Abstract
The mu-opioid receptor encoded by the gene OPRM1 plays a primary role in opiate, alcohol, cocaine and nicotine addiction. Studies using opioid antagonists demonstrate that the mu-opioid receptor (MOP-r) also mediates the hypothalamic–pituitary–adrenal (HPA) axis stress response. A common polymorphism in exon one of the MOP-r gene, A118G, has been shown to significantly alter receptor function and MOP-r gene expression; therefore, this variant likely affects HPA-axis responsivity. In the current study, we have investigated whether the presence of the 118AG variant genotype affects HPA axis responsivity to the stressor metyrapone, which transiently blocks glucocorticoid production in the adrenal cortex. Forty-eight normal and healthy volunteers (32 men, 16 women) were studied, among whom nine men and seven women had the 118AG genotype. The 118G allele blunted the adrenocorticotropic hormone (ACTH) response to metyrapone. Although there was no difference in basal levels of ACTH, subjects with the 118AG genotype had a more modest rise and resultant significantly lower ACTH levels than those with the prototype 118AA at the 8-hour time point (P < 0.02). We found no significant difference between genders. These findings suggest a relatively greater tonic inhibition at hypothalamic–pituitary sites through the mu-opioid receptor and relatively less cyclical glucocorticoid inhibition in subjects with the 118G allele.
Keywords: ACTH, genetics, HPA axis, metyrapone, mu-opioid receptor, OPRM1
INTRODUCTION
The mu-opioid receptor (MOP-r) encoded by the gene OPRM1 is a primary target for opioid drugs (e.g. morphine and heroin) as well as the endogenous peptide beta-endorphin; it also plays a role in addiction to alcohol, cocaine, heroin and other opiates (Kreek 2007; Kreek & LaForge 2007). Gene expression and/or protein products of the MOP-r have been shown to be altered by each of the substances of abuse (Zhou et al. 2010). The A118G single nucleotide polymorphism (SNP) is a common variant of the OPRM1 gene and has been the focus of numerous molecular as well as genetic association studies.
The 118AG variant genotype results in substitution of asparagine to aspartic acid at amino acid position 40. This results in the loss of a putative N-glycosylation site in the variant allele (Bond et al. 1998). Studies have shown that the presence of the A118G variant causes (a) an increased potency of beta-endorphin-induced activation of G-protein-coupled potassium channels; (b) and increased receptor-binding affinity for beta-endorphin, but not for the other endogenous or exogenous opioid ligands; and (c) a reduced expression of the mu-opioid receptor on cell surfaces (Bond et al. 1998; Kroslak et al. 2007). In addition, post-mortem brain samples heterozygous for the 118G allele were shown to have reduced mRNA expression and MOP-r protein levels compared to the 118A prototype (Zhang et al. 2005).
Allelic frequencies of the 118G variant have been reported to range from 9% to 32%, with significant variation in distribution when examined by ethnic group (2–4% in African Americans, 10–25% in Caucasians and Hispanics and up to 50% in Asians; as reviewed by LaForge, Yuferov & Kreek 2000). The 118G variant is the most frequent mutation of the OPRM1 gene and, therefore, is a logical candidate for genetic association studies.
The A118G SNP has been shown to account for up to 21% of the attributable risk for developing heroin addiction, and 11% for developing alcoholism in cohorts from central Sweden, a population in which 80% have only Swedish ancestry (Bart et al. 2004, 2005). Association of the 118G variant allele with heroin addiction was also found in a population of Han Chinese in Hong Kong (Szeto et al. 2001) and in heroin-dependent subjects of Indian background (Kapur et al. 2007). In a Japanese study, alcohol-dependent subjects were shown to have a greater odds ratio of having at least one copy of the 118G allele compared to controls (Nishizawa et al. 2006).
Given that it has been established that the MOP-r modulates the hypothalamic–pituitary–adrenal (HPA) axis (e.g. Schluger et al. 1998), several research groups have examined the effects of the 118AG variant genotype on the HPA axis stress response in normal healthy volunteers using the mu-opioid receptor antagonist naloxone (Wand et al. 2002; Hernandez-Avila et al. 2003, 2007; Chong et al. 2006). All of these studies identified significantly greater cortisol response to naloxone in normal subjects expressing the 118G allele versus those with the 118A prototype. Basal cortisol levels were found to be higher in heterozygous 118G allele carriers in a cohort of 59 normal healthy volunteers of mixed ethnicity (Bart et al. 2006) and also in a smaller study within European American normal healthy subjects (Hernandez-Avila et al. 2007).
