Abstract
Background. Whether the risk of malaria is increased in infants born to mothers who experience malaria during pregnancy is uncertain.
Methods. We investigated malaria incidence among an infant cohort born to 355 primigravidae and 1500 multigravidae with or without placental malaria (PM) in a high malaria transmission area of Ghana. PM was assessed using placental histology.
Results. The incidence of all episodes of malaria parasitemia or clinical malaria was very similar among 3 groups of infants: those born to multigravidae without PM, multigravidae with PM, and primigravidae with PM. Infants born to primigravidae without PM experienced a lower incidence of malaria parasitemia or clinical malaria than the other 3 groups: adjusted hazard ratio, 0.64 (95% confidence interval [CI], .48–.86, P < .01) and 0.60 (95% CI, .43–.84, P < .01), respectively. The incidence of malaria parasitemia or clinical malaria was about 2 times higher in most poor infants compared to least poor infants.
Conclusions. There was no suggestion that exposure to PM directly increased incidence of malaria among infants of multigravidae. In our study area, absence of placental malaria in primigravidae is a marker of low exposure, and this probably explains the lower incidence of malaria-related outcomes among infants of PM-negative primigravidae.
Keywords: malaria, placental malaria, infant malaria, malaria epidemiology, cohort study, Ghana
Malaria in pregnancy leads to maternal anemia and poor pregnancy outcomes such as abortion, preterm delivery [1], or low birth weight [2]. Approximately 75 000–200 000 infant deaths are attributed to malaria in pregnancy annually [2]. Primigravidae (PG) have a higher risk of placental malaria (PM) and a higher risk of poor pregnancy outcomes than do multigravidae (MG) [3]. In Africa, malaria in pregnancy is associated with an increase in the incidence of infant malaria [4–8]. However, there is a paucity of data on the effects of PM on the risk of malaria among infants born to women of different gravidities. The few studies that have been conducted have provided conflicting results, probably due to differences in malaria transmission intensity in the study areas, sample size or exposure, and outcome measures.
Fetal exposure to malaria during pregnancy was assessed by examining placental blood smear in studies undertaken in Tanzania [5] and Gabon [6] and by a mixture of placental histology and placental blood smear in a Mozambican study [7]. Compared to the gold-standard polymerase chain reaction, the sensitivity of a placental blood film in detecting a PM infection is substantially lower (63%) than placental histology (91%) [9–11]. Thus, exposure to PM could have been misclassified in these studies. Second, detailed information on malaria exposure, including differences in malaria transmission at the household level, was not determined, although some studies adjusted for place of residence.
To investigate further the influence of PM, gravidity, and exposure-related factors on the risk of malaria in infancy, we conducted a birth cohort study between 2008 and 2011 in a high malaria transmission area of Ghana.
METHODS
Study Area
A prospective cohort study was conducted in the Brong Ahafo Region of Ghana. Malaria transmission is high and perennial but peaks between April and October [12].
Enrollment and Follow-up of Pregnant Women
Forty-two communities where good follow-up could be obtained were selected from the Kintampo Health and Demographic Surveillance System (KHDSS). All pregnant women resident in the selected communities were identified using vital registers collated by community key informants or by staff of the KHDSS [13] who made home visits. A pregnant woman who reported that she would stay in the study area at least until her baby was 1 year old was eligible for inclusion into the study. There were no other maternal exclusion criteria. At enrollment, information on demographic, socioeconomic, and obstetric characteristics and a finger-prick blood sample were collected. Pregnant women were encouraged to attend antenatal clinics regularly and were also visited at home monthly. Pregnant women were referred to a health facility for treatment if they complained of an illness during a home visit.
Enrollment and Follow-up of Study Infants
Newborns of mothers who were enrolled in the study prior to delivery and whose placental tissue was collected at delivery were included in this cohort study. Infants were actively followed up until 12 months of age through monthly home visits and passively at clinic visits. At each visit, infants were assessed for malaria parasitemia, fever (history of fever in the past 48 hours or an axillary temperature ≥37.5°C), and any other symptom reported. Clinical malaria was defined as fever plus malaria parasitemia of any density. During the monthly home visits, infants who were unwell were referred to the study clinic where a malaria rapid diagnostic test was performed immediately to assist further management, as per the national treatment guidelines, and the same procedure was followed for infants who passively visited the clinic. Blood smears were collected and read later for the study endpoints. To ensure the safety of infants, parents or caregivers of infants were encouraged to report any symptom immediately to study staff. Additionally, project fieldworkers were located within the communities and were able to call a study ambulance to transport an ill infant to the study clinic at any time during the project.
Measurement of Household Exposure to Malaria
A serum sample was collected from all children <5 years of age (other than infants in the study cohort) living in the household of study women. If there was no child <5 years of age in a study woman's household, a blood sample was collected from a child <5 years of age resident in the nearest household.
Laboratory Procedures
Malaria Microscopy
Thin and thick peripheral blood smears were read by 2 microscopists, and by a third microscopist if discordant on positivity or on parasite density, as described by Swysen et al [14]
Hemoglobin Concentration Measurement
Hemoglobin concentrations were determined using an ABX Micros 60 hematology auto analyzer (Horiba ABX).
Placental Malaria
A full-thickness placental biopsy (approximately 2.5 × 2.5 × 2.5 cm3) was taken from the maternal surface halfway between the edge of the placental edge and the insertion of the umbilical cord by a trained fieldworker or birth attendant within 4 hours of placental expulsion. A histopathologist categorized each sample as showing no infection, acute infection, chronic infection, or past infection based on the classification described by Bulmer et al [15] and modified by Ismail et al [16]. Twenty-eight percent (522/1855) of randomly selected placental slides were read by a second histopathologist. The pairwise correlation coefficient between the 2 readings was 0.92.
