Abstract
Background. Diagnoses of genital warts (GW) in genitourinary medicine (GUM) clinics have been increasing in England for many years. In 2008, an HPV immunization program began with a bivalent vaccine (Cervarix). This was expected to markedly reduce infections and disease due to human papillomavirus (HPV) 16/18 but not HPV 6/11 infections or disease. However, from 2009 to 2011 there were decreases in reported diagnoses of GW in young females at GUM clinics.
Methods. Using data from GUM clinics and a sample of general practices (GPs) throughout England, we analyzed rates of GW diagnoses by age, year of diagnosis, and estimated immunization coverage.
Results. The overall reduction in GW diagnoses at GUM clinics between 2008 and 2011 was 13.3% among 16- to 19-year-old females, with the greatest decline of 20.8% in 17-year-olds. Declines were positively associated with estimated immunization coverage. A similar pattern was seen in GP diagnoses, but not among older women, and for other GUM consultations.
Conclusions. Several factors might contribute to declines in GW. However, the size and pattern of the declines strongly suggest that we are observing an unexpected, moderately protective effect of HPV 16/18 vaccination against GW.
Keywords: genital warts, HPV immunization, Cervarix, surveillance
(See the major article by Szarewski et al on pages 1391–6.)
The annual rate of genital warts (GW) cases diagnosed in genitourinary medicine (GUM) clinics in England has, for the most part, been increasing since the early 1970s [1, 2]. A lessening in the rate of increase was observed between 1987 and 1995 and was associated with AIDS awareness campaigns [3]. However, in 2009 there was a slight but notable decrease in diagnoses in females reported by GUM clinics, from 37 062 in 2008 to 35 925 in 2009, which has continued into 2010 and 2011 (<35 000). The decline was confined to young women and was not seen for other sexually transmitted infections (STIs) [4].
The United Kingdom was the first country to introduce a national human papillomavirus (HPV) immunization program using the bivalent HPV 16/18 vaccine (Cervarix; GlaxoSmithKline). Beginning in September 2008, the vaccine has been provided free of charge to girls aged 12–13 years (routine cohort), with catch-up to females up to age 18 years. The program has attained wide coverage, with reported 3-dose coverage in the routine cohorts of more than 80% and approximately 40% or greater in the oldest catch-up cohorts [5].
Both the bivalent HPV 16/18 and the quadrivalent HPV 6/11/16/18 (Gardasil; Merck) vaccines have been shown in clinical trials to be highly effective in preventing high-grade cervical disease caused by HPV 16/18 and by several other closely related HPV types (within the A9 and A7 alpha species) [6]. Clinical trials of the quadrivalent vaccine have shown it to be highly effective in preventing GW, the majority of which are caused by HPV 6 or 11 [7]. In Australia, where an HPV immunization program using the quadrivalent vaccine started in 2007, a consequent substantial decrease in GW was demonstrated [8]. No impact on GW was anticipated from the use of the bivalent vaccine in the immunization program in England [9].
There is some biological plausibility for broad cross-protection from the bivalent vaccine [10, 11]. Recent post hoc analysis of the PATRICIA (PApilloma TRIal against Cancer In young Adults) trial has shown moderate efficacy for the bivalent vaccine against persistent infection with a number of low-risk HPV types [12]. We report ecological analyses to investigate associations between the rate of GW diagnoses in young women and of other STI diagnoses at GUM clinics and coverage of bivalent HPV 16/18 immunization during the 3 years after its introduction in England.
METHODS
Data Sources
HPV Vaccination Coverage
Data on 3-dose coverage achieved by the National HPV Immunisation Programme for each academic year (September to August) and Primary Care Trust (PCT) were obtained from published reports [5, 13] and used to estimate coverage by calendar year of birth and age. For simplicity, we attributed the 3-dose coverage achieved for each academic cohort of girls (September intake) to the following January, that is, slightly overestimating the average 3-dose coverage within each calendar year, as the third dose would not actually have been received until February/March.
Genital Warts
GUM Data
All attendances for care (such as testing for chlamydia and other STIs) and diagnoses of STIs made at GUM clinics in England are reported to Public Health England (formerly the Health Protection Agency) [14]. Before 2008, data were reported by clinics as aggregated counts by reason of attendance, age group (for selected STIs), and gender. Since 2008 (comprehensively since 2009), clinics have reported disaggregated data including age in years, gender, and PCT of residence for each patient attendance [15].
