Cape Town, and the Medical Research Council of South Africa (H. M., A. B., P. A. W., P. S.). Funding for T. G. and J. P. was from the National Institutes of Health Director New Innovator Award (DP2 OD001886) and the National Institutes of Health, National Institute of General Medical Sciences (R01AI079497). Funding to facilitate the collaboration between the researchers was from University of Cape Town's Department of Medicine and the University of Texas Southwestern Medical Center's Office of Global Health.
Potential conflicts of interest. T. G. performed unrelated research work related to antifungals that was sponsored by Merck and has also been a consultant for Merrimack Pharmaceuticals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Visser ME, Grewal HM, Swart EC, et al. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am J Clin Nutr. 2011;93:93–100. doi: 10.3945/ajcn.110.001784. [DOI] [PubMed] [Google Scholar]
- 2.MacKenzie WR, Heilig CM, Bozeman L, et al. Geographic differences in time to culture conversion in liquid media: tuberculosis trials consortium study 28. Culture conversion is delayed in Africa. PLoS One. 2011;6:e18358. doi: 10.1371/journal.pone.0018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calver AD, Falmer AA, Murray M, et al. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg Infect Dis. 2010;16:264–71. doi: 10.3201/eid1602.090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–8. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51:2329–36. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumbo T, Siyambalapitiyage Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: A paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53:3197–204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–9. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered versus directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57:21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chideya S, Winston CA, Peloquin CA, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48:1685–94. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narita M, Hisada M, Thimmappa B, et al. Tuberculosis recurrence: multivariate analysis of serum levels of tuberculosis drugs, human immunodeficiency virus status, and other risk factors. Clin Infect Dis. 2001;32:515–7. doi: 10.1086/318490. [DOI] [PubMed] [Google Scholar]
- 12.Chang KC, Leung CC, Yew WW, et al. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur J Clin Microbiol Infect Dis. 2008;27:467–72. doi: 10.1007/s10096-007-0454-6. [DOI] [PubMed] [Google Scholar]
- 13.Weiner M, Benator D, Burman W, et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis. 2005;40:1481–91. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 14.Heysell SK, Moore JL, Keller SJ, Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis. 2010;16:1546–53. doi: 10.3201/eid1610.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest. 1998;113:1178–83. doi: 10.1378/chest.113.5.1178. [DOI] [PubMed] [Google Scholar]
- 16.Mehta JB, Shantaveerapa H, Byrd RP, Jr., Morton SE, Fountain F, Roy TM. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest. 2001;120:1520–4. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis. 2010;201:1225–31. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–83. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Hall RG, Swancutt MA, Meek C, Leff RD, Gumbo T. Ethambutol pharmacokinetic variability is linked to body mass in overweight, obese, and extremely obese people. Antimicrob Agents Chemother. 2012;56:1502–7. doi: 10.1128/AAC.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelbrot BB. The fractal geometry of nature. New York: W.H. Freeman and Company; 1982. [Google Scholar]
- 21.Campbell D, Farmer D, Crutchfield J, Jen E. Experimental mathematics: the role of computation in nonlinear science. 28 edn. New York, NY: Association for Computing Machinery; 1985. pp. 374–84. [Google Scholar]
- 22.Coffey DS. Self-organization, complexity and chaos: the new biology for medicine. Nat Med. 1998;4:882–5. doi: 10.1038/nm0898-882. [DOI] [PubMed] [Google Scholar]
- 23.Dokoumetzidis A, Iliadis A, Macheras P. Nonlinear dynamics in clinical pharmacology: the paradigm of cortisol secretion and suppression. Br J Clin Pharmacol. 2002;54:21–9. doi: 10.1046/j.1365-2125.2002.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain MK, Pasipanodya JG, Alder L, Lee WM, Gumbo T. Pegylated interferon fractal pharmacokinetics: individualized dosing for hepatitis C virus infection. Antimicrob Agents Chemother. 2012;57:1115–20. doi: 10.1128/AAC.02208-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes D, Ambrose PG, Hammel JP, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother. 2011;55:2113–21. doi: 10.1128/AAC.01430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. Boca Raton: Chapman and Hall/CRC; 1984. [Google Scholar]