Serology
An indirect enzyme-linked immunosorbent assay (ELISA) method based on a modified version of the Afro Immuno Assay ELISA protocol was used to measure MSP1-19 and AMA1 FVO antibody concentrations in samples from siblings and neighbors of study infants [17–19]. The normalized optical density for each antibody was categorized into tertiles. The malaria exposure of infants in the study cohort was scored as low, moderate, or high based on the tertile to which their siblings or neighbors were categorized.
Statistical Analysis
Cleaned data were analyzed using Stata software, version 11.0 (StataCorp). Principal components analysis of women's durable assets was used to derive socioeconomic status (SES) quintiles [20–22]. Kaplan–Meier survival analysis of the probability of infants remaining free of parasitemia or clinical malaria were determined by gravidity and PM status. Cox regression models were used to determine hazard ratios for multiple episodes of malaria parasitemia or clinical malaria, using a robust standard error to account for within-child correlation, and for first or only episode of malaria parasitemia or clinical malaria. Because of the known association between acquisition of immunity to malaria and gravidity, we prespecified the interaction between gravidity and PM infection as an a priori component of the statistical models. The following variables were considered as potential confounders: wealth index of their household, infant insecticide-treated bed net (ITN) use, distance from the house of a participant to the health facility, area of residence, and household malaria exposure. We also explored other factors in multivariate Cox regression models, including potential confounders based on their effect on the main exposures of interest.
Ethical Approval
Ethical approval was obtained from the ethics committees of Kintampo Health Research Centre, Ghana Health Service, London School of Hygiene and Tropical Medicine, and Noguchi Memorial Institute for Medical Research. Written informed consent was obtained from all study women.
RESULTS
Characteristics of Study Women
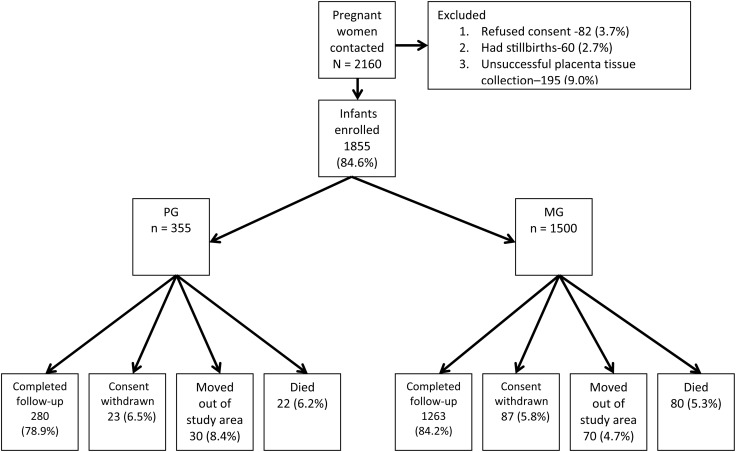
A total of 2160 pregnant women were invited to join the study, and 1855 of their infants (PG 19.1%, MG 80.9%) were eligible for follow-up (Figure 1). The majority (1516/1755 [86.6%]) of women who had used intermittent preventive treatment for malaria in pregnancy (IPTp) had taken 2 or more doses of Sulfadoxine-Pyrimethamine (SP). ITN use among all pregnant women was 47.4%.
Figure 1.
Study flow diagram for infants in the 2008–2011 Kintampo Birth Cohort Study. There were 102 infant deaths (primigravidae [PG] with placental malaria [PM] = 16; PG without PM = 6; multigravidae [MG] with PM = 33; MG without PM = 47), 100 migrations out of the study area, and 110 withdrawals of consent among the cohort of 1855 infants. Eighty-three percent (1543) of the total cohort completed 1 year of follow-up. The final follow-up rate was significantly lower among infants of PG compared those of MG (P = .03). Abbreviations: MG, multigravidae; PG, primigravidae.
There was no statistically significant difference in IPTp use between PG and MG (Table 1). PG were better educated than MG (62.2% vs 43.3% had higher education; P < .01). ITN use was slightly lower in PG than MG (41% vs 49%; P = .03). The proportion of women with a hemoglobin concentration <10 g/dL was higher in PG than MG (49% vs 34%; P < .001). A higher proportion of PG lived in an urban area compared to MG (26% vs 20%; P = .02), but the difference in the proportion of women living >5 km from the health facilities was not statistically significant (Table 1).
Table 1.