We extracted the aggregated counts of first GW diagnoses in females and males for the age groups 15–19 years and 20–24 years from 2002 onward and counts of first GW diagnoses in females and males aged 15–24 years by age and PCT of residence from the subset of clinics that reported disaggregated data since 2008 (171 of 212 clinics). Counts were adjusted for the sampling restriction by multiplying by 1/the proportion of all reported patients in 2010 and 2011 who attended this subset of clinics (1/0 · 855).
Data were analyzed for the whole of England and at the PCT level. For the PCT-level analysis, 28 PCTs were excluded because in 2011 >20% of patients seen in clinics were not included in the disaggregate dataset. An additional 3 PCTs were excluded because of incomplete immunization coverage data (where catch-up immunization was conducted after data were published), leaving 121 PCTs included in this analysis.
Estimates of annual GUM diagnoses rates per 100 000 population were calculated using mid-year population denominators for 2008, 2009, and 2010 (also used for 2011) [16]. Exact Poisson confidence intervals were calculated for incidence rates at 95%.
GP Data
The United Kingdom Clinical Practice Research Datalink (CPRD) includes the world's largest quality-assured database of anonymized longitudinal medical records from general practice (CPRD Gold, formerly the GPRD). The dataset covers approximately 8% of the UK population and is broadly demographically representative [17]. Only data from practices that comply with specific quality measures, defined by the CPRD and based on the overall consistency and completeness of data provided, are included.
Data for first diagnosis of GW in females aged 14–24 years were extracted from CPRD Gold for 2008–2011. GW diagnoses were defined by Read codes as in a previous study [18]. Only the first coding for GW for each patient in the dataset was included in the analysis. Population denominators were counts of all 14- to 24-year-old females registered with a CPRD practice each year.
Other STIs
Data were also extracted for chlamydia screening and chlamydia, gonorrhea, and herpes simplex virus (HSV) diagnoses in GUM clinics for females aged 15–24 years in the same way as for GW diagnoses in GUM clinics as described above.
Data Analysis
HPV immunization status is not recorded in GUM records. To investigate the association between GW diagnoses and immunization coverage, we assumed that age-specific HPV immunization coverage estimates applied to the GUM patient population. With England-level aggregate data, we used negative binomial regression modeling (due to overdispersion of data, Poisson regression modeling was not appropriate) to estimate the incidence rate ratio (IRR) of GW diagnoses associated with year of diagnosis, age group, and HPV immunization coverage using data from 2002 to 2011. To consider the variation in HPV immunization coverage by PCT, we modeled PCT-level data for 2008–2011 to estimate the IRR of GW diagnoses rates associated with coverage and age, adjusted for chlamydia diagnoses rates (to represent shifting service use) and PCT of residence. Vaccine effectiveness (VE) against GW was estimated using the formula VE = 1 −IRR. Analyses were conducted using Stata 11.1.
RESULTS
Association Between GW Diagnoses and HPV Coverage
England-Level Data
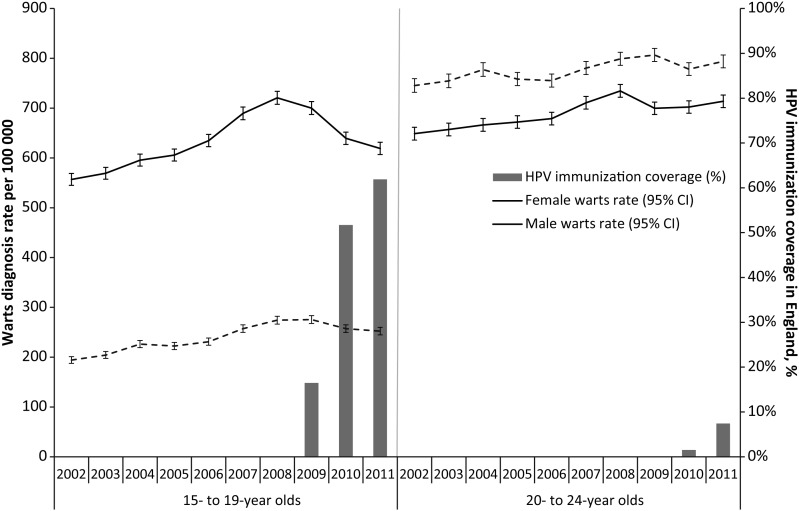
From 2002 to 2011, rates of GW diagnoses in GUM clinics increased significantly with year of diagnosis for males and females of both 15- to 19-year-old and 20- to 24-year-old age groups (Figure 1). The IRR for GW for year of diagnosis was 1.05 (95% confidence interval [CI], 1.04–1.05) in 15- to 19-year-old females, 1.01 (95% CI, 1.01–1.02) in 20- to 24-year-old females, and 1.02 (95% CI, 1.01–1.03) in 15- to 24-year-old males. In 2009, the rates of GW diagnoses among females decreased for both age groups, and, concurrent with marked increases in HPV immunization coverage, GW rates continued to decline in 2010 and 2011 in 15- to 19-year-old females. The IRR associated with estimated immunization coverage of 15- to 19-year-old females, adjusted for year of diagnosis, was 0.66 (95% CI, .62–.71), giving a VE estimate of 34% (95% CI, 29%–38%).
