Abstract
Myofibroblasts accumulate in the spaces between organ structures and produce extracellular matrix (ECM) proteins, including collagen I. They are the primary “effector” cells in tissue remodeling and fibrosis. Previously, leukocyte progenitors termed fibrocytes and myofibroblasts generated from epithelial cells through epithelial-to-mesenchymal transition (EMT) were considered the primary sources of ECM-producing myofibroblasts in injured tissues. However, genetic fate mapping experiments suggest that mesenchyme-derived cells, known as resident fibroblasts, and pericytes are the primary precursors of scar-forming myofibroblasts, whereas epithelial cells, endothelial cells, and myeloid leukocytes contribute to fibrogenesis predominantly by producing key fibrogenic cytokines and by promoting cell-to-cell communication. Numerous cytokines derived from T cells, macrophages, and other myeloid cell populations are important drivers of myofibroblast differentiation. Monocyte-derived cell populations are key regulators of the fibrotic process: They act as a brake on the processes driving fibrogenesis, and they dismantle and degrade established fibrosis. We discuss the origins, modes of activation, and fate of myofibroblasts in various important fibrotic diseases and describe how manipulation of macrophage activation could help ameliorate fibrosis.
Keywords: myofibroblast, pericyte, fibrotic disease, fibrocyte
INTRODUCTION
Fibrosis results when tissues are damaged and normal wound-healing responses persist or become dysregulated, usually in response to some type of repetitive injury. Despite having distinct etiological and clinical features, most chronic fibrotic disorders have in common a persistent irritant that sustains the production of growth factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines, which stimulate the deposition of extracellular matrix (ECM) components (e.g., hyaluronic acid, fibronectin, and interstitial collagens) that progressively remodel tissues. Although mechanical injuries and structural changes can contribute to pathological fibrosis (1), most fibrotic diseases result from chronic exposure to toxins, irritants, immune complexes, or persistent infections. There is also emerging evidence that commensal bacteria living on our skin and mucosal tissues contribute to progressive fibrotic disease by persistently activating the innate immune response (2). Inherited genetic disorders, chronic autoimmune reactions, minor human leukocyte antigen mismatches in transplants, cardiovascular disease, high serum cholesterol levels, obesity, poorly controlled diabetes, and hypertension are also important triggers of fibrosis. In many fibrotic diseases, such as liver cirrhosis, cardiovascular fibrosis, systemic sclerosis, chronic kidney disease, and idiopathic pulmonary fibrosis, pathological tissue remodeling can progress to a stage in which organ failure and death are an inevitable outcome.
When injuries occur, dead or dying structural cells (e.g., epithelial and endothelial cells) release inflammatory mediators that initiate an antifibrinolytic coagulation cascade, which triggers platelet aggregation, clot formation, and development of a provisional ECM. Platelet degranulation also promotes vasodilation, increased blood vessel permeability, and production of enzymes known as matrix metalloproteinases (MMPs), which temporarily disrupt the basement membrane, allowing the efficient recruitment of inflammatory cells to the site of injury. Epithelial and endothelial cells also secrete growth factors, cytokines, and chemokines, which promote the recruitment and activation of leukocytes that participate in wound repair. During this initial inflammatory phase, macrophages and neutrophils debride the wound. They also produce soluble mediators that amplify the wound-healing response by recruiting T cells and other inflammatory cells. These cells, in turn, secrete various wound-healing/profibrotic cytokines, including platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β1, and interleukin (IL)-13 (3). These mediators transform local and recruited fibroblasts into α–smooth muscle actin (α-SMA)-expressing and ECM-producing myofibroblasts. Myofibroblasts are derived primarily from an extensive network of organ resident mesenchymal cells, which include cells known as fibroblasts and those known as pericytes due to their close relationship with the capillary wall. Once activated, myofibroblasts promote wound contraction, a process in which the edges of the wound are physically pulled toward the center. They also secrete factors that are mitogenic and chemotactic for epithelial and endothelial cells, which grow inward, forming new ECM and blood vessels as they migrate toward the center of the wound. In situations wherein the injury is repetitive, this normal wound-healing program becomes dysregulated, causing an excess deposition of ECM components (scar tissue) that progressively distorts normal tissue architecture.
Because myofibroblasts are the key pathogenic cells in all fibrotic diseases, research on the mechanisms of fibrosis has focused on identifying the cellular origins of myofibroblasts, the factors that activate myofibroblasts, and the mechanisms that contribute to myofibroblast deactivation and apoptosis. Recent studies have also identified critical roles for various macrophage subpopulations in the initiation, maintenance, and resolution phases of fibrosis (4). Macrophages are derived either from resident tissue populations or from bone marrow immigrants. In this review, we discuss the roles of the various myofibroblast and macrophage populations in fibrogenesis and briefly describe how this information might be used to generate the first generation of highly effective antifibrotic drugs.
MYOFIBROBLASTS AS EFFECTOR CELLS IN TISSUE REPAIR AND FIBROSIS
Myofibroblasts, originally described in skin wounds in 1972, are defined biochemically by their expression of actin-myosin proteins shared by smooth muscle cells (SMCs), morphologically by the formation of stress fibers, and physiologically by their ability to contract tissues (5, 6). Myofibroblasts are connective tissue fiber–forming cells, that is, cells that generate and deposit pathological ECM proteins and proteoglycans, known as fibrosis, that mature and organize into scar tissue. They accumulate in the virtual spaces between organ structures and produce ECM proteins (7), including collagen I and EDA-containing fibronectin (8). They also produce soluble mediators and reactive oxygen species (ROS). By virtue of these and other salient properties, myofibroblasts are strongly implicated as the primary “effector” cells in tissue remodeling and fibrosis. Myofibroblast activation may be sufficient to explain the cardinal morphologic features of fibrosis in diverse organ systems that are characterized by organ contraction (resulting in reduced size), loss of tissue architecture and cellular homeostasis, deposition of highly cross-linked ECM, and aberrant phenotypes of neighboring cells.
Myofibroblasts as Contractile Cells
Despite the lack of a unique set of biomarkers that define myofibroblasts, the functional capacity to contract tissues is an essential feature. Indeed, the commonly used marker α-SMA, although not specific, is essential for generating maximal myofibroblast contractile activity (9). Although both SMCs and myofibroblasts express α-SMA, their contractile activities are distinct. Whereas SMC contractions are reversible and short lived, myofibroblasts generate isometric contractions that are irreversible and long lived (10). Maintenance of isometric contraction by myofibroblasts is accomplished by a so-called lockstep mechanism that requires continual synthesis and remodeling of the ECM to sustain pericellular tension (10). Indeed, there is growing recognition that biomechanical signaling, via tissue “stiffness,” is a pivotal mechanism for promoting and sustaining the differentiated, contractile myofibroblast phenotype (11, 12). Recent studies indicate that increased matrix stiffness may be sufficient to activate latent TGF-β1 (13), which supports a positive feedback cycle that may perpetuate fibrogenesis. Relaxin, an endogenous peptide hormone, modulates fibrogenic responses to lung injury by inhibiting myofibroblast contractility (14).
Myofibroblasts in Matrix Deposition and Remodeling
Newly secreted matrix undergoes active remodeling by secreted MMPs and tissue inhibitors of metalloproteinases. Myofibroblasts “sense” the biochemical and biophysical properties of the ECM, primarily via cell-surface integrins. Matrix properties are also modified by the action of cross-linking enzymes such as lysyl oxidase (15) and transglutaminases (16). ECM cross-linking may also be mediated by oxidant-dependent mechanisms involving ROS-generating enzymes and extracellular peroxidases (17). Thus, dynamic changes in ECM deposition and remodeling “feed back” on the myofibroblast to regulate its activities, while also influencing the function and fate of neighboring cells.
ORIGINS OF MYOFIBROBLASTS
Historical Context
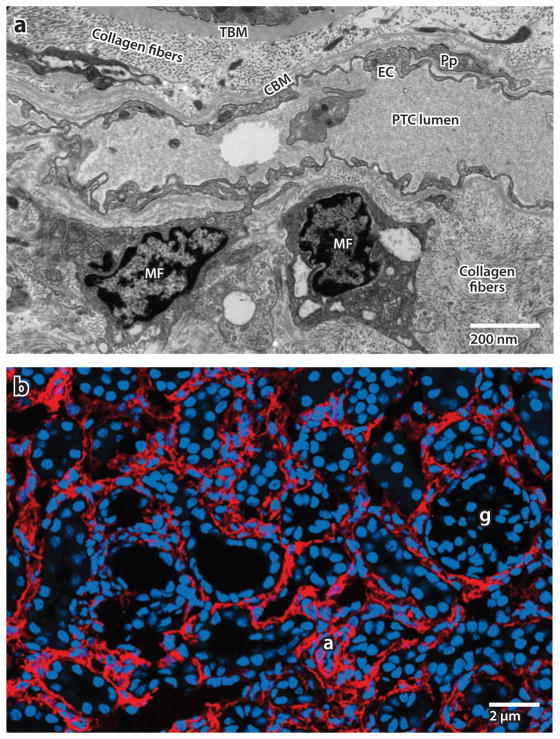
In historical studies dating back to 1867, principally in studies of skin wounding, myofibroblasts were referred to as wound fibroblasts (18) and, until the 1980s, were studied and defined by electron microscopy (EM). In 1970, a set of elegant experiments using parabiotic rats (rats surgically joined together so as to give them a common circulation) showed that wound fibroblasts do not arise from hematogenous precursors but rather are of local origin (18). Thus, the wound fibroblast, defined by EM in skin, derives from local mesenchyme cells. The term myofibroblast, coined in 1972 by Gabbiani & Majno (6) at the University of Geneva, Switzerland, from EM studies of skin, is perhaps now a misnomer because it implies a separate lineage of cells that appear in a diseased tissue; this implication has been refuted by recent fate mapping studies (see later in this section) (19–25). This terminology is, however, firmly embedded in the study of fibrosis and will persist. The distinction between wound fibroblasts and myofibroblasts is made by EM: Wound fibroblasts have dense rough endoplasmic reticulum (ER) and lack lysosomal vacuoles, whereas myofibroblasts also have modestly developed myofilaments with visible stress fibers (Figure 1) (6). In normal internal organs, activated myofibroblasts are believed to be absent.
Figure 1.
(a) Electron microscope image of myofibroblasts (MFs) in the interstitial space of a kidney from a patient with chronic kidney disease. Note the abundance of rough endoplasmic reticulum in these cells due to high ribosomal activity, and note the markedly expanded interstitial space with collagen fibers.
Abbreviations: CBM, capillary basement membrane; EC, endothelial cell; PTC, peritubular capillary; TBM, tubule basement membrane. (b) Confocal image of α–smooth muscle actin–expressing MFs (red ) in adult diseased mouse kidney. Abbreviations: a, arteriole; g, glomerulus.
The Quest for the Myofibroblast Precursor
In the late 1970s to 1980s, investigators found that antibodies that bind specifically to the smooth muscle marker α-SMA specifically label myofibroblasts in the skin. Subsequently, many common and rare diseases of internal organs were discovered to be characterized by fibrosis and the presence of cells equivalent to the skin myofibroblasts (Figure 1) (26–29). Other antibodies against quiescent fibroblasts in vitro, including the calcium binding S100A4 protein, fibroblast-specific protein 1 (FSP1), did not detect a resident population in the internal organs (30). Because of the apparent absence of mesenchymal progenitors in most internal organs, the quest for the myofibroblast progenitor has identified an extensive number of candidates: circulating cells derived from a stromal precursor possibly of bone marrow origin, circulating cells derived from a hematopoietic precursor, epithelial cells, and endothelial cells. Through this quest, investigators have gained many insights into the function, injury responses, and potential plasticity of the respective candidate progenitors. Until very recently, it was widely accepted that in several internal organs (e.g., liver, kidney, and lung), epithelial cells functioned as a primary source of myofibroblasts through a dedifferentiation process known as epithelial-to-mesenchymal transition (EMT), a process that was previously well recognized in embryogenesis (31). Also widely reported is evidence for leukocyte progenitors of solid organ myofibroblasts, which are now known as circulating fibrocytes (32–34). Less well established, but reported to occur in several organs including the heart, is the transition of endothelial cells to myofibroblasts (EndoMT) (35). However, the original studies that initially established the candidacy of these cell types as precursors for myofibroblasts were performed on cells cultured in vitro. In retrospect, these cells appear to have suffered from artifacts of cell culture: Almost all cells, when grown in plastic dishes, can be stimulated to activate α-SMA or FSP1 (S100A4) (22, 36–38), but that does not mean they are pathological fibrillar matrix–forming myofibroblasts in vivo. Moreover, FSP1 (S100A4), now widely used as a surrogate marker of EMT or EndoMT, is in fact highly expressed in subpopulations of activated monocyte–derived macrophages (not myofibroblasts) in vivo, both in liver and in kidney; this observation confounds interpretation of many studies (21, 22, 39). In addition, fate mapping studies that used Tie-1 or Tie-2 promoters to drive Cre recombinase suffered from the well-described activation of Tie-1 and Tie-2 in myeloid lineage cells, which led to an obvious source of artifact without the concurrent use of bone marrow chimerism (40, 41). These potential problems, among others, have been reported in several prominent review articles (42, 43).