Characteristics of Women by Gravidity and Placental Malaria Status
| Characteristic | Total Cohort |
PG |
MG |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PG | MG | P Valuea | PM+ | PM− | P Valuea | PM+ | PM− | P Valuea | |
| Age, y, No., mean (SD) | 354, 19.7 (3.5) | 1486, 28.3 (6.2) | <.01 | 234, 19.3 (3.4) | 120, 20.3 (3.4) | <.01 | 455, 26.7 (6.2) | 1031, 29.0 (6.1) | <.01 |
| IPTp useb | |||||||||
| Yes | 341 (96.1) | 1414 (94.3) | .28 | 225 (96.2) | 116 (96.7) | .81c | 442 (96.1) | 972 (93.6) | .06 |
| No | 13 (3.6) | 84 (5.6) | 9 (3.8) | 4 (3.3) | 18 (3.9) | 66 (6.4) | |||
| Maternal ITN used | |||||||||
| Yes | 146 (41.1) | 734 (48.9) | .03 | 101 (44.7) | 45 (37.5) | .20 | 205 (45.2) | 529 (52.2) | .01 |
| No | 200 (56.4) | 734 (48.9) | 125 (55.3) | 75 (62.5) | 249 (54.8) | 485 (47.8) | |||
| Wealth index | |||||||||
| Least poor | 81 (22.8) | 324 (21.6) | <.01 | 35 (15.0) | 46 (38.0) | <.01 | 80 (17.3) | 244 (23.5) | <.01 |
| Less poor | 74 (20.9) | 294 (19.6) | 46 (19.6) | 28 (23.1) | 81 (17.6) | 213 (20.5) | |||
| Poor | 77 (21.7) | 284 (18.9) | 57 (24.4) | 20 (16.5) | 95 (20.6) | 189 (18.2) | |||
| More poor | 81 (22.8) | 291 (19.4) | 64 (27.3) | 17 (14.1) | 90 (19.5) | 201 (19.3) | |||
| Most poor | 42 (11.8) | 307 (20.5) | 32 (13.7) | 10 (8.3) | 115 (25.0) | 192 (18.5) | |||
| Thatched roof | |||||||||
| No | 259 (73.0) | 1053 (70.2) | .30 | 161 (68.8) | 98 (81.0) | .01 | 313 (67.9) | 740 (71.2) | .19 |
| Yes | 96 (27.0) | 447 (29.8) | 73 (31.2) | 23 (19.0) | 148 (32.1) | 299 (28.8) | |||
| Maternal hemoglobine | |||||||||
| ≥10 g/dL | 168 (47.3) | 938 (62.5) | <.01 | 96 (43.2) | 72 (60.0) | <.01 | 269 (59.8) | 669 (66.9) | <.01 |
| <10 g/dL | 174 (49.0) | 512 (34.1) | 126 (56.8) | 48 (40.0) | 181 (40.2) | 331 (33.1) | |||
| Place of residence | |||||||||
| Urban | 91 (25.6) | 299 (19.9) | .02 | 52 (22.2) | 39 (32.2) | .04 | 94 (20.4) | 205 (19.7) | .77 |
| Rural | 264 (74.4) | 1201 (80.1) | 182 (77.8) | 82 (67.8) | 367 (79.6) | 834 (80.3) | |||
| Distance from nearest health facility | |||||||||
| <5 km | 282 (79.4) | 1136 (75.7) | .14 | 182 (77.8) | 100 (82.6) | .28 | 339 (73.5) | 797 (76.7) | .18 |
| >5 km | 73 (20.56) | 364 (24.3) | 52 (22.2) | 21 (17.4) | 122 (26.5) | 242 (23.3) | |||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IPTp, intermittent preventive treatment of malaria in pregnancy; ITN, insecticide-treated bed net; MG, multigravidae; PG, primigravidae; PM, placental malaria; PM–, no placental malaria; PM+, placental malaria present.
a χ2 P value except.
b IPTp use is defined as at least 1 dose of Sulfadoxine-Pyrimethamine (SP) IPTp.
c Where Fishers exact test was used.
d Maternal ITN was assessed at the last contact with the study mother prior to delivery.
e Maternal hemoglobin concentration measured in the last trimester of pregnancy or at birth.
Mothers without PM were likely to be from higher socioeconomic groups than infected mothers in both gravidity groups, but this was particularly marked among PG women. More than 60% of PG without PM were from the top 2 SES quintiles, with almost 40% from the highest SES quintile. Just 19% of PG without PM lived in houses with a thatched roof, compared to approximately 30% in other groups, and 32% were urban residents (compared to around 20% in other groups; Table 1).
Prevalence of Placental Malaria
Thirty-eight percent (695/1855) of all pregnant women had evidence of past or current PM; 80.3%, 10.2%, and 9.5% had past, chronic, or acute infections, respectively. PM of any category was significantly higher in PG than MG (66.0% vs 30.8%; P < .001). There was a significant difference (P = .02) in the distribution of PM infection types among PG and MG (PG: past, 75.2%; chronic,14.5%; acute, 10.3%; and MG: past, 82.9%; chronic, 8.0%; acute, 9.1%).
Overall Characteristics of Study Infants
A total of 1793 (96.7%) study infants were singletons and 62 (3.3%) were twins. Follow-up to 1 year of age was 83.2% (Figure 1). There were 102 infant deaths (5.5%) with no significant difference in the death rate between infants born to PG (6.2%) or MG (5.3%). The prevalence of low birth weight was highest among PG (Table 2).
Table 2.
Infant Characteristics by Mother's Primigravidae Status and Gravidity Group
| Characteristic | Total Cohort |
PG |
MG |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PG | MG | P Valuea | PM+ | PM− | P Valuea | PM+ | PM− | P Valuea | |
| Sex | |||||||||
| Male | 179 (50.4) | 766 (51.1) | .83 | 119 (50.8) | 60 (49.6) | .82 | 240 (52.1) | 526 (50.6) | .61 |
| Female | 176 (49.6) | 734 (48.9) | 115 (49.2) | 61 (50.4) | 221 (47.9) | 513 (49.4) | |||
| Birth weight | |||||||||
| ≥2.5 kg | 288 (83.5) | 1358 (92.3) | <.01 | 186 (82.3) | 102 (85.7) | .42 | 409 (91.9) | 949 (92.9) | .24 |
| <2.5 kg | 57 (16.5) | 113 (7.7) | 40 (17.7) | 17 (14.3) | 40 (8.9) | 73 (7.1) | |||
| Malaria transmission season at delivery | |||||||||
| Low | 91 (25.6) | 430 (28.7) | .25 | 54 (23.1) | 37 (30.6) | .13 | 133 (28.8) | 297 (28.6) | .92 |
| High | 264 (74.4) | 1070 (71.3) | 180 (76.9) | 84 (69.4) | 328 (71.2) | 742 (71.4) | |||
| Infant ITN useb | |||||||||
| High | 97 (29.4) | 489 (34.1) | .26 | 62 (28.6) | 35 (31.0) | .89 | 128 (29.4) | 361 (36.2) | .01 |
| Moderate | 118 (35.8) | 472 (32.9) | 79 (36.4) | 39 (34.5) | 166 (38.1) | 306 (30.6) | |||
| Low | 115 (34.8) | 473 (40.0) | 76 (35.0) | 39 (34.5) | 142 (32.5) | 331 (33.2) | |||
Data are presented as No. (%).