The influence of either the glucocorticoid negative feedback regulation or the mu-opioid receptor tonic inhibition on the HPA axis can be ascertained in two ways. A mu-opioid receptor antagonist can be administered, usually intravenously for research purposes, which then transiently removes the endogenous mu ligands, primarily beta-endorphin, from the mu receptors. This causes an abrupt rise presumably in corticotrophin releasing factor (CRF) and also in POMC-derived peptides such as the easily measured adrenocorticotropic hormone (ACTH), and, in turn, cortisol. Alternatively, metyrapone can be administered, which blocks the final step in cortisol synthesis (11-beta hydroxylation) and, thus, removes glucocorticoid negative feedback for 6–8 hours, leading to a presumed rise in CRF and a measurable rise in ACTH levels.
Previous research in humans has shown that acute administration of all opiates, and chronic, intermittent administration of short-acting opiates (e.g. heroin or morphine) suppress the HPA axis (Cushman & Kreek 1974; Kosten, Morgan & Kreek 1992; Culpepper-Morgan & Kreek 1997), whereas alcohol and cocaine administration cause HPA-axis activation (as reviewed in Kreek & LaForge 2007). Research conducted in our institution has documented that the HPA axis stress response to metyrapone in former heroin-dependent subjects has stabilized on methadone pharmacotherapy (Kreek 1973; Kreek et al. 1984; Schluger et al. 2001). However, in chronic cocaine-using methadone-maintained subjects, hyperresponsivity of the HPA axis was found (Schluger et al. 2001).
A Swedish study reported an overall blunted HPA axis response to metyrapone in former heroin-dependent subjects maintained on buprenorphine pharmacotherapy, compared to healthy normal volunteers. This essentially is the same finding previously made in active heroin addicts and in early-stage methadone-maintained subjects (Cushman & Kreek 1974). Interestingly, genotypic analysis of the buprenorphine group revealed an even further blunting of ACTH response by heterozygous 118G allele carriers. As there was a small number of subjects with the variant allele within the normal volunteers, genotype differences were not analyzed in this group (Kakko et al. 2008).
The aim of our current study was to characterize the role of the A118G polymorphism of the MOP-r in response to the stressor metyrapone in healthy normal volunteers. Our hypothesis was that we would find decreased ACTH plasma levels in subjects with the 118AG genotype (reflecting tighter binding of beta-endorphin at the MOP-r and, thus, greater inhibition directed at CRF neurons in the absence of cortisol).
METHODS AND MATERIALS
Subjects
Normal healthy volunteers were recruited through newspaper advertisements and flyers. In all, 48 subjects (16 women) with no history of substance abuse or dependence met the inclusion criteria for this study. A written informed consent was obtained from each subject prior to any study procedures. Subjects included in this study had signed a separate informed consent for outpatient genetics testing. The institutional review board of Rockefeller University approved the study protocols and consent forms. This study was carried out in accordance with the precepts of the Declaration of Helsinki (2008).
All subjects were seen in the outpatient clinic on at least two occasions prior to being admitted for inpatient testing. Subjects received medical and psychiatric evaluations by a nurse practitioner, internist, or psychiatrist using clinical interview, physical exam, electrocardiogram and review of laboratory data. Each subject was interviewed using the structured clinical interview for DSM-IV (SCID-I) and did not meet criteria for any axis I diagnosis, including substance and/or alcohol abuse or dependence. In addition, subjects having a history of alcohol drinking to intoxication three or more times per week for at least 6 months, or any drinking of alcohol to intoxication within the last 30 days, were excluded from the study.
Extensive laboratory testing included complete blood cell count, chemistries, endocrine tests, hepatitis serology and an HIV test. Subjects self-reporting HIV-positive status during genetics ascertainment were excluded from screening for neuroendocrine testing since HIV infection is known to alter endocrine function. Urine for toxicology was obtained on each clinic visit and on a 24-hour basis during hospital admission to detect the presence of illicitly-used prescription opioids, heroin, cocaine, cannabinoids, and benzodiazepines. Subjects were included for analysis only if results of all toxicology tests were negative.
A total of 48 consecutive healthy volunteers participated, 32 with the prototype 118A genotype and 16 with a 118G allele in exon 1 of the mu-opioid receptor. We do not recruit by genotype; genetics testing may be performed before or after subjects complete neuroendocrine studies. Therefore, subject data were analyzed only after adequate numbers of normal, healthy controls with the variant allele completed metyrapone testing. All subjects with the variant allele were included for analysis, beginning with the first 118G subject studied with metyrapone on 2 June 1997, and ending with the last 118G subject studied on 25 January 2008. Commencing on the date of the first 118G subject studied, the next 32 consecutive subjects with 118A genotype were included for analysis to achieve a 2:1 ratio (testing dates 25 June 1997–31 October 2002).