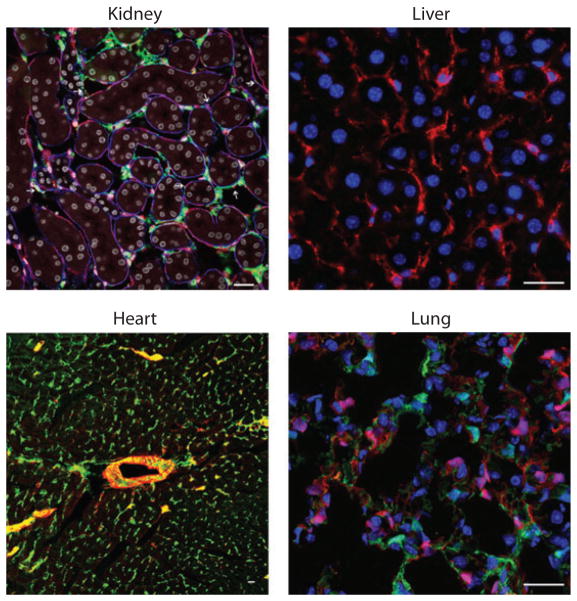
Although these cellular sources may be of merit and are difficult to completely discount, there is an extensive network of embryonic mesenchyme–derived cells throughout all of our internal organs, and these cells become activated in response to tissue injury (Figure 2). Recent advances in immunodetection, fixation methods, and novel genetic methods to study cells in mice have rendered this mesenchymal network much more visible to investigators. More importantly, state-of-the-art genetic fate mapping experiments in several organs, including kidney, lung, central nervous system, skin, liver, and skeletal muscle, now indicate that these mesenchyme-derived cells are the precursors of scar-forming myofibroblasts (19–25, 44–47), and many parallel genetic fate mapping studies show little or no evidence of direct differentiation of epithelial cells or leukocytes, or even endothelial cells, into myofibroblasts (Figure 3) (19–22, 42, 47–54).
Figure 2.
Mesenchyme cells in normal organs of the mouse. Confocal fluorescence images showing normal adult liver, kidney, lung, and heart with fluorescent labels of the resident mesenchyme cells. All the cells lack markers of leukocytes, endothelial cells, and epithelial cells. The kidney image shows pericytes ( green), endothelium (red ), basement membrane (blue), and nuclei (white). The liver image shows hepatic stellate cells labeled with anti-PDGFR-β (platelet-derived growth factor receptor β) antibodies (red ) and nuclei (blue). The heart image shows normal lung fibroblasts labeled with anti-PDGFR-β antibodies ( green) and SM22 (red ). The lung image shows alveolar spaces with two populations of stromal cells, one expressing collagen Iα1 ( green) and the other PDGFR-β (red ). Scale bars, 25 μm. Images reproduced courtesy of Dr. Michelle Tallquist, University of Texas, San Antonio.
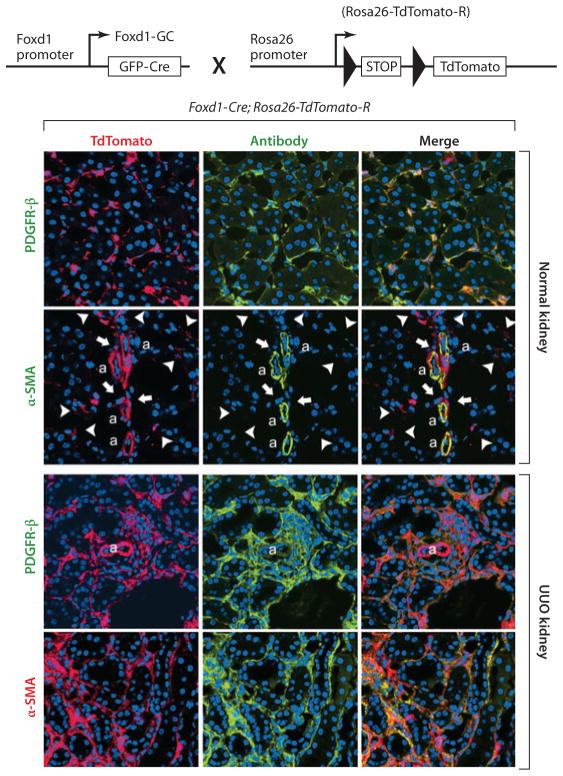
Figure 3.
Genetic fate mapping of mesenchymal progenitors in normal adult and injured kidney by use of the Foxd1-Cre;Rosa26-TdTomato-R mouse. (Top) The cross of the Foxd1–Cre recombinase allele with the TdTomato reporter allele, driven by the universal promoters at the Rosa26 locus. Bigenic mice recombine genomic DNA at the Rosa locus only in cells that have activated Foxd1 in nephrogenesis. (Bottom) Confocal images of kidney cortex in normal adult kidney show large numbers of perivascular cells, all of which coexpress platelet-derived growth factor receptor β (PDGFR-β). Vascular smooth muscle cells of the kidney arterioles are also derived from Foxd1 progenitors and coexpress α–smooth muscle actin (α-SMA) intermediate filament in normal kidney, but none of the Foxd1-derived pericytes (arrowheads) or perivascular fibroblasts (arrows) express α-SMA. In kidney injury [shown here is unilateral ureteral obstruction (UUO) day 7], the pericyte and perivascular fibroblast populations expand and continue to express PDGFR-β. However, the entire expanded population of interstitial Foxd1 progenitor–derived cells coexpress α-SMA, the marker that defines these cells as myofibroblasts. Abbreviations: a, arteriole; GFP, green fluorescent protein.
As of early 2012, there has been a sea change in our thinking about the origin of myofibroblast progenitors in the solid organs. Almost all myofibroblast progenitors probably derive from activation of the embryonic mesenchyme–derived cells within each of our organs. What remains unclear is what contribution, if any, myeloid leukocytes make to the pool of myofibroblasts. There are conflicting reports from studies on the extent of myeloid cells in injured organs that directly lay down pathological matrix, compared with the extent of myeloid cells that drive fibrosis by indirect mechanisms. Perhaps the most compelling studies are those using bone marrow chimera mice that express collagen Iα1–transgenic reporter in bone marrow–derived cells to show myeloid cells that actually make pathological collagen I protein. These studies in kidney, skin, and lung confirm that a rare (<0.1%) myeloid leukocyte subset that expresses CD45 and CD34, known as circulating fibrocytes, can generate low-level transcripts for the collagen I protein in kidney disease, but these cells do not become myofibroblasts (i.e., do not express α-SMA) and probably do not deposit fibrillar matrix (21, 54). In models of liver disease, however, such fibrocytes made up 5% of all collagen-producing cells, and a minority expressed the myofibroblast marker α-SMA (54). There may be organ-to-organ variation in myeloid cell differentiation that makes myeloid leukocytes more likely to directly lay down filamentous matrix. Regardless of such nuances, current data indicate that endothelial cells, epithelial cells, and myeloid leukocytes contribute to fibrogenesis predominantly by indirect mechanisms that involve cell-to-cell signaling, rather than differentiation into a new and distinct cell type.
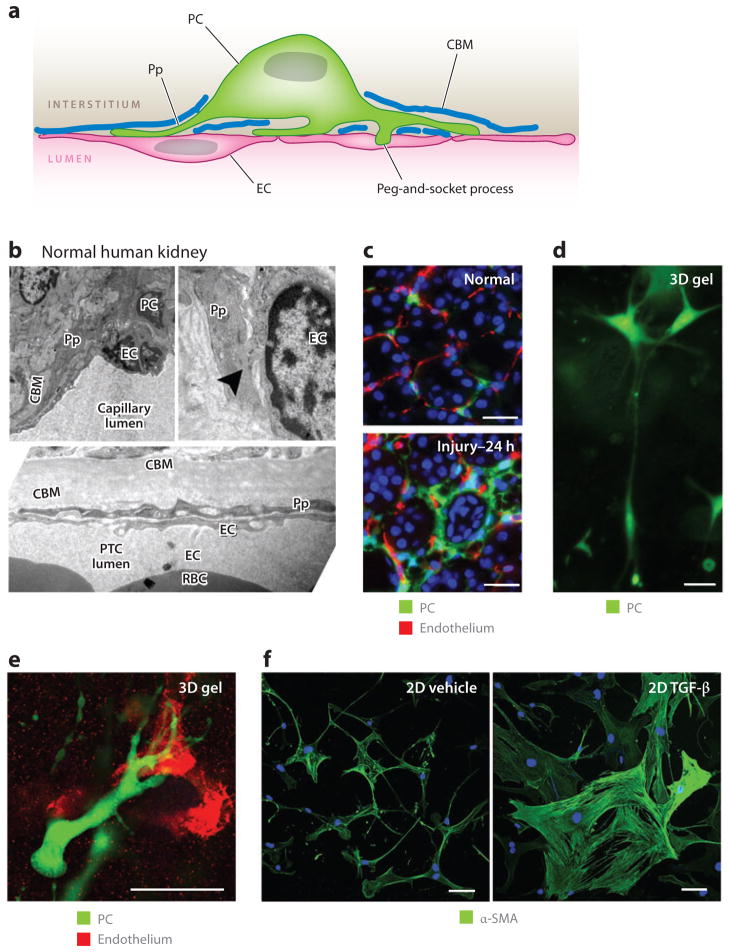
During embryogenesis, mesenchyme-derived cells form an important and large cellular component of the forming organs. Investigators had long assumed that these cells, known in embryonic development as stromal cells (which are embedded in loose connective tissue framework), essentially disappear in adult fully formed organs. However, recent studies indicate that these cells persist in large numbers; although they silence numerous gene markers, rather than become inactive or defunct, they probably play critical roles in organ homeostasis that are currently poorly or only partially understood (21–23, 42, 47, 55–60). In the quiescent state, these mesenchyme-derived cells are extremely discrete; they have extensive, extremely fine cell processes that extend over many cell lengths (Figure 4). During development, embryonic mesenchyme–derived stromal cells play critical roles in organogenesis, angiogenesis, and cell maturation. Strikingly, during embryonic development, these stromal cells express the same markers as myofibroblasts (including collagen transcripts and α-SMA) observed in adult organ disease, yet they do not make scar tissue; rather, one of their roles is to synthesize loose connective tissue or stroma (21). Therefore, it appears that (a) in development, the mesenchymal stromal cells are activated and migratory; (b) in the healthy mature organ, they are less activated and not migratory; but (c) in response to disease, they reactivate and become migratory again. The deposition of pathological filamentous matrix is only one of their functions.
Figure 4.
Pericytes (PCs) in the kidney: definitions, functions, and response to cytokines. (a) A PC attached to an endothelial cell (EC) and partially embedded in a duplication of the capillary basement membrane. Note the specific attachment of PC processes (Pp) and the cell body to the EC at several different sites. (b) Electron microscope images of normal human kidney cortex. Shown are peritubular capillaries (PTCs), in which ECs and PCs or Pp are visible. The tubule basement membrane is clearly visible. Note the peg-and-socket process (arrowhead ) at the upper right. (c) PCs attach to ECs in normal mouse kidney, but only 24 h (prior to mitosis) after the onset of obstructive injury, the PCs detach, spread, and migrate from the ECs. (d) PCs in three-dimensional (3D) collagen gel that exhibit long cytoplasmic processes that extend the length of more than 10 cell bodies. (e) PCs in 3D collagen gel home to and bind by attachment specifically to capillary tubes composed of endothelial cells. (f) Kidney PCs cultured on gelatin matrix, stained for α–smooth muscle actin (α-SMA). Note that, in control conditions, PCs show weak α-SMA expression and many long fine processes and elongations, but 24 h after exposure to transforming growth factor (TGF)-β, the PCs change shape, spread, lose their long cytoplasmic processes, upregulate α-SMA, and show distinct cytoplasmic filaments. Scale bars, 25 μm. Abbreviations: 2D, two-dimensional; CBM, capillary basement membrane; RBC, red blood cell.
Adult Mesenchymal Cells
The mature organ mesenchyme–derived cell populations (also known as stromal cells) bear remarkable similarities across organs but also have distinct differences. All these cell populations have long processes, which are characteristic of neurons and neuronally associated cells (Figure 4). They express many neuronal markers (Table 1), and although the stroma of each organ is widely believed to be derived from the mesenchyme that is local to that organ (and therefore is thought to be imprinted), there is tantalizing evidence that early neural crest precursors may migrate to and contribute to the mesenchymal precursors throughout the body (47, 61). Whereas the lymphoid subpopulations are well characterized, mesenchymal characterization is still in its infancy, held back by the failure over many years to adequately identify these cells and by the lack of a broad array of antibodies against cell-surface markers.
Table 1.
Markers expressed by resident mesenchymal cells, including cells known as pericytes and resident fibroblasts (differ between organs and between subpopulations)
| Restricted by cell type, expressed in homeostasis | Restricted by cell type, expressed only in activated state | Not restricted by cell type, expressed in homeostasis |
|---|---|---|
| PDGFR-β | NG2 | VEGFA |
| PDGFR-α | P75 NGFR | ANGPT1 |
| CD248 | α-SMA | CTGF |
| CD73a | ADAMTS1a | TIMP-3 |
| CD105 | CD44 | |
| Glial fibrillary acidic protein | ||
| Synaptophysin | ||
| gp38 (synaptopodin) | ||
| Sprouty1a | ||
| ColIa1 | ||
| RGS5 | ||
| Desmin |
Somewhat restricted. Abbreviations: CTGF, connective tissue growth factor; NGFR, nerve growth factor receptor; PDGFR, platelet-derived growth factor receptor; RGS, regulator of G protein signaling; SMA, smooth muscle actin; TIMP, tissue inhibitor of metalloproteinase; VEGF, vascular endothelial growth factor.