Figure 1.
Rates of genital warts diagnoses in genitourinary medicine clinics in England from 2002 to 2011 and HPV immunization coverage of females in England, by age group and year.
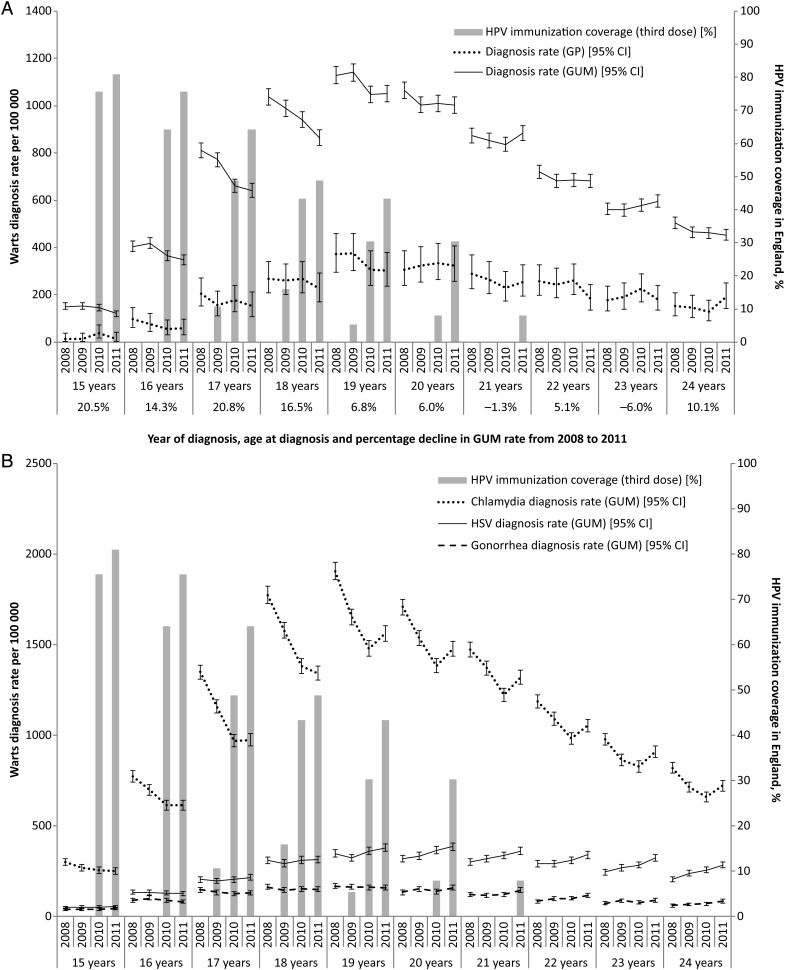
Rates of GW diagnoses in females, at GUM clinics and by general practitioners (GPs), by age from 2008 to 2011, together with the estimated coverage of the national HPV immunization program in England, are shown in Figure 2A. The overall reduction in GW diagnoses at GUM clinics between 2008 and 2011 was 13.3% among 16- to 19-year-olds. The greatest decline (20.8%) was in 17-year-olds, for whom vaccine coverage in 2011 was estimated at 64%. The data from GPs show no evidence of an increase that could be compensating for the decrease from GUM clinics, rather the data reflect the pattern seen at GUM clinics. The unadjusted and adjusted IRR of GW diagnoses at GUM clinics associated with coverage are given, by age, in Table 1. The data show the IRR of GW in vaccinated compared with unvaccinated female cohorts to be significantly less than 1 (ie, protective effect associated with vaccination) in 15-, 16-, and 17-year-old females, using England-level data.