Abbreviations: ITN, insecticide-treated bed net; MG, multigravidae; PG, primigravidae; PM, placental malaria; PM–, no placental malaria; PM+, placental malaria present.
a χ2 P value.
b Fieldworkers determined infant ITN use during monthly home visits. The mean score of ITN use per month was graded into tertiles (high, moderate and low users).
Incidence of Malaria Parasitemia or Clinical Malaria in Study Infants
Fifty-two percent (957/1855) of study infants experienced at least 1 episode of malaria parasitemia and 41% (760/1855) of infants experienced at least 1 episode of clinical malaria before their first birthday. The incidence of all episodes of malaria parasitemia (first and subsequent) was 1.31 per child-year (95% confidence interval [CI], 1.26–1.36) and that of clinical malaria 0.70 per child-year (95% CI, .66–.74). The incidence of malaria parasitemia in infants born to PM-positive women was 1.26 per child-year (95% CI, 1.20–1.33) compared to an incidence of 1.39 per child-year (95% CI, 1.30–1.48) in infants of women without PM. The corresponding figures for clinical malaria were 0.73 per child-year (95% CI, .68–.81) and 0.68 per child-year (95% CI, .63–.73), respectively. Stratified by both gravidity and PM status, the incidence of all episodes of malaria parasitemia and all episodes of clinical malaria were lowest among infants of PG without PM (PG/PM−), but were similar among infants of PG with PM (PG/PM+), MG without PM (MG/PM−), and MG with PM (MG/PM+) (Table 3).
Table 3.
Incidence of Malaria Parasitemia and Clinical Malaria in Infants According to Their Mothers' Placental Malaria (PM) Status
| Status | No. | Person-years | Incidence Rate per Infant per Year (95% CI) |
|---|---|---|---|
| All episodes of parasitemia | |||
| MG PM− | 1306 | 983.3 | 1.32 (1.25–1.40) |
| MG PM+ | 623 | 448.3 | 1.39 (1.28–1.50) |
| PG PM− | 80 | 114.8 | 0.69 (.56–.85) |
| PG PM+ | 309 | 223.2 | 1.38 (1.24–1.54) |
| Total | 2318 | 1769.5 | 1.31 (1.26–1.36) |
| All episodes of clinical malaria | |||
| MG PM− | 706 | 983.3 | 0.72 (.67–.77) |
| MG PM+ | 328 | 448.3 | 0.73 (.66–.82) |
| PG PM− | 40 | 114.8 | 0.34 (.26–.48) |
| PG PM+ | 168 | 223.2 | 0.75 (.65–.87) |
| Total | 1242 | 1769.5 | 0.70 (.66–.74) |
| First or only episode of parasitemia | |||
| MG PM− | 537 | 759.61 | 0.71 (.65–.77) |
| MG PM+ | 249 | 334.44 | 0.74 (.65–.84) |
| PG PM− | 47 | 96.40 | 0.49 (.37–.55) |
| PG PM+ | 124 | 171.26 | 0.72 (.61–.86) |
| Total | 957 | 1361.70 | 0.70 (.66–.75) |
| First or only episode of clinical malaria | |||
| MG PM− | 426 | 830.37 | 0.51 (.47–.56) |
| MG PM+ | 204 | 368.49 | 0.55 (.48–.64) |
| PG PM− | 32 | 104.49 | 0.30 (.21–.43) |
| PG PM+ | 98 | 188.03 | 0.52 (.43–.64) |
| Total | 760 | 1491.40 | 0.51 (.47–.55) |
Abbreviations: CI, confidence interval; IPTp, intermittent preventive treatment of malaria in pregnancy; ITN, insecticide-treated bed net; MG, multigravidae; PG, primigravidae; PM, placental malaria; PM–, no placental malaria; PM+, placental malaria present.
Factors Associated With All Episodes of Malaria Parasitemia or Clinical Malaria
The relationship between PM and the incidence of all episodes of malaria parasitemia was modified by gravidity (likelihood ratio test, P < .01). The incidence of all episodes of parasitemia was significantly lower among PG/PM– infants than among MG/PM– infants (adjusted hazard ratio [AHR], 0.64 [95% CI, .48–.86], P < .01). The incidence of all episodes of parasitemia among infants of PG/PM+ (AHR, 1.01 [95% CI, .86–1.19], P = .93) or MG/PM+ (AHR, 0.98 [95% CI, .85–1.12], P = .72) was not significantly different from that among infants of MG/PM− (Table 4). Similarly, the incidence of all clinical malaria episodes was significantly lower in PG/PM− infants (AHR, 0.60 [95% CI, .43–.84], P < .01) than in MG/PM– infants (Table 4). The AHR for all episodes of clinical malaria among PG/PM+ (AHR, 1.00 [95% CI, .83–1.21], P = .97) or MG/PM+ (AHR, 0.94 [95% CI, .81–1.10], P = .44) infants were, however, not different from MG/PM− infants.