Pericytes
Pericytes, first described nearly 140 years ago (62), are mesenchymal cells that are defined electron micrographically by the direct communication between pericyte processes and endothelial cells. Pericytes are sheathed with basement membrane (Figure 4) (64–66), a duplication of capillary basement membrane (CBM). Almost all microvasculature has a discontinuous coating of mesenchymal cells known as mural cells or pericytes (Figure 4) (64, 65). Pericytes contribute to the synthesis and maintenance of the CBM (69). The CBM coverage between pericytes and endothelial cells is frequently incomplete, which allows for direct cell-cell contact (64–66), and invaginations in either cell type known as peg-and-socket junctions may contain tight, gap, and adherens junctions and may be sites of signaling (Figure 4). Although pericyte processes are always covered by the CBM, this coverage may be incomplete in the area of the cell body (62, 67, 68). Due to a lack of well-characterized markers, pericytes remain defined histologically by their relationship to the CBM. Given that most studies of resident fibroblasts do not visualize the CBM, many mesenchymal cells in solid organs that are reported as resident fibroblasts may also have pericyte functions.
Pericyte Functions
Over the past 20 years, studies of pericytes, derived predominantly from brain and eye, but more recently described in cancer biology, have established a broad range of functions for these cells that relate mainly to the vasculature. A key property of pericytes is that they migrate to and bind to capillaries (Figure 4). However, they are critical for building new blood vessels, stabilizing them, and actively regulating their functions (Table 2)
Table 2.
Pericyte functions in development and homeostasis
| Function | Reference(s) |
|---|---|
|
| |
| Migrate to and bind to capillaries | 87, 95 |
|
| |
| Regulate angiogenesis by cell cross talk involving PDGFR-β, Tie-2, TGF-β receptor, VEGFR, S1P | 98 |
|
| |
| Regulate vascular permeability | 89, 93 |
|
| |
| Regulate capillary flow by contractile functions | 85, 86 |
|
| |
| Stabilize vessels: | |
| Inhibit and regulate angiogenesis | — |
| Synthesize the CBM and stimulate ECs to deposit the CBM | 73, 87, 95–97 |
| Prevent hemorrhage, aneurysm, dilatation, rarefaction, EC death | 72, 90 |
Abbreviations: CBM, capillary basement membrane; EC, epithelial cell; PDGFR, platelet-derived growth factor receptor; S1P, sphingosine 1-phosphate; TGF, transforming growth factor; VEGFR, vascular endothelial growth factor receptor.
Pericytes as Myofibroblast Precursors
Although pericytes were described in the kidney 30 years ago (22, 67) and have been little studied until recently, it was studies in the kidney that first uncovered the pericyte origin of myofibroblasts (21, 22). Through the use of a transgenic mouse expressing green fluorescent protein (GFP) under the regulation of the collagen I promoter (Coll-GFP), numerous pericytes and fibroblasts that were closely associated with the vasculature were identified in normal kidney cortex and medulla. The pericytes were attached by multiple long processes to peritubular capillaries embedded in the CBM (Figure 4) (21, 70). In response to kidney injury, adult kidney pericytes detached from capillaries, rapidly upregulated collagen Iα1 expression, migrated from the endothelium, and reactivated expression of the pericyte markers P75 nerve growth factor receptor and α-SMA within a few hours of the onset of injury (Figure 4) (21). Four days after injury, the population of active, collagen Iα1–expressing interstitial pericyte cells had expanded markedly, and the vast majority of these cells activated the expression of α-SMA, thereby defining them as myofibroblasts. A mathematical kinetic analysis of the expansion of Coll-GFP-expressing pericytes with time after injury strongly suggested that the appearance of Coll-GFP-expressing myofibroblasts could be explained by detachment, migration, and expansion of the original population of mesenchyme-derived pericytes (21).
To characterize the fate of kidney pericytes more definitively, Humphreys et al. (22) adopted a genetic fate mapping approach. These authors generated mice expressing the DNA recombinase enzyme Cre at the loci of transcription factors expressed by discrete subpopulations of embryonic kidney precursors (stem cells) in metanephric mesenchyme (22). The Foxd1 transcription factor gene locus was selected because Foxd1 progenitors become kidney pericytes as well as mesangial cells and vascular SMCs (22, 36, 71–73). Expression of the Cre transgene under regulation of the Foxd1 locus in Rosa26 reporter mice allowed all pericytes, vascular SMCs, and mesangial cells of the kidney to be labeled (Figure 3) (22). In response to kidney injury (ischemia reperfusion injury, ureteral obstruction, or nephrotoxic serum nephritis), over 2 to 3 weeks there was a 15-fold increase in the Foxd1 reporter–labeled progeny, and all these cells activated the myofibroblast marker α-SMA (Figure 3) (22)—a fate that is identical to that of Coll-GFP cells in the Coll-GFP mouse (21). These findings strongly suggest that pericytes and perivascular fibroblasts are the predominant source of myofibroblasts in mouse kidney injury. The fate mapping findings are further supported by definitive cohort labeling obtained through the conditional, tamoxifen-sensitive, CreER recombinase at the Foxd1 locus (22).
Another research group identified the same cells by using a different reporter system, and these investigators drew similar conclusions: All myofibroblasts in kidney disease come from activation of the PDGF receptor (PDGFR)-β+ CD73+ mesenchymal cells found in the normal kidney. Not long after these experiments were performed, investigators studying spinal cord, lung, skin and skeletal muscle, and intestine also identified pericytes as the major population of myofibroblast precursors (21–23, 42, 47, 55–60, 74).
A role for the hepatic stellate cell (HSC) of liver in fibrogenesis has long been suspected (75). However only recently has the fate of HSCs as the major source of myofibroblasts been definitively established (19, 39, 48, 50, 76). In parallel with studies in the kidney, fate mapping studies have comprehensively demonstrated that epithelial cells are not a source of myofibroblasts but that, in most liver diseases, myofibroblasts derive almost exclusively from HSCs and SMCs of the larger vessels (19, 39, 48, 50, 76). These studies used Cre/Lox and inducible Cre systems to map epithelial cells, HSCs, or portal fibroblasts. Studies of biliary tract disease show that portal fibroblasts also contribute to the total population of myofibroblasts, although in this disease, HSCs are nevertheless major contributors to the total myofibroblast population. HSCs express PDGFR-β and glial fibrillary acidic protein but have a different lineage from that of Foxd1 progenitors in the kidney (19, 39, 48, 50, 76). HSCs lie in apposition with sinusoidal endothelial cells, and their long cell processes lie within a very loose CBM. They play an important homeostatic role in the maintenance of sinusoidal endothelial cell fenestrations by delivering vascular endothelial growth factor (VEGF); when they become myofibroblasts, this function is lost, which causes capillarization of the sinusoid (77). HSCs have direct angiogenic functions similar to those of pericytes (78, 79). HSCs are, therefore, a modified form of pericytes. Although much work remains to be done, it is likely that pericytes throughout the body are major sources of myofibroblast precursors.
RESIDENT FIBROBLASTS AND OTHER CELLS
In many tissues, including skin, heart, and lung, the presence of poorly defined resident fibroblasts has been well described, and these cells have been relatively easy to culture and study in vitro. However, until very recently the precise nature of resident fibroblasts [cells embedded in connective tissue (stroma) that produce collagen and other fibers] was poorly understood (70, 74). EM studies have revealed that many of these cells have close relationships with epithelial or endothelial cells. In lung development, the critical role of stromal cells in epithelial differentiation has been well described. Recent studies that have enabled easy visualization of these cells indicate that they are much more extensively distributed than previously thought and that they may have critical functions in homeostasis, including pericyte functions. Many resident fibroblasts also have close associations with epithelial cells and may function as epithelial pericytes.
Surrounding the arterioles are perivascular fibroblasts or fibrocytes (21, 70, 74). These cells may be termed adventitial cells in other texts, and evidence suggests they have important immunomodulatory functions and that some serve as vessel wall progenitors (80). In some tissues, including skin and liver, some resident adult microvascular wall mesenchymal cells (i.e., pericytes) have progenitor cell functions (81); that is, they can differentiate into mature cells of the tissue, including vascular smooth muscle, white adipocytes, and possibly neurons. It is unclear whether all adult mesenchyme cells have this ability to act as progenitors for other cell types within their organ, or whether there are more restricted subpopulations of perivascular cells that have this capacity.
Studies over the past several years have investigated some of the signaling pathways involved in pericyte-to-myofibroblast transition. Although this area is rapidly evolving, it appears that the same signaling pathways that regulate angiogenesis in cross talk between endothelial cells and pericytes are critical in pericyte and fibroblast activation. Those pathways include TGF-β, PDGFR-β, PDGFR-α, and VEGF receptor 2 (VEGFR2). Indeed, loss-of-function studies of these pathways have shown them to be critical in the early events of pericyte activation, the appearance of myofibroblasts, and fibrogenesis. Other developmental pathways that are important in building the vasculature may also be significant in the development of a myofibroblast. These pathways include angiopoietin signaling; sphingosine kinase signaling; and the developmental pathways WNT, Hedgehog, and Notch. Current evidence suggests that in development these signaling pathways are carefully regulated but that in disease they are markedly dysregulated, resulting in an overactivated phenotype relative to development. It may be desirable to “dial down” these over-activated developmental signaling pathways to counteract the appearance of myofibroblasts in tissues. Also, extracellular regulators of the VEGFR signaling pathway and metalloproteinases play important roles in the activation of pericyte function. Pericytes must detach from capillaries, spread, and migrate and must withdraw from the CBM, which requires proteolytic activity. One such factor that is activated strongly and early after injury in kidney pericytes is the metalloproteinase ADAMTS1 (87). In different organs, distinct metalloproteinases probably play important roles in pericyte acquisition of the myofibroblast phenotype, and these metalloproteinases may prove to be useful targets in reversal of phenotype.
EPITHELIAL-TO-MESENCHYMAL TRANSITION VERSUS EPITHELIAL SIGNALING IN FIBROGENESIS
Although recent comprehensive studies (of up to 3 months’ duration) in animal disease models indicate that epithelial cells do not become scar-forming myofibroblasts in solid organs (20), ample data suggest that activation or injury to epithelial cells alone is sufficient to drive fibrogenesis in many organs, including liver, lung, and kidney (82). Injured epithelial cells undoubtedly undergo profound phenotypic changes, which include the acquisition of a migratory phenotype (83). However, in the relevant animal models, this activated migratory phenotype is restricted to migration to areas of denuded or damaged epithelial basement membrane, where repair and restitution occur. This activated migratory phenotype in response to injury may be termed EMT, given that injured epithelial cells (a) activate a program of gene expression, which includes the transcription factor SNAIL and the intermediate filament vimentin, and (b) acquire a more mesenchymal appearance and migrate. To many investigators, EMT has become synonymous with the appearance of myofibroblasts in the interstitial space. Furthermore, injured epithelial cells do not express many of the genes that characterize the embryonic mesenchyme, and they do activate many genes that are not developmentally active.
Injured epithelial cells share only a few features with the embryonic mesenchyme. Increasing examples of epithelial stress, including ER stress, dysregulation of energy metabolism, and arrest in G2 of the cell cycle, are sufficient to activate intracellular signaling programs that confer a profibrotic phenotype on the epithelial cell without necessarily activating the developmental EMT genes (84). Although there is no consensus, perhaps the term EMT should be restricted to situations wherein the EMT transcriptional program is selectively activated, rather than describing all activated, injured epithelial cells that are profibrotic. Only malignant epithelial cells migrate outside the confines of the epithelial structure. In the absence of epithelial cell differentiation into the cells that actually deposit pathological fibrillar matrix between cell structures, the epithelium must signal to the interstitial and perivascular spaces to activate stromal cells to become myofibroblasts.
Thanks to many years of EMT research, we know of several candidate molecules that drive fibrogenesis and may act as soluble factors that activate the pericyte and fibroblast populations (Table 3) (Figure 5). One of these soluble factors is TGF-β. Indeed, antibodies against TGF-β and antibodies against the TGF-β-activating αvβ6 integrin are in clinical trials as antifibrotics. Injured epithelial cells are a major source of both cytokines and activating integrin receptors. TGF-β acts as an important factor in vascular development through the signaling of endothelial cells to pericytes (85, 86). In fact, TGF-β mutant mice and TGF-β-activated kinase mutant mice die during fetal development due to vascular defects (87–89). Many other epithelium-derived factors probably play a role in epithelial cell signaling to the perivascular and interstitial spaces.
Table 3.
Candidate epithelial signaling molecules
| Developmental pathway factors | Cytokines | Chemokines, small-molecular mediators |
|---|---|---|
| Hedgehog | IL-1β | CCL3–6 |
| VEGF | Oxygen radicals | CCL8 |
| PDGF | IL-6 | |
| TGF-β | S1P | |
| CTGF family | Nitric oxide | |
| WNT | TNF-α | |
| Notch | ||
| FGF |
Abbreviations: CTGF, connective tissue growth factor; FGF, fibroblast growth factor; IL, interleukin; PDGF, platelet-derived growth factor; S1P, sphingosine 1-phosphate; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
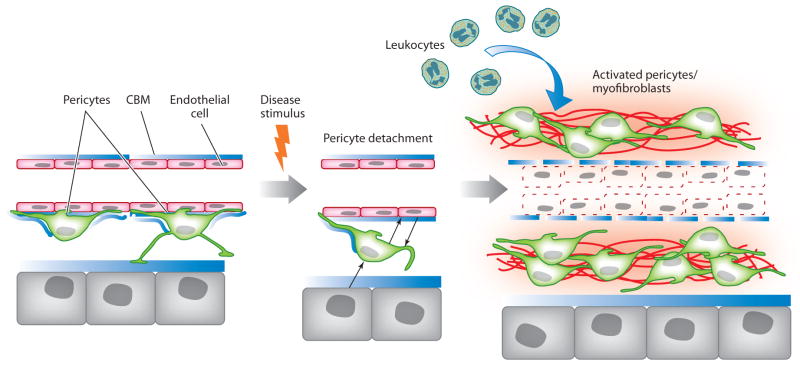
Figure 5.