Figure 2.
(A) Rates of first genital wart diagnosis in females (with error bars showing 95% confidence intervals) and human papillomavirus immunization coverage in England, by age and year. (B) Rates of diagnosis for human simplex virus, Neisseria gonorrhoeae, and chlamydia in genitourinary medicine clinics and HPV immunization coverage in England, by year and age. Abbreviations: CI, confidence interval; CT, Chlamydia trachomatis; GP, general practitioner; GUM, genitourinary medicine; HPV, human papillomavirus; HSV, human simplex virus.
Table 1.
Incidence Rate Ratios of Genital Warts Diagnoses in Females in Vaccinated Compared With Unvaccinated Female Cohorts, by Age, Adjusted for Chlamydia Diagnoses Rates
| IRR (95% CI) of GW |
||||||
|---|---|---|---|---|---|---|
| England-level Analysis |
PCT-level Analysisb |
|||||
| Age, y | n | IRR (95% CI) | Adjusteda IRR (95% CI) | n | IRRb (95% CI) | Adjustedc IRR (95% CI) |
| 15 | 1731/1 212 679 | 0.83 (.73, .95) | 0.84 (.74, .95) | 1344/994 464 | 0.84 (.74, .97) | 0.81 (.71, .93) |
| 16 | 4792/1 247 308 | 0.81 (.73, .89) | 0.84 (.77, .91) | 3703/1 022 137 | 0.87 (.75, 1.01) | 0.89 (.77, 1.03) |
| 17 | 9233/1 278 085 | 0.69 (.62, .76) | 0.78 (.71, .86) | 7157/1 046 426 | 0.74 (.60, .90) | 0.76 (.62, .92) |
| 18 | 12 586/1 314 995 | 0.73 (.65, .83) | 0.89 (.79, 1.00) | 9781/1 075 034 | 0.75 (.58, .97) | 0.77 (.60, .99) |
| 19 | 14 684/1 344 061 | 0.97 (.86, 1.09) | 1.10 (1.00, 1.21) | 11 367/1 094 272 | 0.86 (.65, 1.13) | 0.87 (.67, 1.15) |
| 20 | 13 860/1 358 690 | 0.90 (.74, 1.10) | 0.99 (.86, 1.14) | 10 652/1 102 375 | 0.95 (.64, 1.40) | 0.96 (.65, 1.40) |
Negative binomial regression modeling was used to estimate the IRR of GW diagnoses associated with age group and human papillomavirus immunization coverage.
Abbreviations: CI, confidence interval; GW, genital warts; IRR, incidence rate ratio; PCT, primary care trust.
a Adjusted for chlamydia diagnoses rate.
b Adjusted for PCT.
c Adjusted for chlamydia diagnoses rate and PCT.
In males, GW diagnoses declined slightly in 15- to 19-year-olds from 2008 to 2011, and this decline was also significantly associated with HPV vaccination of 15- to 19-year-old females (IRR, 0.70; 95% CI, .63–.78). In 20- to 24-year-old males, as in females, diagnoses rates showed no sustained decline.
PCT-Level Data
Analysis of GW rates for 15- to 20-year-olds and of immunization coverage at the PCT level found the IRR for HPV vaccination to be 0.83 (95% CI, .77–.90) and hence the VE estimate to be 16.9% (95% CI, 9.9%–23.4%). Inclusion of an interaction between age and coverage did not improve the model significantly; however, with inclusion of this interaction term, the pattern of VE by age was similar to that seen in the England-level analysis.
Trends in GUM Clinic Attendance for Reasons Other Than GW
England-Level Data
Figure 2B shows the rates of chlamydia, gonorrhea, and HSV diagnoses among young females; these rates are comparable to those for GW shown in Figure 2A. There was a decline in the rates of chlamydia and gonorrhea diagnoses in females aged <20 years between 2008 and 2010, then there were flat or increasing rates to 2011. Rates of HSV diagnoses declined between 2008 and 2009 for late teens before increasing from 2009. Rates of HSV diagnoses in women aged >20 years increased each year.