- 27.Steinberg D, Colla P. CART: Tree-structured non-parametric data analysis. San Diego: Salford Systems; 1995. [Google Scholar]
- 28.Kim H, Loh WY. Classification trees with unbiased multiway splits. J Am Stat Assoc. 2001;88:457–67. [Google Scholar]
- 29.World Health Organization, International Union Against Tuberculosis and Lung Disease, Royal Netherlands Tuberculosis Association. Revised international definitions in tuberculosis control. Int J Tuberc Lung Dis. 2001;5:213–5. [PubMed] [Google Scholar]
- 30.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother. 2006;50:1170–7. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIlleron H, Wash P, Burger A, Folb P, Smith P. Widespread distribution of a single drug rifampicin formulation of inferior bioavailability in South Africa. Int J Tuberc Lung Dis. 2002;6:356–61. [PubMed] [Google Scholar]
- 32.Los Angeles: Biomedical Simulations Resource; 2009. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. [computer program] [Google Scholar]
- 33.Drusano GL, Forrest A, Yuen G, Plaisance K, Leslie J. Optimal sampling theory: effect of error in a nominal parameter value on bias and precision of parameter estimation. J Clin Pharmacol. 1994;34:967–74. doi: 10.1002/j.1552-4604.1994.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins JJ, Langdon G, McIlleron H, Pillai GC, Smith PJ, Simonsson US. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol. 2006;62:727–35. doi: 10.1007/s00228-006-0141-z. [DOI] [PubMed] [Google Scholar]
- 35.Chigutsa E, Visser ME, Swart EC, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother. 2011;55:4122–7. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkins JJ, Langdon G, McIlleron H, Pillai GC, Smith PJ, Simonsson US. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol. 2011;72:51–62. doi: 10.1111/j.1365-2125.2011.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breiman L. Technical note: Some properties of splitting criteria. Machine Learning. 1996;24:41–7. [Google Scholar]
- 38.Lewis RJ. An introduction to classification and regression tree (CART) analysis. 2000 Annual Meeting of the Society for Academic Emergency Medicine (San Francisco); Des Plaines, IL: Society for Academic Emergency Medicine; 2000. http://www.srnr.arizona.edu/rnr/rnr573/Readings/lewis1.pdf. Accessed 7 April 2013. [Google Scholar]
- 39.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 40.Milán Segovia RC, Domínguez Ramírez AM, Jung Cook H, et al. Population pharmacokinetics of rifampicin in Mexican patients with tuberculosis. J Clin Pharm Ther. 2013;38:56–61. doi: 10.1111/jcpt.12016. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, Starke JR, Burman WJ, et al. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy. 2002;22:686–95. doi: 10.1592/phco.22.9.686.34067. [DOI] [PubMed] [Google Scholar]
- 42.Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–7. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–54. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 44.Mitchison DA. Basic mechanisms of chemotherapy. Chest. 1979;76(6 Suppl):771–81. doi: 10.1378/chest.76.6_supplement.771. [DOI] [PubMed] [Google Scholar]
- 45.Almeida D, Nuermberger E, Tasneen R, et al. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–84. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother. 2012;56:4806–15. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother. 2010;54:1484–91. doi: 10.1128/AAC.01474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad Z, Peloquin CA, Minkowski A, Grosset JH, Nuermberger EL, Ahmad Parry Z. The anti-tuberculous activity of pyrazinamide is driven by AUC/MIC in murine tuberculosis [O_01]. program and abstracts of the 3rd International Workshop on Clinical Pharmacology of Tuberculosis Drugs; Boston, MA. 2010. [Google Scholar]
- 49.Salfinger M, Heifets LB. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother. 1988;32:1002–4. doi: 10.1128/aac.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conte JE, Jr., Golden JA, Duncan S, McKenna E, Zurlinden E. Intrapulmonary concentrations of pyrazinamide. Antimicrob Agents Chemother. 1999;43:1329–33. doi: 10.1128/aac.43.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.