Schema of pericyte activation by a disease stimulus (based around kidney injury). In response to injury, pericytes become activated and detach from capillaries. This process requires bidirectional signaling between endothelial cells and pericytes. Epithelial cells can also signal to pericytes, and it is unknown whether pericytes signal to epithelial cells. In the presence of persistent injury, activated pericytes proliferate, migrate, and activate genes that give them the myofibroblast phenotype, including upregulated expression of pathological matrix genes, contractile machinery, and immune response genes. This process results in pathological matrix deposition in the virtual interstitial space; recruitment of inflammatory cells; and the loss of pericyte coverage of the endothelial cells, which causes an unstable endothelium that in turn leads to dysangiogenesis and, potentially, rarefaction. Abbreviation: CBM, capillary basement membrane.
Recent studies indicate that active TGF-β is an important cytokine in pericyte- and fibroblast-to-myofibroblast transition, but across different organ settings it does not stimulate proliferation (90). Another epithelial factor that can signal to pericytes or fibroblasts is PDGF. PDGF signaling from the endothelium to pericytes is also critical in vascular development, particularly through PDGFR-β (91, 92). Injured or activated epithelial cells are a potent source of PDGFs, and the PDGFR signaling pathways are important in the initiation and progression of fibrogenesis in response to tissue injury (70, 90, 93). Several studies have implicated connective tissue growth factor (CTGF) and CyR61, two related ECM proteoglycans in fibrogenesis, and these factors are upregulated in injured epithelial cells across several organs. Other potential factors that are currently under scrutiny are the developmental signaling pathways Notch, WNT, and Hedgehog, as well as the nerve growth factor signaling pathway (94–96). Clearly, injured epithelium is a source of ligands for each of these pathways, and they may be important factors in driving fibrogenesis. In addition to developmental signaling pathways, epithelial cells are significant sources of proinflammatory cytokines and chemokines, which contribute directly to fibrogenesis in several organs (97).
EVIDENCE FOR INNATE IMMUNE RESPONSES IN MYOFIBROBLASTS AND THEIR STROMAL PROGENITORS
New lines of investigation have highlighted an important but previously poorly appreciated function of activated mesenchymal cells: their role as innate immune cells. Myofibroblasts in the liver, which we now know derive either from HSCs or from biliary duct fibroblasts, are highly phagocytic, and the glomerular myofibroblast precursor in the kidney, the mesangial cell, is also highly phagocytic (98, 99). Although myofibroblasts lack the specialized machinery of macrophages, this immune function is an important component of their activated state. Whether this function reflects simply a clearance pathway for bacteria and tissue debris, or whether activated mesenchymal cells have antigen presentation roles, remains to be determined.
In recent gain-of-function studies using mutant, constitutively active PDGFR-β, Olson & Soriano (81) showed that PDGFR-β-activated brain pericytes in newborn mice generated many immune response factors. The most enriched factors were antigen presentation genes, pattern-recognition receptor (PRR) genes, interferon (IFN) signaling, chemokines, and the complement pathway (Table 4). This pattern of expression was associated with marked recruitment of leukocytes to the perivascular area. These observations are complemented by studies of PDGFR-β blockade in adult kidney injury. PDGFR-β blockade prevents pericyte activation in the injured kidney and prevents myofibroblast appearance, fibrosis, and microvascular rarefaction. However, blockade of this single receptor, which is restricted to mesenchymal cells in kidney, is potently anti-inflammatory, causing a 70% reduction in leukocyte recruitment to the injured kidney (70, 90).
Table 4.
Immune response gene categories activated in pericytes in response to injury or platelet-derived growth factor receptor (PDGFR) signaling
| Adult kidney pericytes in response to organ injury | Newborn brain pericytes in response to PDGFR-β overactivity |
|---|---|
| Immune response | Antigen presentation pathway |
| Response to wounding | Interferon signaling |
| Defense response | Dendritic cell maturation |
| Inflammatory response | Pattern-recognition receptors |
| Response to stress | Complement system |
| Regulation of cytokine production | |
| Innate immune response | |
| Adaptive immune response | |
| Cytokine activity | |
| Chemotaxis |
Similar to the developmental studies, a more recent transcriptional array of adult kidney pericytes in vivo has shown that activated pericytes generate a wide range of inflammatory response genes (Table 4) (87). Together with results from recent studies of skin pericytes or skin myofibroblasts (100), these data indicate that activated pericytes and activated fibroblasts have critical immune response functions that had previously been unappreciated. Strikingly, chemokine receptor blockade in liver fibrosis exerts anti-inflammatory effects and antifibrotic effects through blockade of chemokine signaling in liver myofibroblasts (97). Anti-inflammatory strategies that have been directed at leukocytes may confer unexpected benefits by additionally targeting inappropriately activated mesenchymal cells.
THE FATE OF MYOFIBROBLASTS: KEY DETERMINANT BETWEEN NORMAL REPAIR AND FIBROSIS
The activation of tissue myofibroblasts is a highly conserved and stereotypic response to injury and normal wound healing (7, 101). Resolution of the reparative phase of wound healing requires apoptotic clearance of myofibroblasts (102). The mechanisms that cause myofibroblast apoptosis in physiologic wound repair, however, remain unclear; similarly, the mechanisms underlying an apparently apoptosis-resistant myofibroblast phenotype in progressive fibrotic disorders are not well defined (103).
In addition to inducing myofibroblast differentiation, TGF-β1 itself promotes myofibroblast survival. TGF-β1 activates two prosurvival signaling pathways, focal adhesion kinase and PKB/AKT, by mechanisms that involve cell adhesion and release of soluble growth factors, respectively (104, 105), and contribute combinatorially to myofibroblast survival (106). Importantly, the administration of a protein kinase inhibitor that modulates the activities of these prosurvival pathways attenuates fibrosis in a model of bleomycin-induced lung fibrosis (107). Therapeutic approaches that induce apoptosis of myofibroblasts and/or interfere with myofibroblast contractility and tissue stiffness may prove effective in the treatment of progressive fibrotic disorders.
Myofibroblasts are highly synthetic and secretory cells that elaborate a wide range of soluble lipid mediators and ROS, as well as cytokines and chemokines. This property has led to the characterization of myofibroblasts as inflammatory cells (108). Myofibroblasts may directly participate in innate immunity by responding to bacterial components via Toll-like receptors and activating inflammatory responses (109). Myofibroblasts may acquire an immune-privileged phenotype by expressing Fas ligand (FasL), killing Fas+ lymphocytes, and resisting Fas-induced apoptosis (110). Paracrine effects of FasL-expressing myofibroblasts may also induce apoptosis of adjacent epithelial cells (111); additional myofibroblast-secreted factors implicated in epithelial cell death include angiotensin peptides (112), TGF-β1 (113), and hydrogen peroxide (H2O2) (114).
NOX ENZYMES AND REACTIVE OXYGEN SPECIES IN INNATE IMMUNITY AND FIBROSIS
NADPH oxidase (NOX) enzymes and ROS play essential roles in innate immunity and host defense against pathogens across the plant and animal kingdoms. In plants, the generation of NOX-dependent ROS at the site of pathogen contact elicits both a localized hypersensitivity response and a systemic immune response (115, 116); this localized response includes ECM cross-linking, akin to a fibrotic response, to limit the spread of the pathogen. In fish, wounding triggers activation of a NOX family enzyme, DUOX, in epithelial cells; DUOX releases H2O2, thereby serving as both a local microbicidal agent and a neutrophil chemoattractant (117).
For several decades, investigators have known that the phagocyte NOX2 enzyme plays a key role in mammalian innate immunity by mediating ROS-dependent pathogen killing (118). More recently, a family of NOX enzymes that participate in expanded roles in innate immunity and other physiological functions was identified (119, 120). To date, seven isoforms of NOX enzymes have been identified in mammals (120).
Fibroblasts may express multiple NOX isoforms; however, the NOX4 isoform has been specifically implicated in myofibroblast differentiation and lung fibrosis (121–123). Transcriptomal analyses (using Affymetrix GeneChips) of human lung fibroblasts treated with TGF-β1 (123) identified NOX4 as one of the most highly upregulated genes. NOX4 activation mediates the generation of H2O2, myofibroblast differentiation, contractility, and ECM production in human lung fibroblasts (123). In tissues from human idiopathic pulmonary fibrosis (IPF) patients, the expression of NOX4 is localized to myofibroblasts, both within fibroblastic foci and in epithelial cells associated with aberrant bronchiolization (123, 124). Therapeutic delivery of NOX4 small interfering RNA protects against fibrosis in two different animal models of injury-provoked pulmonary fibrosis (123); protection has also been observed in mice with genetic deficiency in NOX4 (124). In addition to its effects on mesenchymal cells, NOX4 may induce apoptosis of lung epithelial cells, thereby contributing to lung fibrosis (124). These observations, in addition to NOX4’s potential roles in vascular remodeling and pulmonary hypertension (125, 126), suggest that NOX4 mediates effects on multiple cell types and tissue compartments that contribute to organ fibrosis.
CYTOKINES THAT REGULATE MYOFIBROBLAST ACTIVATION
The proinflammatory cytokines tumor necrosis factor (TNF)-α and IL-1β are important drivers of fibrosis (127). TNF-α was identified as a key player in murine models of silica-and bleomycin-induced pulmonary fibrosis (128, 129). TNF-α may also play a critical role in radiation-induced fibrosis, Crohn’s disease–induced intestinal fibrosis, CCL4- and cholestasis-induced liver fibrosis, and nonalcoholic steatohepatitis (NASH) (130–133). A similar role has been established for IL-1β and NALP3/ASC inflammasome signaling (134–136). Pulmonary fibrosis induced by bleomycin and silica, liver fibrosis in hypercholesterolemic mice, renal interstitial fibrosis arising from unilateral ureteric obstruction, and cardiovascular fibrosis following myocardial infarction were reduced in IL-1β-deficient mice (137–141). Like TNF-α, IL-1β is a potent proinflammatory mediator that promotes epithelial changes including EMT and myofibroblast activation through a TGF-β1-dependent mechanism (142, 143). Both cytokines also induce the production of IL-6, which exhibits autocrine growth factor activity in fibroblasts (144, 145). IL-6 is an important mediator of fibrosis in diffuse systemic sclerosis, liver fibrosis following CCL4 exposure, and fibrosis in chronic cardiac allograft rejection (146–148). Thus, several proinflammatory cytokines appear to participate in the pathogenesis of fibrosis by promoting the differentiation and activation of myofibroblasts. Consequently, clinical trials have been initiated to evaluate whether neutralizing antibodies to TNF-α or other proinflammatory cytokines could be beneficial in the treatment of pulmonary fibrosis and other fibrotic diseases (149).
The CD4+ T helper 17 (Th17) cell subset that expresses the proinflammatory cytokine IL-17A is also emerging as an important initiator of fibrosis. IL-17A expression has been implicated in the pathogenesis of pulmonary fibrosis (141, 150, 151), chronic allograft rejection (152, 153), fibrosis in orthotopic lung transplantation (154), myocardial fibrosis (155), and hepatitis-induced hepatic fibrosis (156). In most cases, IL-17A expression is associated with significant neutrophil recruitment (157, 158), and exaggerated neutrophil responses may contribute to the development of fibrosis by triggering vascular endothelial cell apoptosis (159). Importantly, neutrophilia is a reliable predictor of early mortality in IPF patients (160). Mechanistic studies investigating the IL-17 pathway of fibrosis identified the proinflammatory cytokine IL-1β and the Th17-inducing cytokine IL-23 as important upstream initiators of profibrotic Th17 responses (141, 161). A link between IL-17A expression and the profibrotic cytokine TGF-β1 has also been identified (141, 153, 162). TGF-β1 is important for the development of the Th17 response in mice exposed to bleomycin, whereas the development of IL-17-driven fibrosis depends on TGF-β1. In addition to its role in promoting inflammation, IL-17A directly induces MMP-1 expression in primary human cardiac fibroblasts (163), which suggests that IL-17A promotes fibrosis both by exacerbating the neutrophil-dominated inflammatory response and by regulating the downstream activation of fibroblasts. Together, these data characterize the IL-1β/IL-17A/TGF-β1 cytokine axis as an important pathway driving the development of fibrosis.