As expected from previously documented allelic frequencies, fewer African Americans have the variant compared to Caucasians and Hispanics. Demographic data are shown in Table 1. There were no subjects homozygous for the variant.
Table 1.
Demographic characteristics of subjects by OPRMI genotype.
| A118A (n = 32) No. (%) |
A118G (n = 16) No. (%) |
|
|---|---|---|
| Male | 23 (72) | 9 (56) |
| Female | 9 (28) | 7 (44) |
| Ethnicity, no. (%) | ||
| Caucasian | 11 (34) | 7 (44) |
| African American | 15 (47) | 3 (19) |
| Hispanic | 2 (6) | 5 (31) |
| Other (more than one) | 4 (13) | 1 (6) |
| Mean age (95%CI) | 33.8 (30.7–36.8) | 31.8 (27.9–35.6) |
Procedures
Subjects were admitted to the stress-minimized inpatient unit at the Rockefeller University Hospital the evening prior to neuroendocrine testing. The following morning, a standard dose of metyrapone 2.25 g (Novartis, Ireland) was given orally followed by 30 ml of an antacid to minimize adverse gastrointestinal effects. Subjects fasted for at least 9 hours prior to the start of testing and were allowed to eat 2 hours after metyrapone administration. Blood samples were uniformly obtained at specific time points immediately prior to and following metyrapone administration and analyzed for plasma ACTH and cortisol levels. The following time points were included for the analysis: 0 (immediately prior to metyrapone administration), 4 and 8 hours post-metyrapone administration. Subjects were genotyped for the A118G polymorphism either by fluorescent polymerase chain reaction (PCR) in a TaqMan protocol using TaqMan MGB probes and primers designed according to specifications of Applied Biosystems (Foster City, CA, USA), or by Sanger direct sequencing of the PCR products.
Hormone assays
Plasma concentrations of ACTH and cortisol were measured using duplicate radioimmunoassay procedures with slight modifications (ACTH-Nichols Institute, San Juan Capistrano, CA, USA, and RIMA-DiaSorin Stillwater, MN, USA; cortisol: Diagnostic Products, Los Angeles, CA, USA). Intra- and interassay coefficients of variation were 12.4% and 13.6%, respectively, for ACTH and 8.9% and 9.8%, respectively, for cortisol.