Table 4.
Hazard Ratio for All Episodes of Parasitemia or Clinical Malaria in the First Year of Life
| All Parasitemia |
All Clinical Malaria |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | AHR (95% CI) | P Value | HR (95% CI) | P Value | AHR (95% CI) | P Value | |
| Gravidity and PM | ||||||||
| MG PM− | … | … | … | … | ||||
| MG PM+ | 1.03 (.89–1.19) | .68 | 0.98 (.85–1.12) | .73 | 1.00 (.86–1.17) | .99 | 0.94 (.81–1.10) | .44 |
| PG PM− | 0.52 (.39– .70) | <.01 | 0.64 (.48–.86) | <.01 | 0.48 (.34–.69) | <.01 | 0.60 (.43–.84) | <.01 |
| PG PM+ | 1.04 (.86–1.24) | .70 | 1.01 (.86–1.19) | .93 | 1.04 (.85–1.28) | .71 | 1.00 (.83–1.21) | .97 |
| IPTp use | ||||||||
| Yes | 1 | … | … | 1 | ||||
| No | 0.99 (.82–1.18) | .88 | 1.23 (.93–1.59) | .15 | ||||
| Maternal ITN use | ||||||||
| Yes | 1 | … | 1 | |||||
| No | 0.90 (.83–.98) | .02 | .57 | 0.88 (.79–.99) | .03 | |||
| Wealth index | ||||||||
| Least poor | 1 | … | 1 | … | 1 | … | 1 | … |
| Less poor | 1.77 (1.51–1.93) | <.01 | 1.54 (1.23–1.93) | <.01 | 1.69 (1.29–2.21) | <.01 | 1.44 (1.11–1.87) | <.01 |
| Poor | 2.47 (1.98–3.09) | <.01 | 1.88 (1.50–2.35) | <.01 | 2.43 (1.87–3.16) | <.01 | 1.8 (1.38–2.35) | <.01 |
| More poor | 2.62 (2.11–3.24) | <.01 | 1.86 (1.50–2.31) | <.01 | 2.70 (2.11–3.45) | <.01 | 1.86 (1.44–2.39) | <.01 |
| Most poor | 3.27 (2.64–4.05) | <.01 | 2.21 (1.77–2.76) | <.01 | 3.02 (2.36–3.87) | <.01 | 2.00 (1.55–2.59) | <.01 |
| Thatched roof | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.71 (1.51–1.93) | <.01 | 1.32 (1.16–1.49) | <.01 | 1.70 (1.49–1.95) | <.01 | 1.29 (1.13–1.49) | <.01 |
| Maternal hemoglobin | ||||||||
| ≥10 g/dL | 1 | … | … | 1 | 1 | |||
| <10 g/dL | 1.16 (1.06–1.26) | <.01 | 1.21 (1.07–1.35) | <.01 | 1.13 (.98–1.30) | .08 | ||
| Place of residence | ||||||||
| Urban | 1 | … | 1 | 1 | 1 | |||
| Rural | 2.77 (2.43–3.21) | <.01 | 2.06 (1.7–2.57) | <.01 | 3.02 (2.47–3.68) | <.01 | 2.25 (1.74–2.90) | <.01 |
| Distance from nearest health facility | ||||||||
| <5 km | 1 | … | 1 | 1 | 1 | |||
| >5 km | 1.24 (1.12–1.35) | <.01 | 0.93 (.81–1.07) | 0.31 | 1.35 (1.19–1.52) | <.01 | 1.01 (.87–1.17) | .91 |
| Infant Sex | ||||||||
| Male | 1 | … | … | 1 | … | … | ||
| Female | 1.07 (.99–1.16) | .10 | 1.00 (.90–1.12) | .93 | ||||
| Birth weight | ||||||||
| ≥2.5 kg | 1 | … | … | 1 | … | … | ||
| <2.5 kg | 0.94 (.82–1.09) | .44 | 0.87 (.71–1.07) | .20 | ||||
| Malaria transmission season at delivery | ||||||||
| Low | 1 | … | … | 1 | … | … | ||
| High | 0.99 (.91–1.09) | .90 | 1.07 (.94–1.21) | .30 | ||||
| Infant ITN use | ||||||||
| High | 1 | 1 | 1 | 1 | ||||
| Moderate | 1.08 (.98–1.20) | .20 | 1.15 (.99–1.32) | .06 | 1.13 (.98–1.30) | .08 | 1.21 (1.03–1.41) | .02 |
| Low | 1.16 (1.04–1.28) | <.01 | 1.15 (1.00–1.32) | .05 | 1.20 (1.04–1.37) | .01 | 1.20 (1.02–1.40) | .03 |
| Malaria exposure score | ||||||||
| Low | 1 | 1 | 1 | 1 | ||||
| Moderate | 1.30 (1.12–1.52) | <.01 | 1.28 (1.10–1.47) | <.01 | 1.17 (.98–1.38) | .07 | 1.15 (.98–1.35) | .09 |
| High | 1.18 (1.00–1.38) | .04 | 1.15 (.99–1.32) | .08 | 1.40 (.96–1.36) | .12 | 1.12 (.95–1.32) | .17 |
AHRs were generated using 1 model for malaria parasitemia or clinical malaria.