Numerous studies have suggested that the type 2 cytokine response can also serve as a key driver of progressive fibrosis (164–169). Th2 responses are defined by the production of IL-4, IL-5, and IL-13 (170), and although all three cytokines have been linked with the development of fibrosis in various model systems (171–173), IL-13 has emerged as the dominant mediator of fibrotic tissue remodeling in several experimental and natural models of fibrosis (173, 174). IL-13 is implicated in the development of fibrosis in chronic asthma (174, 175), IPF (176), models of experimental lung fibrosis (177), systemic sclerosis (178), atopic dermatitis–induced skin fibrosis (179, 180), radiation-induced fibrosis (181), intestinal fibrosis associated with Crohn’s disease and ulcerative colitis (182), and liver fibrosis resulting from persistent infections (173, 183) and from NASH (184). Mechanistically, IL-13 induces fibrosis by stimulating the production and activation of TGF-β (185, 186). However, studies have suggested that IL-13 can also promote fibrosis independently of TGF-β (93, 187). Indeed, IL-13 directly stimulates the synthetic and proliferative properties of fibroblasts, epithelial cells, and SMCs (173, 176, 186, 188–193). Additional mechanistic studies have established that the profibrotic activity of IL-13 is controlled by the relative expression of IL-13Rα1 (signaling receptor) versus IL-13Rα2 (decoy receptor) on important target cells such as myofibroblasts, epithelial cells, and SMCs (194, 195). When decoy receptor expression (IL-13Rα2) is low on these cells, IL-13-dependent fibrosis is exacerbated due to enhanced IL-13/IL-13Rα1-mediated signaling (196). These findings suggest that therapeutics that target the IL-13 pathway could be a viable strategy to ameliorate fibrosis.
THE ROLE OF MONOCYTE AND MACROPHAGE POPULATIONS IN FIBROSIS PROGRESSION AND RESOLUTION
At a basic level, all multicellular organisms recognize injuries through the neoepitope exposure of normally sequestered intracellular biochemical molecules. Examples include specific phospholipids that are normally expressed on the inner cell membrane that flip to the outer cell membrane during stress and apoptosis (e.g., phosphotidyl serine and phosphotidyl ethanolamine), as well as nuclear components such as DNA, histones, and other nuclear proteins that are exposed only during necrotic or apoptotic cell death. These so-called damage-associated molecular patterns (DAMPs) are up-regulated at sites of injury and/or infection and stimulate a highly conserved innate cellular response controlled by monocyte-derived cells. Whether this innate cellular response causes mild acute inflammation and healing, robust chronic inflammation and autoimmunity, or subchronic inflammation and fibrosis is determined by the type of cellular debris, the cellular activation pathways that are used to recognize these basic DAMP signals, and the surrounding cytokine and growth factor milieu in which they are found.
Monocyte-derived cell populations (macrophages, dendritic cells, and fibrocytes) can dynamically control the fibrotic process through both direct effects on matrix remodeling and indirect effects on the regulation of activated myofibroblasts, their precursor populations, and endothelial cells (4, 197–203). Monocyte-derived cells play an important role in inflammation, and the subsequent development of fibrosis, in a range of organ pathologies (4, 199–201, 204–208). For example, macrophages and fibrocytes in vivo are often found in close association with collagen-producing myofibroblasts, and alternatively they can produce cytokines and growth factors that either stimulate or suppress myofibroblast activity. Importantly, the identification of distinct functional subsets of macrophages (M1, inflammatory; M2a-like, profibrotic; Mreg/M2c-like, regulatory) (4, 198, 200, 201, 203, 205–207) and their relative impact on fibrosis progression and resolution indicate that the equilibrium between these different macrophage populations and other monocyte-derived cells (such as fibrocytes) probably determines whether the outcome of an injury response is productive reepithelialization and healing or pathogenic scarring.
Monocytes promote progression of fibrotic disease through differentiation into M2a-like macrophages and fibrocytes that produce various fibroblast stimulatory growth factors and cytokines, such as TGF-β1, PDGF, FGF2 (fibroblast growth factor 2), insulin-like growth factor–binding protein 5, CCL18, and Galectin-3 (Figure 6) (4, 208–216); increased levels of these macrophage-secreted factors can constitute peripheral biomarkers of fibrotic disease progression (217). Through the stimulation of additional local tissue injury, M1-type macrophages may also provoke a fibrotic wound-healing response in the neighboring tissue cells that is independent of the production of cytokines that drive myofibroblast activation. Indeed, conditional ablation of macrophages at early stages of fibrosis blocks fibrosis progression in several fibrosis model systems (4, 206, 218). Many individual and redundant stimuli, including cytokines such as IL-4 and IL-13, growth factors such as macrophage colony-stimulating factor, and chemokines such as CCL17 and CCL2, contribute to the milieu in fibrosis that activates the differentiation of monocytes into the downstream fibrocyte and profibrotic macrophage cell populations (4, 219–223). In addition, once activated, the fibrocytes and profibrotic macrophages amplify the level and number of profibrotic cytokines and growth factors produced, thereby driving and accelerating myofibroblast activation (Figure 6).
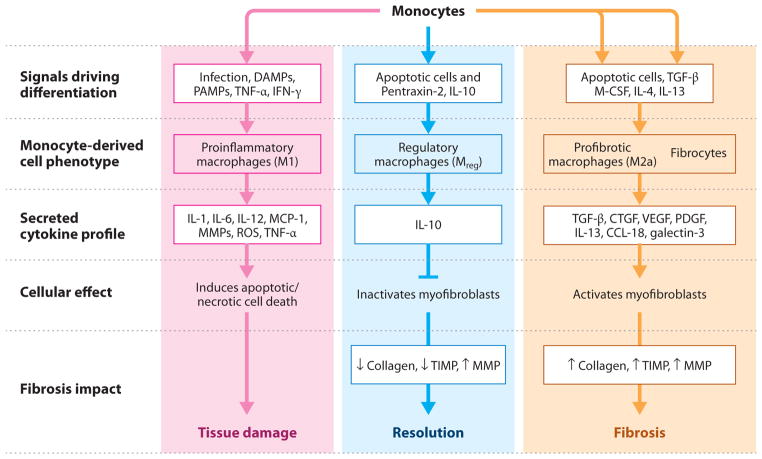
Figure 6.
Monocyte/macrophage activation pathways involved in fibrosis progression and resolution. Monocytes are recruited to sites of tissue injury and differentiate into distinct specialized effector macrophage populations, depending on the extracellular milieu present at the site of injury. These different effector cell populations can have dramatically different impacts on fibrosis initiation, propagation, and resolution. (Left) Monocytes promote the initiation of fibrosis through differentiation into M1-type macrophages that release cytokines and reactive oxygen species (ROS) that cause additional local tissue injury, and they promote myofibroblast resistance to apoptosis. (Center) Monocytes promote the resolution of fibrotic disease through differentiation into regulatory macrophages (Mreg) that inactivate myofibroblasts and inhibit M1- and M2-type macrophages through local production of interleukin (IL)-10 and/or Arginase-1. (Right) Monocytes promote the progression of fibrotic disease through differentiation into profibrotic (M2a-like) macrophages and fibrocytes that produce various fibroblast stimulatory growth factors and cytokines. Abbreviations: CTGF, connective tissue growth factor; DAMP, damage-associated molecular pattern; IFN, interferon; M-CSF, macrophage colony-stimulating factor; MCP, monocyte chemotactic protein; MMP, matrix metalloproteinase; PAMP, pathogen-associated molecular pattern; PDGF, platelet-derived growth factor; TIMP, tissue inhibitor of metalloproteinase; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Fibrocytes constitute a distinct subset of collagen-producing, fibroblast-like cells derived from peripheral-blood monocytes that enter sites of tissue injury to promote angiogenesis, scar production, and collagen contraction (202, 224). They differentiate from a CD14+ peripheral-blood monocyte precursor population and express markers of both hematopoietic cells (CD45, major histocompatibility class II, CD34 cells) and stromal cells (collagen I and III and fibronectin). In humans, fibrocytes have been detected in fibrotic tissue from many different sources, including cutaneous wounds, hypertrophic scars, scleroderma skin lesions, asthma, IPF, nephrogenic fibrosing dermopathy, and solid tumors (202, 224). Increased levels of fibrocyte precursors have also been detected in the peripheral blood of IPF patients and scleroderma patients with lung fibrosis (225–227). Interestingly, subsequent studies in IPF patients indicated a significant correlation between higher fibrocyte levels in blood and both exacerbation of disease and mortality (228). In animal models, fibrocytes are associated with experimental fibrosis induced by irradiation damage, bleomycin injections into skin or lung, intimal hyperplasia of the carotid artery, systemic acetaminophen administration, chronic granuloma formation following Schistosoma japonicum infection, and cutaneous wounds (202, 224). They have also been detected in other organs, including diseased kidney and liver, but their role in the direct deposition of fibrogenic matrix in these organ settings is controversial (21, 204). Fibrocytes may represent a subset of macrophages and dendritic cells, similar to M2a macrophages that exert profibrotic effects by mechanisms other than direct deposition of pathological matrix (21).
The identification of Mreg/M2c-like macrophages that can promote resolution of fibrotic disease is a relatively recent advance in the field of fibrotic research. Macrophage depletion at the start of the recovery phase of liver injury severely suppresses ECM degradation and the reduction in myofibroblasts necessary for resolution (205), whereas transfer of nonfibrotic macrophages into mice reduces fibrosis in both the kidney and lung fibrosis models (222, 229). Macrophage-mediated resolution of liver fibrosis has also been directly linked to the production of MMP-13 (207), and MMP-9 overexpression in macrophages substantially reduces lung fibrosis (230). In models of liver fibrosis, macrophage phagocytosis of apoptotic hepatocytes also reduces inflammation and prevents the development of fibrosis (231), and phagocytosis of apoptotic cholangiocytes reverses existing fibrosis (232). A defining marker of regulatory macrophage function is the secretion of IL-10; direct IL-10 treatment, genetically modified or transfused IL-10-stimulated macrophages, or in vivo induction of macrophage IL-10 expression can ameliorate fibrosis and inflammation in kidney, gut, and brain (201, 204, 233–235). Arginase-1-expressing M2 macrophages have also been implicated in the amelioration of liver fibrosis induced by chronic S. mansoni infection (236). Therefore, monocytes can promote resolution of fibrotic disease by differentiating into regulatory macrophages that locally produce suppressor cytokines, including IL-10, by producing MMPs that can directly degrade interstitial collagen (e.g., MMP-1, MMP-2, MMP-8, MMP-9, and MMP-13), by locally depleting essential amino acids required for T cell and fibroblast proliferation, by actively promoting apoptosis of myofibroblasts, and by phagocytosing ECM and cellular debris that would otherwise stimulate inflammatory and fibrotic cell activation. This critical discovery has led investigators to search for mechanisms through which regulatory macrophage activity can be stimulated or amplified to naturally treat fibrotic disease.
THE ROLE OF PENTRAXIN-2 IN FIBROSIS REGULATION
Pentraxin-2 (PTX-2), commonly referred to as serum amyloid P, is a naturally circulating plasma protein and a soluble PRR of the innate immune system that controls monocyte differentiation and activation in response to damaged tissue. PTX-2 potently inhibits the differentiation of M2a-like macrophages and fibrocytes (34, 222), as well as M1 macrophages (237), while promoting the differentiation of IL-10-secreting Mreg/M2c-like macrophages (204, 222, 238). The unique pentameric structure of PTX-2 allows recognition of DAMP ligands through one face of the molecule to localize PTX-2 specifically to damaged tissue at sites of injury (204), and subsequent Fcγ receptor (FcγR) binding to the opposite face of the molecule promotes phagocytosis (239–244) and regulatory monocyte function at those sites (200, 204).
Initial studies in preclinical models of pulmonary fibrosis demonstrated that purified PTX-2 exerts potent therapeutic effects in blocking fibrotic collagen deposition; these effects strongly correlated with both improved lung function and decreased numbers of fibrocytes, macrophages, and myofibroblasts in the lung (245). Subsequent studies confirmed that PTX-2 has preventative and therapeutic antifibrotic effects in multiple models of fibrosis in lung (218, 222, 227, 245), heart (246, 247), skin (33), and kidney (204, 248), as well as strong anti-inflammatory effects in an EAE (experimental autoimmune encephalomyelitis) model of multiple sclerosis (237). Importantly, the therapeutic effect of PTX-2 was consistent across both acute and chronic model systems; the widely varying modes of induction included obstructive injury, toxin exposure, reperfusion, allergic autoimmunity, and chronic transgenic TGF-β1 expression. In animals that have been treated with PTX-2, macrophages isolated from injured kidney (204), monocytes stimulated in vitro with cytokine cocktails (222), and macrophages analyzed in situ from injured lung (218, 222, 227) demonstrate a substantial suppression of profibrotic and proinflammatory activation markers. PTX-2 promoted a regulatory macrophage phenotype associated with increased expression of the antifibrotic cytokine IL-10 (204, 222, 227, 248) and the antifibrotic chemokine IP-10 (IFN-γ-induced protein 10) (218). Furthermore, the therapeutic effect of PTX-2 on kidney fibrosis was attenuated in IL-10−/−, whereas IL-10 transgenic expression was itself therapeutic in vivo and directly inhibited myofibroblast expression of collagen in vitro (204). PTX-2 therapeutic activity was also substantially reduced in both cardiac (247) and kidney (204) fibrosis models when FcγR signaling was compromised (FcγRγ−/− mice), and adoptive transfer of monocytes treated in vitro with PTX-2 was therapeutic in reducing fibrosis in a fungus-induced asthma model (222). Together, these data indicate that PTX-2 couples recognition of DAMP ligands bound to one face of the molecule with binding to monocyte FcγRs at the opposite face of the pentraxin molecule, thereby affecting monocyte signaling events specifically at the sites of tissue injury (204, 249) and promoting a proresolution macrophage phenotype.