DISCUSSION
The United Kingdom is the first country to deliver a high-uptake national immunization program using the bivalent HPV vaccine, thus offering the first chance to observe the effects of widespread use of this vaccine at the population level. One unexpected event that was curiously concurrent with bivalent immunization in England was a moderate decrease in the rate of GW diagnoses among females in the age range eligible for immunization and a smaller decrease among same-aged males. Our ecological analysis of variations in immunization coverage by year and PCT suggest that this decrease in GW in young females may be associated with bivalent HPV immunization.
This study made use of England's comprehensive surveillance system for STIs in GUM clinics. These clinics see approximately 95% of individuals in England with GW [18]. After application of our data quality criteria, our analysis included approximately 85% of GUM clinic attendances for GW between 2008 and 2011. In addition, we accessed data on GP consultations for a representative sample of the population to check for potential shifts in service use by patients with GW from GUM clinics to GPs. However, it is possible that trends in GW diagnosed only in other healthcare services that did not report to PHE over this time period, such as community contraception and sexual health services [19], could have been different. Also, some cases of recurrent GW may have been included due to coding errors in GUM clinic data and limited clinical history in the GP data.
We used estimates of HPV immunization coverage from administrative data collected by the HPV Immunisation Programme. While these data are complete and of high quality for the total population, it is possible that they do not reflect coverage in those who attend GUM clinics. Furthermore, this measurement error may vary by age. For example, if the probability of receiving HPV immunisation is associated with socioeconomic status [20] and GW is more strongly associated with lower socio-economic status in younger girls, the population coverage estimates may be less accurate for younger girls attending GUM than for older girls.
Using data submitted from 2002 to 2011, we were able to investigate the secular trends in GW diagnoses prior to the introduction of the national HPV immunization program, albeit limited to available age groupings. Variations in preimmunization rates of GW by PCT of residence were not available for analysis, as collection of patient residence data from GUM clinics began in the same year as HPV immunization in the UK (2008).
A number of factors could explain some or all of the observed decreases in GW that could not be formally included in our analysis. These are described in the following paragraphs.
Use of Quadrivalent HPV 16/18/6/11 Vaccine
The quadrivalent HPV 16/18/6/11 vaccine is highly effective against GW, and the impact of its use on GW diagnoses in STI clinics in Australia has been reported [8]. The quadrivalent vaccine is available for purchase within the United Kingdom and can be prescribed on the National Health Service (NHS) in certain circumstances. Prescribing data from the NHS Business Services Authority show that 8300, 10 200, 5200, and 3700 doses of the quadrivalent vaccine were prescribed by the NHS in 2008, 2009, 2010, and 2011, respectively. This likely underestimates the use of this vaccine, as the system does not capture private healthcare for which data are not available. For quadrivalent vaccine use in England to fully explain the observed decline in GW in young females via direct vaccine-induced protection, the uptake would have to be similar to the decrease, that is, 14%–34% of the population within specific birth cohorts, implying use of approximately 125 000–300 000 doses for a single birth cohort. This percentage could be slightly lower if private uptake of the quadrivalent vaccine was biased toward higher-risk but, as yet, uninfected females. However, anecdotally we understand there has been demand for this vaccine among men and women who are older than those included in the catch-up program. Also, given the high cost of private immunization, it seems very unlikely that the vaccine was used this heavily among those who are eligible for free immunization with the bivalent vaccine or among the higher-risk groups. However, private use of the quadrivalent vaccine among young males could be a plausible alternative explanation for the small declines in GW in young males.
Theoretically, we could also expect some indirect protection of UK residents from widespread use of the quadrivalent vaccine in countries with frequent travel to and from the United Kingdom. However, the impact of this herd immunity is likely to be relatively small, and we would expect it to be at least equally strong among males and to impact more on individuals who are slightly older for whom travel abroad is more common.