Abbreviations: AHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; IPTp, intermittent preventive treatment of malaria in pregnancy; ITN, insecticide-treated bed net; MG, multigravidae; PG, primigravidae; PM, placental malaria; PM–, no placental malaria; PM+, placental malaria present.
The incidence of malaria in infants was influenced by a number of factors in addition to PM (Table 4). The incidence of all episodes of malaria parasitemia or clinical malaria was twice as high among infants born to the most poor women compared to infants born to the least poor (AHR, 2.21 [95% CI, 1.77–2.76], P < .01; and AHR, 2.0 [95% CI, 1.55–2.59], P < .01), respectively (Table 4). There was also a strong association with place of residence: infants who lived in rural areas experienced a higher incidence of malaria parasitemia (AHR, 2.09 [95% CI, 1.70–2.57], P < .01) and clinical malaria (AHR, 2.25 [95% CI, 1.74–2.90], P < .01) compared to infants who lived in an urban area (Table 4). Infants who lived in households with moderate or high malaria exposure were more likely to experience malaria than those who lived in households with low malaria exposure, but the difference was significant only for those in households with medium exposure (AHR for all malaria parasitemia, 1.28 [95% CI, 1.10–1.47], P < .01 and AHR for all clinical malaria, 1.15 [95% CI, .98–1.35], P = .09; Table 4). There was no significant association between birth weight, period of delivery, and infant ITN use and overall episodes of parasitemia or clinical malaria (Table 4). Among PG infants, past and chronic infections were significantly associated with higher incidence of malaria parasitemia or clinical malaria episodes (Supplementary Table 1).
Factors Associated With First or Only Episode of Malaria Parasitemia or Clinical Malaria
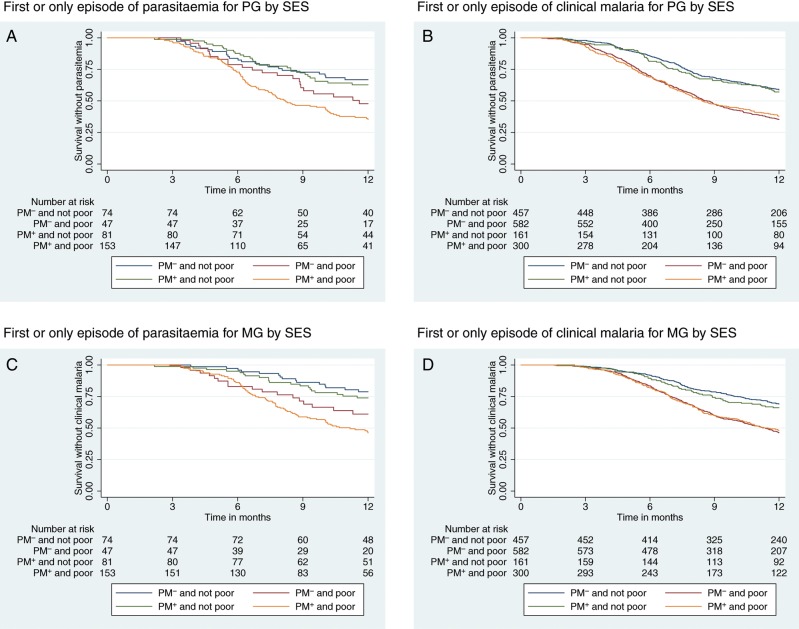
Kaplan–Meier plots indicate clear differences in the incidence of malaria outcomes among infants born to PG/PM− and the other groups (PG/PM+, MG/PM−, MG/PM+; log-rank test P < .01; Figure 2). When mothers were classified into 2 SES groups (poor or not poor), there was a significant association between the risk of parasitemia or clinical malaria and the wealth index among both PG and MG infants (log-rank test P < .01; Figure 3).
Figure 2.
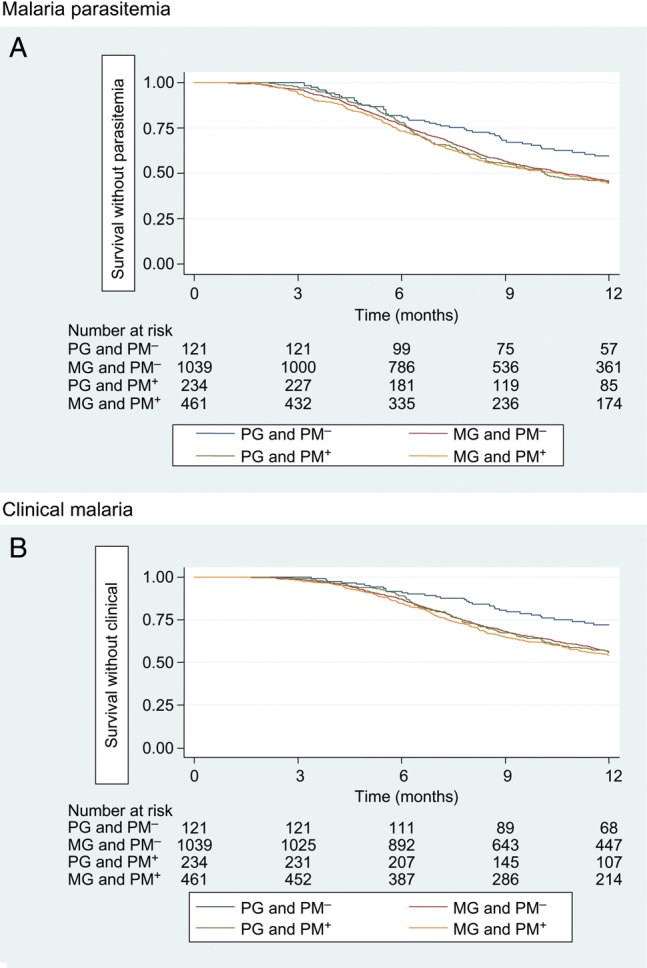
Kaplan–Meier curves for the risk of the first or only episode of parasitemia (A) or clinical malaria (B) in infants by gravidity and placental malaria status. Abbreviations: MG, multigravidae; PG, primigravidae; PM− indicates no placental malaria; PM+ indicates placental malaria present.