Initial human studies have also demonstrated a potential association between decreased PTX-2 levels in blood and fibrotic pathology. Patient and control samples were collected in two independent studies, and the serum levels of PTX-2 were determined (204, 218). In the circulation of patients with kidney disease, the level of PTX-2 was lower than in patients with minimal kidney disease (204), and it correlated with the glomerular filtration rate (a measure of kidney function). This finding suggests that, like complement proteins, binding and turnover at sites of inflammation may lead to consumption from the circulation (204). Human kidney biopsy specimens were also tested and showed deposition of PTX-2 in areas of damage in a range of kidney diseases (200, 204). PTX-2 concentrations were also reduced in the circulation of patients with histologically confirmed IPF, compared with concentrations in age-matched controls, and in the sera of IPF patients inversely correlated with disease severity (218). Given the associations between dysregulated M2 monocyte and fibrocyte levels that were also observed in IPF patients (202, 213, 217, 225–228), these data suggest that an inverse relationship may exist between PTX-2 levels in patients and insufficiently controlled monocyte biology in human fibrotic disease and that potential therapeutic intervention with PTX-2 may be warranted. A fully recombinant form of the human PTX-2 protein, PRM-151, is currently in multiple human clinical trials of fibrotic disease (http://www.promedior.com).
Several other signaling pathways in dendritic cells and macrophages regulate the monocyte response to activation by pathogen-associated molecular patterns (PAMPs) or DAMPs. Studies in several organs show that the canonical WNT signaling pathway transcriptional regulator β-catenin can play a pivotal role in myeloid cells in the switch to an Mreg-type leukocyte in response to DAMP or PAMP activation. This transcriptional regulator may be activated by numerous cell signaling pathways, including the WNT pathway, as well as by other signaling pathways that act through the inhibitory cytosolic protein glycogen synthase kinase 3β (250). The effect of manipulating this pathway in myeloid cells in chronic inflammation and the consequences for fibrosis have not been established. Recent studies have established that signaling through the autophagy pathway in macrophages can promote an Mreg phenotype by mechanisms that are not yet understood (251, 252). Promoting signaling through these pathways during tissue injury may promote resolution of inflammation and inhibit fibrogenesis.
THERAPEUTIC APPROACHES TO THE TREATMENT OF FIBROSIS
On the basis of the large number of biochemical pathways reviewed in the sections above, an equally large number of therapeutic approaches to treat fibrotic pathology are currently in various stages of development (Table 5). To date, despite the enormous unmet medical need for an effective antifibrotic agent, the only approved drug treatment for fibrotic pathology in the United States is direct injection of collagenase (http://www.Xiaflex.com) into the joints of patients with Dupuytren’s contracture, a proliferative fibrotic disorder that involves the palmar fascia of the hand, causing the fingers to curl. All other potential drug products are still undergoing clinical trials to prove their efficacy and safety.
Table 5.
Antifibrotics in clinical development
| Company | Compound | Mechanism of action | Route | Indications | Status of project |
|---|---|---|---|---|---|
| Intermune, Shionogi | Pirfenidone | Unknown, reduces growth factors/cytokines | Oral | IPF, DN, FSGS | Approved in Japan and Europe for IPF, Phase III for IPF in United States, Phase II for DN and FSGS |
| Intermune | ActImmune™ | Recombinant human IFN- | Subcut. | IPF | Failed in Phase III for IPF |
| Actelion Pharmaceuticals | Bosentan (Tracleer™) | Endothelin antagonist | Oral | IPF | Failed in Phase III for IPF |
| Gilead | Ambrisentan (Letiaris) | Endothelin antagonist | Oral | IPF | Phase III |
| Pfizer | Sildenafil (Revatio) | PDE5 inhibitor | Oral | PAH in IPF | Phase III |
| Boehringer Ingelheim | BIBF-1120 (Vargatef ) | VEGFR, FGFR, PDGFR inhibitor | Oral | IPF | Phase III |
| Abbott/Reatta | Bardoxolone methyl | Nrf2 agonist, PDJ2 mimetic | Oral | CKD in DN | Phase III |
| Novartis | Imatinib mesylate (Gleevec) | PDGFR-α/β inhibitor | Oral | IPF, DN | Failed in Phase II for IPF, Phase II for DN |
| Celgene | Thalidomide (Thalomid) | Unknown, reduces collagen production | Oral | IPF, MF | Phase II in IPF, Phase II in MF |
| Celgene | CC-930 | JNK inhibitor | Oral | IPF | Phase II |
| Gilead/Arresto | GS-6624 | Anti-LOXL2 monoclonal antibody | IV | MF, LF, IPF | Phase II in MF, Phase I/II in LF, and Phase I in IPF |
| Amarillo Biosciences | IFN-α | Anti-inflammatory | Oral | IPF | Phase II |
| Wyeth | Etanercept (Enbrel) | Soluble TNF-α receptor | Subcut. | IPF | Phase II |
| Cornerstone Therapeutics/Skyepharma | Zileuton (Zyflo) | Leukotriene synthesis inhibitor, 5-LO inhibitor | Oral | IPF | Phase II |
| Adeona/Pipex Therapeutics | Tetrathiomolybdate (Coprexa) | Copper chelator | Oral | IPF, DN | Phase II |
| Centocor | CNTO-888 | Anti-CCL2 monoclonal antibody | IV | IPF | Phase II |
| Novartis | QAX576 | IL-13 inhibitor | IV | IPF, asthma | Phase II |
| Pharmaxis | PXS25 | Leukocyte extravasation inhibitor | IV | IPF | Phase II |
| Mondobiotech | Vasoactive intestinal peptide | Anti-inflammatory | Inhaled | IPF | Phase II |
| Lilly | LY2382770 | Anti-TGF-β monoclonal antibody | Subcut. | DN | Phase II |
| Genzyme | GC-1008 (fresolimumab) | Anti-TGF-β monoclonal antibody | IV | IPF, MF | Phase II in IPF, Phase I in MF |
| FibroGen | FG-3019 | Anti-CTGF human monoclonal antibody | IV | IPF, DN, LF in HBV | Phase II in IPF, Phase II in DN, Phase II in LF |
| Insys Therapeutics | Cintredekin besudotox | IL-13 receptor+ cell destruction | Inhaled | IPF | Phase II, appears discontinued |
| Stromedix | STX-100 | Anti–αvβ6 integrin monoclonal antibody | IV | IPF | Phase Ib/IIa |
| Promedior | PRM-151 | Recombinant human SAP, monocyte inhibitor | IV | IPF | Phase Ib |
| Bristol-Myers Squibb | — | Anti-IL-4/IL-13 receptor monoclonal antibody | IV | IPF | Phase I |
Abbreviations: CTGF, connective tissue growth factor; DN, diabetic nephropathy; FGFR, fibroblast growth factor receptor; FSGS, focal segmental glomerulsclerosis; IFN, interferon; IL, interleukin; IPF, idiopathic pulmonary fibrosis; IV, intravenous; LF, liver fibrosis; MF, myelofibrosis; PAH, pulmonary arterial hypertension; PDE, phosphodiesterase; PDGFR, platelet-derived growth factor receptor; SAP, serum amyloid protein; subcut., subcutaneous; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGFR, vascular endothelial growth factor receptor; HBV, hepatitis B virus infection.
One busy clinical area has been the study of treatments for IPF. Notably, outside the United States another drug, pirfenidone (http://www.intermune.com/pirfenidone), a relatively primitive p38 kinase inhibitor that reduces TGF-β synthesis, has been approved for the treatment of IPF in Japan (trade name, PirespaTM) and more recently in Europe (trade name, EsbrietTM). Interestingly, the use of pirfenidone in the United States was rejected by the Food and Drug Administration in 2011 due to failure in one of two Phase III clinical studies; this drug is currently being retested in a new Phase III clinical trial. Some other notable failures in clinical development for IPF include recombinant human IFN-γ (trade name, ActImmuneTM; http://www.intermune.com), bosentan (trade name, TracleerTM, an endothelin antagonist; http://www.actelion.us), and imatinib mesylate (trade name, Gleevec, a mixed kinase inhibitor with selectivity for PDGFRs, Abl, and c-kit; http://www.novartis.com).
However, there is evidence that other molecules are making progress in the clinic. Vargatef (BIBF-1120, a mixed kinase inhibitor with selectivity for PDGFR, VEGFR, and FGF receptor; http://www.boehringer-ingelheim.com) recently showed a statistically significant delay in the rate of decline in lung function in IPF patients in a Phase II study and has advanced into Phase III testing. Many additional drugs based on more recently identified targets are in the initial stages of development; these include drugs that target inhibition of the TGF-β1, IL-13, LPA (lysophosphatidic acid), CTGF, αvβ6 integrin, Galectin-3, LOXL2, transglutaminase 2, NOX4, and JNK pathways, as well as those that stimulate the PGE2 (prostaglandin 2), HGF (hepatocyte growth factor), Nrf2, and PTX-2 pathways (Table 5). Only time will tell which pathways and molecules show the best promise, but given our rapid progress in the field of fibrotic research, the future looks bright indeed.
CONCLUSIONS
Although myofibroblasts from distinct organ sources are the primary drivers of fibrosis, it is becoming increasingly clear that various monocyte and macrophage populations also play key roles in the pathogenesis of fibrosis. Inflammatory macrophages promote fibrosis by producing specific MMPs, such as MMP-2 and MMP-9, that degrade the basement membrane, allowing inflammatory cells and fibroblasts to migrate into areas of tissue injury. They also produce ROS and nitrogen species that are toxic to invading organisms. However, these cells also induce significant bystander damage to neighboring healthy tissues if they are not quickly controlled. Macrophages subsequently take on features of wound-healing macrophages. These cells remove cellular debris that would otherwise perpetuate the inflammatory response, antagonize the activity of M1 macrophages, engulf and digest ECM components, and induce the transformation of fibroblasts into ECM-secreting myofibroblasts. They also secrete various profibrotic mediators, such as TGF-β1, PDGF, and chemokines that recruit and activate cells involved in wound repair. In the final phase, macrophages take on a regulatory phenotype and express various mediators, such as IL-10, MMP-13, Relma-α, PD-L2, and Arginase-1, which are critical to the resolution of the fibrotic response and can direct the reversal of established fibrosis. Thus, future efforts should focus on better characterizing the macrophage subpopulations that induce, maintain, suppress, and ultimately reverse the profibrotic activity of myofibroblasts. This information could help guide the development of novel antifibrotic treatments, which would be based on modifying the wound-healing response so that tissue regeneration is favored over pathological fibrosis.
SUMMARY POINTS.
Myofibroblasts are the primary effector cells in tissue remodeling and fibrosis.
Genetic fate mapping experiments suggest that mesenchyme-derived cells known as resident fibroblasts and pericytes are the primary precursors of scar-forming myofibroblasts.
Epithelial cells, endothelial cells, and myeloid leukocytes (fibrocytes) contribute to fibrogenesis by producing key fibrogenic cytokines and by promoting cell-to-cell communication.
Hematopoietic cells participate in the activation of myofibroblasts by producing various cytokines, including TGF-β1, TNF-α, IL-1, IL-6, PDGF, IL-17A, and IL-13.
Increased matrix stiffness can also perpetuate the activation of myofibroblasts by promoting the activation of latent TGF-β1.
Resolution of the reparative phase of wound healing requires apoptotic clearance of myofibroblasts. Therapeutic approaches that induce apoptosis of myofibroblasts and/or interfere with myofibroblast contractility and tissue stiffness may prove effective in progressive fibrotic disorders.
Circulating bone marrow–derived monocytes differentiate into macrophages and dendritic cells and can regulate all stages of fibrogenesis and repair. They can amplify epithelial cell damage by functioning as classical inflammatory cells; they can promote myofibroblast activation and proliferation through release of profibrotic cytokines and growth factors; and they can also reverse the disease by dismantling and degrading established fibrosis and by stimulating inactivation of myofibroblasts through production of IL-10.
Many antifibrotic drugs are at various stages of development, including those targeting the TGF-β1, IL-13, LPA, CTGF, αvβ6 integrin, Galectin-3, LOXL2, transglutaminase-2, NOX4, and JNK pathways, as well as those stimulating the PGE2, HGF, Nrf2, and PTX-2 pathways.
Acknowledgments
J.S.D. is funded by National Institutes of Health (NIH) grants DK84077, DK87389, and DK93493; a University of Washington Genzyme Research-in-Progress Grant; the Nephcure Foundation; and a research agreement from Regulus Therapeutics. V.J.T. is funded by NIH grants HL067967 and HL107181. T.A.W. is supported by the intramural research program of the National Institute of Allergy and Infectious Diseases/NIH, and his research has benefited from cooperative research and development agreements with Pfizer, MedImmune, Amgen, Regeneron, Centocor, and Genentech. The authors thank Dr. Shuyu Ren, Dr. Claudia Schrimpf, and Dr. Michelle Tallquist for generating images.
Glossary
- ECM
extracellular matrix
- Matrix metalloproteinases (MMPs)
proteases that degrade ECM proteins, process numerous bioactive molecules, and cleave cell-surface receptors
- TGF
transforming growth factor
- α-SMA
α–smooth muscle actin
- Pericytes
cells that surround the endothelial cell layers of the capillary network and play a critical role in scar formation
- ROS
reactive oxygen species
- Epithelial-to-mesenchymal transition (EMT)
a process in which epithelial cells are stimulated to differentiate into myofibroblast-like cells
- Fibrocyte
a monocyte-derived cell that expresses both hematopoietic cell markers (CD34 and CD45) and collagen I
- GFP
green fluorescent protein
- NADPH oxidase 4 (NOX4)
an enzyme that produces ROS that exhibit antimicrobial activity but promote myofibroblast differentiation
- IPF
idiopathic pulmonary fibrosis
- Pentraxin-2 (PTX-2)
a naturally circulating plasma protein that can inhibit the differentiation of profibrotic macrophages and fibrocytes
Footnotes
DISCLOSURE STATEMENT
V.J.T. holds a patent with the University of Michigan on treatment of fibrotic diseases with RNAi approaches targeting NOX4. T.A.W. holds patents related to the targeting of IL-13 and IL-21. J.S.D. holds patents related to the targeting of DKK proteins and bone marrow progenitor cells in the treatment of fibrotic diseases. J.S.D. is on the scientific advisory board of Regulus Therapeutics and Promedior, Inc. M.L. holds patents related to the use of PTX-2 therapeutics and is an employee of Promedior, Inc.