Changes in Use of GUM Services
The large increase in chlamydia screening of young people over the last few years and specific targets for testing outside of GUM clinics (introduced in 2008/2009) have resulted in a shift of chlamydia testing to community healthcare and nonhealthcare settings [21]. This may be responsible, at least in part, for the declines in chlamydia diagnoses in young women, although the number of tests performed in GUM clinics has not declined. Such a shift in diagnoses away from GUM clinics may have led, inadvertently, to a reduction in opportunistic GW diagnoses on attendance for chlamydia screening at GUM clinics in proportion with reduced chlamydia diagnoses. However, our analyses, which included chlamydia diagnoses as an independent variable, found the association with HPV immunization to remain significant. Moreover, for the decline in GW diagnoses to be a direct consequence of reduced chlamydia diagnoses, the overlapping risks for and occurrence of these 2 diagnoses would need to be higher than other evidence suggests [22]. The lack of a compensating increase in GP diagnoses of GW also argues against this.
Improvements in Sexual Health
It has been argued that sexual behavior might change as a result of the offer or receipt of HPV immunization. There have been concerns voiced about the possible risk of HPV vaccination leading to a false sense of protection against STIs and subsequent disinhibition of sexual behaviors, leading indirectly to higher rates of STIs and unwanted pregnancies. The reverse is also possible as increased awareness of the potential consequences of STIs and health education messages received in addition to immunization could result in reduced risk-taking behaviors among vaccinated females. However, there is little evidence to suggest that sexual behaviors are affected either way by HPV immunization [23]. There may have been other causes of changes in sexual behavior in females at this age, although none are known or evident in data for other STIs.
Direct Protection From the Bivalent Vaccine
Biological plausibility is required for discussion of the possible direct impact of the bivalent vaccine on GW. The extent to which HPV 16/18 cause GW, either directly or synergistically by interaction with HPV 6/11, is unclear. Two studies found HPV 16/18 in a substantial proportion of GWs. Sturegard et al found HPV16/18 in 49/296 (17%) of GW, in half of which HPV 16/18 was the only type [24]. Ball et al found HPV 16/18 in 11/31 (35%) of GW, although only 1 sample was negative for both types 6 and 11 [25]. If HPV types 16/18 have a direct role in causing up to 10% of GW, which seems to be an upper plausible limit, at most a 5% decline in GW among young females (given the immunization coverage achieved) might be expected due to direct protection against HPV 16/18–associated GW.
Data on vaccine efficacy against persistent infection with low-risk HPV types has been released recently from a clinical trial of the bivalent vaccine. Post hoc analyses have shown a VE against types 6/11 of 34.5% (95% CI, 11.3–51.8) among women who are DNA negative for HPV at trial entry [12]. The mechanism of this partial protection is not clear, although some studies of T-cell responses are consistent with some HPV 6/11 efficacy from the bivalent vaccine. This could explain the decline we have seen, given the age at immunization and coverage of females in England to date. If so, we would expect slightly greater declines in years to come when girls immunized at age 12 years with >80% coverage reach ages of risk for GW. However, in September 2012 the UK HPV immunization program changed to using the quadrivalent vaccine, and we expect to see a further and much greater reduction in GW when girls who receive this vaccine reach sexually active ages (around 2016/2017).
While several factors might contribute to the observed declines in GW, it seems likely that we are observing some direct protection from bivalent immunization. We are conducting a case-control study of GW cases among females targeted for bivalent vaccination in England to investigate this further. To the extent that the bivalent vaccine does prevent GW (and possibly associated Recurrent Respiratory Papillomatosis [RRP]), this would improve the cost effectiveness of immunization programs that use this vaccine. Better understanding of the mechanism of this partial protection may help improve our understanding of how HPV vaccines confer protection.
Notes
Acknowledgments. We thank Stephen Duffel and all clinics that report data to the Genitourinary Medicine Clinic Activity Dataset (GUMCAD), the Clinical Practice Research Datalink Division (CPRD), and all practices that contribute to the CPRD for providing the data for this study. We are grateful to Stefano Conti for advice on statistical analyses and to Peter Goon and Andreas Kaufman for helpful discussions about plausible biological explanations for our observations.
Financial support. CPRD is funded by the Medicines and Healthcare products Regulatory Agency (MHRA) and the NHS National Institute for Health Research (NIHR). The funders had no role in the study design; in the collection, analysis, and interpretation of data; in writing the manuscript; or in the decision to submit the paper for publication.