Figure 3.
Kaplan–Meier curves for the risk of the first or only episode of parasitemia (A for primigravidae [PG] and B for multigravidae [MG]) or first or only episode of clinical malaria (C for PG and D for MG) in infants by placental malaria status and wealth index. There was a clear association between the risk of first infant parasitemia or clinical malaria and wealth index among PG and MG. P value for log-rank test was <.01 for all survival graphs. Abbreviations: PM– indicates no placental malaria; PM+ indicates placental malaria present.
Compared to MG/PM– infants, the AHR for first or only episode of parasitemia was lower among PG/PM– infants (AHR, 0.77 [95% CI, .57–1.04], P = .09) but the difference was not significant at the 5% level. The incidence in PG/PM+ and MG/PM+ was very similar to that among infants of MG/PM– (AHR, 1.02 [95% CI, .84–1.24], P = .86 and AHR, 1.02 [95% CI, .88–1.19], P = .79, respectively; Supplementary Table 2). This pattern was similar in the analysis of first or only episode of clinical malaria as an outcome (Supplementary Table 2).
DISCUSSION
We investigated the hypothesis that PM influences the incidence of malaria in infants during the first year of life. We found that rates of malaria infection and clinical malaria were similar in infants of PG with PM and MG with or without PM but were substantially lower in infants of PG without PM. Incidence of malaria was significantly higher in poor households and in rural areas.
A previous study from Kenya suggested that a subgroup of infants exposed to malaria in utero became tolerant to malaria antigens, and were subsequently more susceptible to malaria compared to infants not exposed to malaria in utero [23]. In our study, infants born to PG without PM experienced significantly lower incidence of malaria compared to infants of PG with PM, but it is not clear if this is due to a direct benefit of not being exposed in utero, or simply because these infants were less exposed once they were born. There was no difference in the incidence of malaria among infants born to MG with or without PM. If development of a tolerant phenotype in some exposed children does explain some differences in malaria in infancy, it is not clear why this would not apply also to offspring of MG women who were exposed to malaria in utero.
Absence of PM in a first pregnancy is likely to be a marker of lower than average exposure, particularly in our study area where transmission is very high, because acquired immunity to malaria in pregnancy is uncommon in PG. Among MG, absence of PM is not such a clear indicator of low exposure, because some of the MG without PM may have acquired immunity from exposure in previous pregnancies, which would prevent infection in the current pregnancy despite exposure. This conclusion is supported by the significant difference in malaria incidence in infants between the 2 uninfected groups (PG/PM− and MG/PM− infants), which may not be due to any effect of PM. This observation is further supported by the fact that PG/PM− mothers were predominantly of higher SES, were better educated, lived in better quality housing, and were more likely to live in an urban area than others in the cohort. Nonbiological factors, such as higher SES, and maternal education have been shown to be associated with a lower risk of malaria parasitemia among children [24–28]. Given these factors, we consider that infants born to PG/PM− mothers were less likely to be exposed to malaria compared to the other 3 groups, and that these factors may explain the differences in malaria incidence observed. Our analyses attempted to adjust for potential confounding by exposure. However, because PM is a direct consequence of malaria exposure, it is unlikely that complete control of confounding is achieved by adjusting for only proxy measures of exposure such as wealth index, housing construction, and location.
These results do not support or refute the hypothesis of an immune tolerant phenotype, because we did not distinguish infants exposed to malaria in utero as sensitized or nonsensitized as in the Kenya study [23]. However, our results suggest that, even under high transmission, heterogeneity in exposure is likely to be the dominant factor influencing incidence of malaria in infancy. Removal of any risk in infancy that is attributable to immune modulation of the unborn infant in utero will not remove the large differences in exposure that different children will face after birth.
Our findings are contrary to the results of a Tanzanian study that found PM to be associated with a decreased risk of malaria parasitemia among infants of PG with PM [5]. In another study in Gabon, the risk of clinical malaria up to 30 months of life was significantly higher in children born to MG with PM than infants of MG without PM, but there was no difference in the risk of malaria between infants of PG with and without PM as found in our study [6]. It is likely that the Gabon study was not adequately powered to detect differences in PG, given the low numbers followed and the low malaria incidence. In our study, a larger cohort of infants was recruited, a more sensitive technique (placental histology) was used to identify PM, and other important exposure-related covariates such as wealth index and household construction were systematically measured and controlled for in analysis.