LITERATURE CITED
- 1.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burmolle M, Thomsen TR, Fazli M, Dige I, Christensen L, et al. Biofilms in chronic infections—a matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59:324–36. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabbiani G, Majno G. Dupuytren’s contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972;66:131–46. [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of α–smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–63. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castella LF, Buscemi L, Godbout C, Meister JJ, Hinz B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci. 2010;123:1751–60. doi: 10.1242/jcs.066795. [DOI] [PubMed] [Google Scholar]
- 11.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–61. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 13.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Gai Y, Yang N, Lu B, Samuel CS, et al. Relaxin regulates myofibroblast contractility and protects against lung fibrosis. Am J Pathol. 2011;179:2751–65. doi: 10.1016/j.ajpath.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150:497–507. [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, et al. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:699–707. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larios JM, Budhiraja R, Fanburg BL, Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor β1–stimulated fibroblasts. J Biol Chem. 2001;276:17437–41. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 18.Ross R, Everett NB, Tyler R. Wound healing and collagen formation. VI The origin of the wound fibroblast studied in parabiosis. J Cell Biol. 1970;44:645–54. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–98. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–36. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S-L, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–27. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. Shows that pericytes are the predominant ECM-producing myofibroblasts in kidney fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:e1475–83. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffield JS. Starting the scar: a primary role for pericytes? Nat Med. 2011;17:1052–53. doi: 10.1038/nm0911-1052. [DOI] [PubMed] [Google Scholar]
- 25.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol. 1992;2:190–97S. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph R, McClure WJ, Woodward M. Contractile fibroblasts in chronic alcoholic cirrhosis. Gastroenterology. 1979;76:704–9. [PubMed] [Google Scholar]
- 28.Rudolph R. Inhibition of myofibroblasts by skin grafts. Plast Reconstr Surg. 1979;63:473–80. doi: 10.1097/00006534-197904000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Bioulac-Sage P, Quinton A, Saric J, Grimaud JA, Mourey MS, Balabaud C. Chance discovery of hepatic fibrosis in patient with asymptomatic hypervitaminosis A. Arch Pathol Lab Med. 1988;112:505–9. [PubMed] [Google Scholar]
- 30.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat. 1996;156:173–86. doi: 10.1159/000147845. [DOI] [PubMed] [Google Scholar]
- 32.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 33.Naik-Mathuria B, Pilling D, Crawford JR, Gay AN, Smith CW, et al. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16:266–73. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 36.Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011;38:417–23. doi: 10.1111/j.1440-1681.2011.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123:335–46. doi: 10.1007/s00418-005-0788-z. [DOI] [PubMed] [Google Scholar]
- 38.Mlodzik K, Loffing J, Le Hir M, Kaissling B. Ecto-5′-nucleotidase is expressed by pericytes and fibroblasts in the rat heart. Histochem Cell Biol. 1995;103:227–36. doi: 10.1007/BF01454028. [DOI] [PubMed] [Google Scholar]
- 39.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA. 2011;108:308–13. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–76. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 41.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Investig. 2011;121:468–74. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–53. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 44.Peduto L, Reuter VE, Sehara-Fujisawa A, Shaffer DR, Scher HI, Blobel CP. ADAM12 is highly expressed in carcinoma-associated stroma and is required for mouse prostate tumor progression. Oncogene. 2006;25:5462–66. doi: 10.1038/sj.onc.1209536. [DOI] [PubMed] [Google Scholar]
- 45.Dulauroy S, Vives FL, Eberl G, Peduto L. Lineage tracing of ADAM12+ perivascular cells reveals a dominant profibrotic pathway in injured tissues. Nat Med. 2012;18 doi: 10.1038/nm.2848. In press. [DOI] [PubMed] [Google Scholar]
- 46.Rojas A, Chang FC, Lin SL, Duffield JS. Contribution of perivascular cells in kidney interstitial injury. Clin Nephrol. 2012;77:400–8. doi: 10.5414/CN107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asada N, Takase M, Nakamura J, Oguchi A, Asada M, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Investig. 2011;121:3981–90. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–95. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology. 2010;51:737–40. doi: 10.1002/hep.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffield JS. Epithelial to mesenchymal transition in injury of solid organs: fact or artifact? Gastroenterology. 2010;139:1081–83. doi: 10.1053/j.gastro.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, et al. Tubular overexpression of transforming growth factor β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–43. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Investig. 2010;120:4040–54. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–78. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, et al. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179:189–98. doi: 10.1016/j.ajpath.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2010;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 56.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geerts A, Niki T, Hellemans K, De Craemer D, Van Den Berg K, et al. Purification of rat hepatic stellate cells by side scatter–activated cell sorting. Hepatology. 1998;27:590–98. doi: 10.1002/hep.510270238. [DOI] [PubMed] [Google Scholar]
- 58.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, et al. Platelet-derived growth factor receptor β signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103:1393–401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–37. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2010;138:371–84. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith CL, Tallquist MD. PDGF function in diverse neural crest cell populations. Cell Adhes Migr. 2010;4:561–66. doi: 10.4161/cam.4.4.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouget C. Memoire sur le developpement, la structure et les proprietés physiologiques des capillaires sanguins et lymphatiques. Arch Physiol Norm Pathol. 1873;5:603–63. [Google Scholar]
- 63.Zimmerman K. Der feinere Bau der Blutcapillaren. Z Anat Entwickl. 1923;68:29–36. [Google Scholar]
- 64.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 65.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 66.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2009;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 67.Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- 68.Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–73. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- 69.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–23. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol. 2012;27:183–93. doi: 10.1007/s00467-011-1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor BF-2. Genes Dev. 1996;10:1467–78. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H, Palmer R, Gao X, Kreidberg J, Gerald W, et al. Transcriptional activation of placental growth factor by the forkhead/winged helix transcription factor FoxD1. Curr Biol. 2003;13:1625–29. doi: 10.1016/j.cub.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 74.Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarstrom C, et al. Inflammatory bowel disease–associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–15. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–62. [PubMed] [Google Scholar]
- 76.Kisseleva T, Cong M, Paik Y-H, Scholten D, Jiang C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–53. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaffner F, Popper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–42. [PubMed] [Google Scholar]
- 78.Das A, Shergill U, Thakur L, Sinha S, Urrutia R, et al. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk-dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol. 2010;298:908–15. doi: 10.1152/ajpgi.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–79. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–54. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olson LE, Soriano P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20:815–26. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II–mediated renal injury. Am J Physiol Renal Physiol. 2012;302:742–49. doi: 10.1152/ajprenal.00583.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–43. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schor SL. Fibroblast subpopulations as accelerators of tumor progression: the role of migration stimulating factor. EXS. 1995;74:273–96. doi: 10.1007/978-3-0348-9070-0_14. [DOI] [PubMed] [Google Scholar]
- 86.Sato S, Kubota T, Suzuki Y. Composition and function of noncollagenous proteins in alveolar bone. Bull Kanagawa Dent Coll. 1990;18:119–25. [PubMed] [Google Scholar]
- 87.Jadrich JL, O’Connor MB, Coucouvanis E. The TGFβ activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–41. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- 88.Oshima M, Oshima H, Taketo MM. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 89.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor β 1 knock out mice. Development. 1995;121:1845–54. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 90.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–81. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 91.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-β-deficient mice. Science. 1997;277:242–45. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 92.Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–73. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Meyer C, Muller A, Herweck F, Li Q, et al. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J Immunol. 2011;187:2814–23. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 94.Fabian SL, Penchev RR, Jacques BS, Rao AN, Sipila P, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180:1441–53. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dill MT, Rothweiler S, Djonov V, Hlushchuk R, Tornillo L, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:967–77. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 97.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Investig. 2009;119:1858–70. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–29. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 99.Hughes J, Liu Y, Van Damme J, Savill J. Human glomerular mesangial cell phagocytosis of apoptotic neutrophils: mediation by a novel CD36-independent vitronectin receptor/thrombospondin recognition mechanism that is uncoupled from chemokine secretion. J Immunol. 1997;158:4389–97. [PubMed] [Google Scholar]
- 100.Sargent JL, Milano A, Bhattacharyya S, Varga J, Connolly MK, et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Investig Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981;54:183–94. [PubMed] [Google Scholar]
- 102.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 103.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–56. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, et al. Myofibroblast differentiation by transforming growth factor β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–89. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 105.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, et al. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor β1 in mesenchymal cells is mediated by p38 MAPK–dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–67. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, et al. Combinatorial activation of FAK and AKT by transforming growth factor β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–71. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–75. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Phan SH, Zhang K, Zhang HY, Gharaee-Kermani M. The myofibroblast as an inflammatory cell in pulmonary fibrosis. Curr Top Pathol. 1999;93:173–82. doi: 10.1007/978-3-642-58456-5_18. [DOI] [PubMed] [Google Scholar]
- 109.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–78. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 110.Wallach-Dayan SB, Golan-Gerstl R, Breuer R. Evasion of myofibroblasts from immune surveillance: a mechanism for tissue fibrosis. Proc Natl Acad Sci USA. 2007;104:20460–65. doi: 10.1073/pnas.0705582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Golan-Gerstl R, Wallach-Dayan SB, Amir G, Breuer R. Epithelial cell apoptosis by Fas ligand–positive myofibroblasts in lung fibrosis. Am J Respir Cell Mol Biol. 2007;36:270–75. doi: 10.1165/rcmb.2006-0133OC. [DOI] [PubMed] [Google Scholar]
- 112.Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, et al. Human lung myofibroblast–derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol Lung Cell Mol Physiol. 1999;277:1158–64. doi: 10.1152/ajplung.1999.277.6.L1158. [DOI] [PubMed] [Google Scholar]
- 113.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, et al. TGF-β1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168:6470–78. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 114.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, et al. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–56. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 115.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–93. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 116.Shirasu K, Dixon RA, Lamb C. Signal transduction in plant immunity. Curr Opin Immunol. 1996;8:3–7. doi: 10.1016/s0952-7915(96)80097-5. [DOI] [PubMed] [Google Scholar]
- 117.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–99. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Babior BM, Curnutte JT, McMurrich BJ. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Investig. 1976;58:989–96. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 120.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 121.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, et al. NAD(P)H oxidase 4 mediates transforming growth factor β1–induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 122.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–38. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, et al. NADPH oxidase 4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–81. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–19. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pache JC, Carnesecchi S, Deffert C, Donati Y, Herrmann FR, et al. NOX-4 is expressed in thickened pulmonary arteries in idiopathic pulmonary fibrosis. Nat Med. 2011;17:31–32. doi: 10.1038/nm0111-31. [DOI] [PubMed] [Google Scholar]
- 126.Mittal M, Roth M, Konig P, Hofmann S, Dony E, et al. Hypoxia-dependent regulation of non-phagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–67. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1β and tumor necrosis factor α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol. 1993;150:4188–96. [PubMed] [Google Scholar]
- 128.Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–47. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- 129.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–63. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nawroth I, Alsner J, Behlke MA, Besenbacher F, Overgaard J, et al. Intraperitoneal administration of chitosan/DsiRNA nanoparticles targeting TNFαprevents radiation-induced fibrosis. Radiother Oncol. 2010;97:143–48. doi: 10.1016/j.radonc.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 131.Bahcecioglu IH, Koca SS, Poyrazoglu OK, Yalniz M, Ozercan IH, et al. Hepatoprotective effect of infliximab, an anti-TNF-α agent, on carbon tetrachloride–induced hepatic fibrosis. Inflammation. 2008;31:215–21. doi: 10.1007/s10753-008-9067-1. [DOI] [PubMed] [Google Scholar]
- 132.Gabele E, Froh M, Arteel GE, Uesugi T, Hellerbrand C, et al. TNFα is required for cholestasis-induced liver fibrosis in the mouse. Biochem Biophys Res Commun. 2009;378:348–53. doi: 10.1016/j.bbrc.2008.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, et al. Tumour necrosis factor α signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–24. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–54. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gasse P, Mary C, Guenon I, Noulin N, Charron S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Investig. 2007;117:3786–99. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–44. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Piguet PF, Vesin C, Grau GE, Thompson RC. Interleukin 1 receptor antagonist (IL-1RA) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine. 1993;5:57–61. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- 138.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp. 2009;57:165–76. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones LK, O’Sullivan KM, Semple T, Kuligowski MP, Fukami K, et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24:3024–32. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 140.Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55:1086–94. doi: 10.1016/j.jhep.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, et al. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–52. doi: 10.1084/jem.20092121. Identifies the IL-1/IL-17A/TGF-β1 cytokine axis as a critical driver of bleomycin-induced pulmonary fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gobe G, Johnson DW. Interleukin-1β induces human proximal tubule cell injury, α–smooth muscle actin expression and fibronectin production. Kidney Int. 2002;62:31–40. doi: 10.1046/j.1523-1755.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- 143.Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor β1–dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–31. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 144.Lonnemann G, Engler-Blum G, Muller GA, Koch KM, Dinarello CA. Cytokines in human renal interstitial fibrosis. II Intrinsic interleukin (IL)-1 synthesis and IL-1-dependent production of IL-6 and IL-8 by cultured kidney fibroblasts. Kidney Int. 1995;47:845–54. doi: 10.1038/ki.1995.127. [DOI] [PubMed] [Google Scholar]