Potential conflicts of interest. S. W. was a GlaxoSmithKline employee and has stock in the company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Health Protection Agency. Vol. 1. Health Protection Report; 2007. Trends in genital warts and genital herpes diagnoses in the United Kingdom. [Google Scholar]
- 2.Health Protection Agency. Vol. 3. Health Protection Report; 2009. Trends in genital herpes and genital warts infections, United Kingdom: 1999 to 2008. [Google Scholar]
- 3.Nicoll A, Hughes G, Donnelly M, et al. Assessing the impact of national anti-HIV sexual health campaigns: trends in the transmission of HIV and other sexually transmitted infections in England. Sex Transm Infect. 2001;77:242–7. doi: 10.1136/sti.77.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Protection Agency. Vol. 6. Health Protection Report; 2012. Sexually transmitted infections in England, 2011. [Google Scholar]
- 5.Department of Health. Annual HPV vaccine uptake in England: 2010/11. http://media.dh.gov.uk/network/211/files/2012/03/120319_HPV_UptakeReport2010-11-revised_acc.pdf . Accessed April 2013.
- 6.Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11 doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacey CJN, Lowndes CM, Shah KV. Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87:544–7. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 9.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtinen M, Palmroth J, Merikukka M, Kaasila M, Apter D, Paavonen J. Competition of vaccine and non-vaccine HPV types before and after mass-vaccination. Eurogin 2011, Lisbon [SS 2-1] [Google Scholar]
- 11.Hepburn HM, Seipel M, Schwarz T, Pawlita M, Waterboer T, Kaufmann AM. Ex vivo monitoring of cellular memory responses in young women immunized with either Gardasil or Cervarix four years prior to enrollment. IPV 2009, Malmo [P-13.13] [Google Scholar]
- 12.Szarewski A, Skinner SR, Garland S, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low risk HPV types (PATRICIA randomised trial): an unexpected observation. 2013;208:1391–6. doi: 10.1093/infdis/jit360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Health. Annual HPV vaccine coverage in England in 2009/2010. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123795 . Accessed August 2011.
- 14.Savage EJ, Marsh K, Duffell S, Ison CA, Zaman A, Hughes G. Rapid increase in gonorrhoea and syphilis diagnoses in England in 2011. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- 15.Health Protection Agency. Genitourinary Medicine Clinic Activity Dataset (GUMCAD) http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1201265888302 . Accessed August 2011.
- 16.Annual Mid-year Population Estimates for England and Wales. Available at: http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-england-and-wales/mid-2002-to-mid-2010-revised–national-/stb—mid-2002-to-mid-2010-revised-population-estimates-for-england-and-wales.html . Accessed August 2013. [PubMed]
- 17.Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3:89–99. doi: 10.1177/2042098611435911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K. Genital warts and cost of care in England. Sex Transm Infect. 2011;87:464–8. doi: 10.1136/sti.2010.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yung M, Denholm R, Peake J, Hughes G. Distribution and characteristics of sexual health service provision in primary and community care in England. Int J STD AIDS. 2010;21:650–2. doi: 10.1258/ijsa.2010.010198. [DOI] [PubMed] [Google Scholar]
- 20.Hughes A, Mesher D, White J, Soldan K. Coverage of the English National HPV Immunisation Programme (2008–2011) among 12–17 year old females by area-level deprivation score. Submitted for publication 2012. [DOI] [PubMed]
- 21.Health Protection Agency. Vol. 5. Health Protection Report; 2011. Further increase in chlamydia screening coverage in 2010/11; pp. 21–3. [Google Scholar]
- 22.Hughes G, Catchpole M, Rogers PA, et al. Comparison of risk factors for four sexually transmitted infections: results from a study of attenders at three genitourinary medicine clinics in England. Sex Transm Infect. 2000;76:262–7. doi: 10.1136/sti.76.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster AS, Marlow LA, Stephenson J, Wardle J, Waller J. Human papillomavirus vaccination and sexual behaviour: cross-sectional and longitudinal surveys conducted in England. Vaccine. 2012;30:4939–44. doi: 10.1016/j.vaccine.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Sturegard E, Johnsson A, Gustafsson E, Dillner J. Condyloma typing important for follow up of HPV vaccination. A condyloma reporting project. Lakartidningen. 2008;105:3648–50. [PubMed] [Google Scholar]
- 25.Ball SLR, Winder DM, Vaughan K, et al. Analysis of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol. 2011;83:1345–50. doi: 10.1002/jmv.22111. [DOI] [PubMed] [Google Scholar]