ITNs were distributed freely in this area during the study. Nevertheless, maternal and infant ITN use was relatively low irrespective of mother's gravidity, and ITNs did not influence the association between PM and infant malaria outcomes. Similarly, despite high coverage of 2 courses of Sulfadoxine-Pyrimethamine (SP)-IPTp, prevalence of placental malaria at delivery was high. These results suggest that coverage of these interventions needs to be improved to reduce the risk to mothers or infants posed by malaria in pregnancy in high transmission settings. Given the differences in risk of malaria in different socioeconomic groups and different areas, these results underline the importance of ensuring that resources for malaria control reach the rural poor for maximum impact.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to the community leaders in the study area, and study parents and their infants who participated in the study; the Ghana Health Service facilities in the study area; the Pathology Department of University of Ghana Medical School, especially Mr Dzomeku; the director and staff of Noguchi Memorial Institute for Medical Research; Mr Martin Adjuik, INDEPTH Network, Ghana; Drs Jane Bruce, Teun Bousema, and Chris Drakeley of the London School of Hygiene and Tropical Medicine; and Dr Martha Sedegah of Naval Medical Research Centre (NMRC). We also thank Dr Evelina Angov and Dr Sheetij Dutta of Medical Command (MEDCOM) Walter Reed Army Institute of Research (WRAIR) for the supply of MSP1–19 and AMA1 antigens that were used for estimation of malaria-related exposure.
Authors contribution. K. P. A., S. O.-A., K. K., B. Gr., and D. C. conceived the idea and designed the study. K. P. A., S. O.-A., E. A. B., D. K. D., and G. A. collected data. D. D., R. G., B. Gy., A. A.-B., T. D., N. A., and D. K. D. performed biological specimen analysis and its interpretation. K. P. A., M. C., and E. M. performed statistical analysis. K. P. A. wrote the first draft of the manuscript with support from M. C. All authors contributed to the data interpretation and manuscript writing. All authors read and approved the final version of the manuscript.
Financial support. This work was supported by the Division of Microbiology and Infectious Disease, National Institute of Allergy and Infectious Diseases, US National Institutes of Health (contract number HHSN266200400016C), which funded the Kintampo Birth Cohort Study; the Gates Malaria Partnership, London School of Hygiene and Tropical Medicine, which funded placental tissue histology processing and analysis; and the Malaria Capacity Development Consortium, which funded some personnel costs for field and laboratory activities.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 2.Ordi J, Ismail MR, Ventura PJ, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 3.McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans R Soc Trop Med Hyg. 1983;77:232–44. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 4.Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol. 1997;146:826–31. doi: 10.1093/oxfordjournals.aje.a009200. [DOI] [PubMed] [Google Scholar]
- 5.Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47:1017–25. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 7.Bardaji A, Sigauque B, Sanz S, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis. 2011;203:691–9. doi: 10.1093/infdis/jiq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Port A, Watier L, Cottrell G, et al. Infections in infants during the first 12 months of life: role of placental malaria and environmental factors. PLoS One. 2011;6:e27516. doi: 10.1371/journal.pone.0027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–4. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okoko BJ, Ota MO, Yamuah LK, et al. Influence of placental malaria infection on foetal outcome in the Gambia: twenty years after Ian Mcgregor. J Health Popul Nutr. 2002;20:4–11. [PubMed] [Google Scholar]
- 11.Bako BG, Audu BM, Geidam AD, et al. Prevalence, risk factors and effects of placental malaria in the UMTH, Maiduguri, north-eastern, Nigeria: a cross-sectional study. J Obstet Gynaecol. 2009;29:307–10. doi: 10.1080/01443610902878783. [DOI] [PubMed] [Google Scholar]
- 12.Dery DB, Brown C, Asante KP, et al. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malar J. 2010;9:314. doi: 10.1186/1475-2875-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owusu-Agyei S, Nettey OE, Zandoh C, et al. Demographic patterns and trends in central Ghana: baseline indicators from the Kintampo Health and Demographic Surveillance System. Glob Health Action. 2012;5:1–11. doi: 10.3402/gha.v5i0.19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swysen C, Vekemans J, Bruls M, et al. Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J. 2011;10:223. doi: 10.1186/1475-2875-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993;22:219–25. doi: 10.1111/j.1365-2559.1993.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 16.Ismail MR, Ordi J, Menendez C, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 17.Lusingu JP, Vestergaard LS, Alifrangis M, et al. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar J. 2005;4:48. doi: 10.1186/1475-2875-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nebie I, Diarra A, Ouedraogo A, et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2008;76:759–66. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodoo D, Aikins A, Kusi KA, et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houweling TA, Kunst AE, Mackenbach JP. Measuring health inequality among children in developing countries: does the choice of the indicator of economic status matter? Int J Equity Health. 2003;2:8. doi: 10.1186/1475-9276-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe LD, Galobardes B, Matijasevich A, et al. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–86. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asante KP, Zandoh C, Dery DB, et al. Malaria epidemiology in the Ahafo area of Ghana. Malar J. 2011;10:211. doi: 10.1186/1475-2875-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krefis AC, Schwarz NG, Nkrumah B, et al. Principal component analysis of socioeconomic factors and their association with malaria in children from the Ashanti Region, Ghana. Malar J. 2010;9:201. doi: 10.1186/1475-2875-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Custodio E, Descalzo MA, Villamor E, et al. Nutritional and socio-economic factors associated with Plasmodium falciparum infection in children from Equatorial Guinea: results from a nationally representative survey. Malar J. 2009;8:225. doi: 10.1186/1475-2875-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somi MF, Butler JR, Vahid F, Njau J, Kachur SP, Abdulla S. Is there evidence for dual causation between malaria and socioeconomic status? Findings from rural Tanzania. Am J Trop Med Hyg. 2007;77:1020–7. [PubMed] [Google Scholar]
- 28.Slutsker L, Khoromana CO, Hightower AW, et al. Malaria infection in infancy in rural Malawi. Am J Trop Med Hyg. 1996;55(1 suppl):71–6. doi: 10.4269/ajtmh.1996.55.71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.