- 145.Lonnemann G, Shapiro L, Engler-Blum G, Muller GA, Koch KM, Dinarello CA. Cytokines in human renal interstitial fibrosis. I Interleukin-1 is a paracrine growth factor for cultured fibrosis–derived kidney fibroblasts. Kidney Int. 1995;47:837–44. doi: 10.1038/ki.1995.126. [DOI] [PubMed] [Google Scholar]
- 146.Natsume M, Tsuji H, Harada A, Akiyama M, Yano T, et al. Attenuated liver fibrosis and depressed serum albumin levels in carbon tetrachloride–treated IL-6-deficient mice. J Leukoc Biol. 1999;66:601–8. [PubMed] [Google Scholar]
- 147.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transpl. 2009;9:1773–83. doi: 10.1111/j.1600-6143.2009.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–55. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 150.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–65. [PMC free article] [PubMed] [Google Scholar]
- 151.Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, et al. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2009;179:705–16. doi: 10.1164/rccm.200811-1700OC. [DOI] [PubMed] [Google Scholar]
- 152.Dudas PL, Sague SL, Elloso MM, Farrell FX. Proinflammatory/profibrotic effects of interleukin-17A on human proximal tubule epithelium. Nephron Exp Nephrol. 2011;117:e114–23. doi: 10.1159/000320177. [DOI] [PubMed] [Google Scholar]
- 153.Faust SM, Lu G, Marini BL, Zou W, Gordon D, et al. Role of T cell TGFβ signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183:7297–306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–22. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feng W, Li W, Liu W, Wang F, Li Y, Yan W. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol. 2009;87:212–18. doi: 10.1016/j.yexmp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Wang LY, Chen SJ, Xu KS. IL-17 expression is correlated with hepatitis B–related liver diseases and fibrosis. Int J Mol Med. 2011;27:385–92. doi: 10.3892/ijmm.2011.594. [DOI] [PubMed] [Google Scholar]
- 157.Miyamoto M, Prause O, Sjöstrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–72. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 158.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjöstrand M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 159.Zhu F, Wang Q, Guo C, Wang X, Cao X, et al. IL-17 induces apoptosis of vascular endothelial cells—a potential mechanism for human acute coronary syndrome. Clin Immunol. 2011;141:152–60. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 160.Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE., Jr Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 2008;133:226–32. doi: 10.1378/chest.07-1948. [DOI] [PubMed] [Google Scholar]
- 161.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS ONE. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mi S, Li Z, Yang HZ, Liu H, Wang JP, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–14. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 163.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-β, NF-κB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:3356–65. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 164.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–96. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 165.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663–68. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mavalia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, et al. Type 2 helper T cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–58. [PMC free article] [PubMed] [Google Scholar]
- 167.Furuie H, Yamasaki H, Suga M, Ando M. Altered accessory cell function of alveolar macrophages: a possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur Respir J. 1997;10:787–94. [PubMed] [Google Scholar]
- 168.Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101:436–41. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Westermann W, Schobl R, Rieber EP, Frank KH. Th2 cells as effectors in postirradiation pulmonary damage preceding fibrosis in the rat. Int J Radiat Biol. 1999;75:629–38. doi: 10.1080/095530099140276. [DOI] [PubMed] [Google Scholar]
- 170.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reiman RM, Thompson RW, Feng CG, Hari D, Knight R, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–79. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ong C, Wong C, Roberts CR, Teh HS, Jirik FR. Anti-IL-4 treatment prevents dermal collagen deposition in the tight-skin mouse model of scleroderma. Eur J Immunol. 1998;28:2619–29. doi: 10.1002/(SICI)1521-4141(199809)28:09<2619::AID-IMMU2619>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 173.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2–dominated inflammatory response. J Clin Investig. 1999;104:777–85. doi: 10.1172/JCI7325. Shows for the first time that antagonists of the IL-13 signaling pathway can significantly ameliorate fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Investig. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–32. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 176.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFβ1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–82. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 177.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–76. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 178.Fuschiotti P. Role of IL-13 in systemic sclerosis. Cytokine. 2011;56:544–49. doi: 10.1016/j.cyto.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 179.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–42. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184:1526–35. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- 181.Han G, Zhang H, Xie CH, Zhou YF. Th2-like immune response in radiation-induced lung fibrosis. Oncol Rep. 2011;26:383–88. doi: 10.3892/or.2011.1300. [DOI] [PubMed] [Google Scholar]
- 182.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NKT cells. Immunity. 2002;17:629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 183.Weng HL, Liu Y, Chen JL, Huang T, Xu LJ, et al. The etiology of liver damage imparts cytokines transforming growth factor β1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–43. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
- 184.Shimamura T, Fujisawa T, Husain SR, Kioi M, Nakajima A, Puri RK. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J Immunol. 2008;181:4656–65. doi: 10.4049/jimmunol.181.7.4656. [DOI] [PubMed] [Google Scholar]
- 185.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, et al. The contribution of inter-leukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001;25:385–91. doi: 10.1165/ajrcmb.25.3.4437. [DOI] [PubMed] [Google Scholar]
- 187.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J Immunol. 2004;173:4020–29. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 188.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–89. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 189.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, et al. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol. 2001;25:474–85. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- 190.Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–14. [PubMed] [Google Scholar]
- 191.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Inter-leukin (IL)-4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Investig. 1998;101:2129–39. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292:988–94. [PubMed] [Google Scholar]
- 193.Jakubzick C, Choi ES, Kunkel SL, Joshi BH, Puri RK, Hogaboam CM. Impact of interleukin-13 responsiveness on the synthetic and proliferative properties of Th1- and Th2-type pulmonary granuloma fibroblasts. Am J Pathol. 2003;162:1475–86. doi: 10.1016/S0002-9440(10)64280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, et al. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2–dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. Identifies the IL-13Rα2 subunit as a decoy receptor and endogenous inhibitor of IL-13-driven fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mentink-Kane MM, Cheever AW, Wilson MS, Madala SK, Beers LM, et al. Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Rα2. Gastroenterology. 2011;141:2200–9. doi: 10.1053/j.gastro.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 198.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–46. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 199.Lupher ML, Jr, Gallatin WM. Regulation of fibrosis by the immune system. Adv Immunol. 2006;89:245–88. doi: 10.1016/S0065-2776(05)89006-6. [DOI] [PubMed] [Google Scholar]
- 200.Duffield JS, Lupher ML., Jr PRM-151 (recombinant human serum amyloid P/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010;23:305–15. doi: 10.1358/dnp.2010.23.5.1444206. [DOI] [PubMed] [Google Scholar]
- 201.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–25. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 202.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548–56. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Castano A, Lin SL, Surowy T, Nowlin BT, Turlapathi S, et al. Serum amyloid P inhibits fibrosis through FcγR-dependent monocyte/macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. Demonstrates that PTX-2 (serum amyloid P) mediates its antifibrotic effects by inducing a regulatory macrophage phenotype, identified through specific upregulation of IL-10 in tissue macrophages following therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Investig. 2005;115:56–65. doi: 10.1172/JCI22675. Shows that distinct macrophage populations are required to both induce and suppress fibrogenesis at different stages of the wound-healing response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase 13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–95. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 208.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–43. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 209.Bonner JC, Osornio-Vargas AR, Badgett A, Brody AR. Differential proliferation of rat lung fibroblasts induced by the platelet-derived growth factor AA, AB, and BB isoforms secreted by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:539–47. doi: 10.1165/ajrcmb/5.6.539. [DOI] [PubMed] [Google Scholar]
- 210.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 212.Martinet Y, Rom WN, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987;317:202–9. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- 213.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 214.Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-β and regulation by TGF-β. Ann NY Acad Sci. 1990;593:188–96. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 215.Xing Z, Tremblay GM, Sime PJ, Gauldie J. Overexpression of granulocyte-macrophage colony–stimulating factor induces pulmonary granulation tissue formation and fibrosis by induction of transforming growth factor β1 and myofibroblast accumulation. Am J Pathol. 1997;150:59–66. [PMC free article] [PubMed] [Google Scholar]
- 216.Yasuoka H, Yamaguchi Y, Feghali-Bostwick CA. The profibrotic factor IGFBP-5 induces lung fibroblast and mononuclear cell migration. Am J Respir Cell Mol Biol. 2009;41:179–88. doi: 10.1165/rcmb.2008-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Prasse A, Müller-Quernheim J. Non-invasive biomarkers in pulmonary fibrosis. Respirology. 2009;14:788–95. doi: 10.1111/j.1440-1843.2009.01600.x. Shows a direct correlation between the level of M2 macrophage precursor cells in the blood of IPF patients, lung function, prognosis. Together with Reference 235, shows that changes in the monocyte compartment identified in preclinical systems are also present in human fibrotic disease. [DOI] [PubMed] [Google Scholar]
- 218.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, et al. TGF-β driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 219.Baran CP, Opalek JM, McMaken S, Newland CA, O’Brien JM, Jr, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Crawford JR, Pilling D, Gomer RH. Improved serum-free culture conditions for spleen-derived murine fibrocytes. J Immunol Methods. 2010;363:9–20. doi: 10.1016/j.jim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol. 2007;18:2350–58. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 222.Moreira AP, Cavassani KA, Hullinger R, Rosada RS, Fong DJ, et al. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore–induced allergic airway disease. J Allergy Clin Immunol. 2010;126:712–21. doi: 10.1016/j.jaci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 223.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–33. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pilling D, Gomer R. Regulatory pathways for fibrocyte differentiation. In: Bucala R, editor. Fibrocytes: New Insights into Tissue Repair and Systemic Fibroses. Singapore: World Sci; 2007. pp. 37–60. [Google Scholar]
- 225.Homer RJ, Herzog EL. Recent advances in pulmonary fibrosis: implications for scleroderma. Curr Opin Rheumatol. 2010;22:683–89. doi: 10.1097/BOR.0b013e32833ddcc9. [DOI] [PubMed] [Google Scholar]
- 226.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 227.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS ONE. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. Shows a direct correlation between the level of fibrocyte precursor cells in the blood of IPF patients at baseline and subsequent mortality. Together with Reference 224, shows that changes in the monocyte compartment identified in preclinical systems are also present in human fibrotic disease. [DOI] [PubMed] [Google Scholar]
- 229.Nishida M, Okumura Y, Fujimoto S, Shiraishi I, Itoi T, Hamaoka K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–16. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 230.Cabrera S, Gaxiola M, Arreola JL, Ramirez R, Jara P, et al. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol. 2007;39:2324–38. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 231.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515–25. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Popov Y, Sverdlov DY, Bhaskar KR, Sharma AK, Millonig G, et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;298:323–34. doi: 10.1152/ajpgi.00394.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Koronyo-Hamaoui M, Ko MK, Koronyo Y, Azoulay D, Seksenyan A, et al. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. J Neurochem. 2009;111:1409–24. doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- 234.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.van Strien ME, Mercier D, Drukarch B, Breve JJ, Poole S, et al. Anti-inflammatory effect by lentiviral-mediated overexpression of IL-10 or IL-1 receptor antagonist in rat glial cells and macrophages. Gene Ther. 2010;17:662–71. doi: 10.1038/gt.2010.8. [DOI] [PubMed] [Google Scholar]
- 236.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, et al. Arginase-1-expressing macrophages suppress Th2 cytokine–driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. Identifies Arginase-1-expressing M2 macrophages as a suppressor of Th2-driven fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ji Z, Ke ZJ, Geng JG. SAP suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Immunol Cell Biol. 2011;90:388–95. doi: 10.1038/icb.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang M, Zhang J, Yan M, Luo D, Zhu W, et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2010;118:672–78. doi: 10.1016/j.ophtha.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 239.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fcγreceptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–41. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 240.Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine FcγRs. J Immunol. 2001;166:1200–5. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 241.Bijl M, Horst G, Bijzet J, Bootsma H, Limburg PC, Kallenberg CG. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003;48:248–54. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- 242.Ciurana CL, Hack CE. Competitive binding of pentraxins and IgM to newly exposed epitopes on late apoptotic cells. Cell Immunol. 2006;239:14–21. doi: 10.1016/j.cellimm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 243.Familian A, Zwart B, Huisman HG, Rensink I, Roem D, et al. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001;167:647–54. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- 244.Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcγreceptors. J Autoimmun. 2002;19:147–54. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- 245.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. Demonstrates that therapeutic dosing with PTX-2 (serum amyloid P) is effective at both reducing fibrosis and restoring lung function in rats that are exposed to bleomycin-mediated lung injury and that it correlates with reductions in macrophages and fibrocytes within the lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, et al. Bone marrow–derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–89. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA. 2008;105:10179–84. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang W, Wu J, Qiao B, Xu W, Xiong S. Amelioration of lupus nephritis by serum amyloid P component gene therapy with distinct mechanisms varied from different stage of the disease. PLoS ONE. 2011;6:e22659. doi: 10.1371/journal.pone.0022659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–92. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–53. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li B, Castano AP, Hudson TE, Nowlin BT, Lin SL, et al. The melanoma-associated transmem-brane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010;24:4767–81. doi: 10.1096/fj.10-154757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, et al. Microtubule-associated protein 1 light chain 3 α (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci USA. 2011;108:17396–401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]