Statistical analysis
There have been reports of gender differences in HPA axis reactivity within normal, healthy volunteers (Uhart et al. 2006), as well as in ACTH response to metyrapone challenge in depressed patients and in healthy controls (Young & Ribeiro 2006). Therefore, we included gender as a factor in our analyses.
Two approaches were taken in the analysis of ACTH response to metyrapone. First, a three-way analysis of variance (ANOVA), Genotype × Gender × Timepoint with repeated measures on the third factor, was conducted followed by planned comparisons where appropriate. Then, area under the curve (AUC) for ACTH from time 0 to 8 hours post-metyrapone was calculated. A two-way ANOVA, Genotype × Gender, was used to examine the differences in ACTH AUCs.
RESULTS
Timepoint analysis of ACTH response
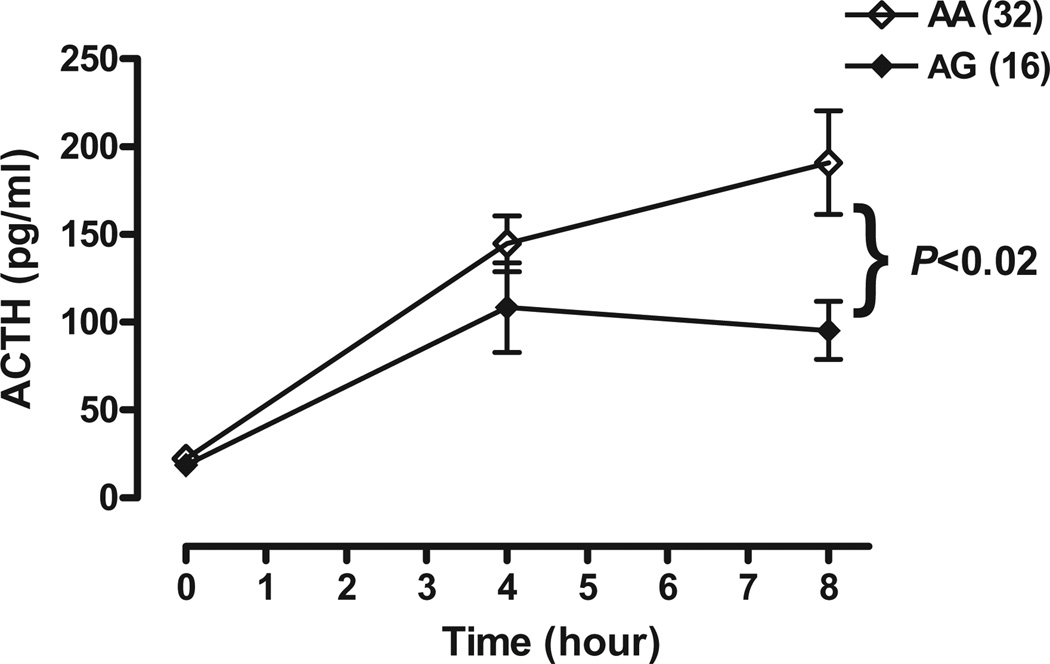
ACTH response at each timepoint, 0 (before metyrapone), 4 and 8 hours after metyrapone, for each genotype is shown in Fig. 1. A three-way ANOVA, Genotype × Gender × Timepoint, showed that ACTH levels were significantly lower in subjects with a 118G allele than in those with the prototype AA genotype (main effect F1,44 = 5.81, P < 0.005; with no main effect of gender, F1,44 < 1.00. However, there was a significant Genotype × Timepoint interaction (F2,88 = 4.85, P < 0.02). Whereas there was no significant difference in ACTH levels between genotypes before metyrapone (at the 0 time point, by the 8th hour after metyrapone, ACTH levels were significantly lower in subjects with the 118G allele than in those with the prototype AA genotype (Fig. 1).
Figure 1.
Mean plasma ACTH levels (±SEM) at 0900 hours (just prior to metyrapone administration) and at 1300 and 1700 hours in OPRM1 118A/G genotype normal volunteers
Analysis of ACTH response as measured by the AUC (0–8 hours)
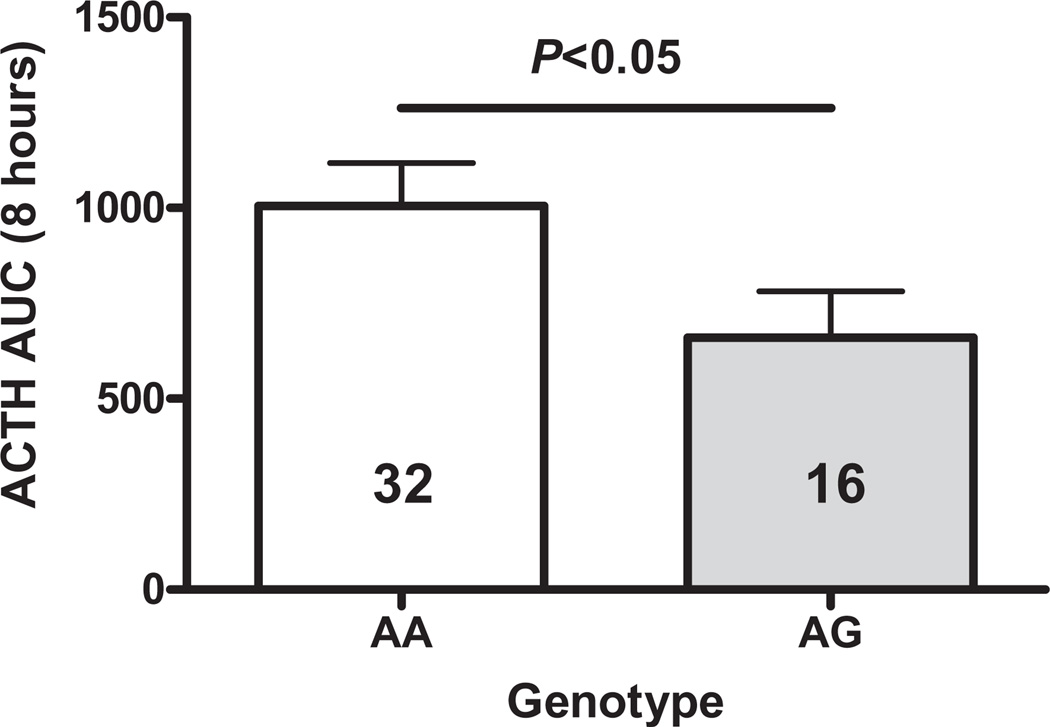
The ACTH AUC response to metyrapone is shown in Fig. 2. A two-way ANOVA showed that by this measure also, the ACTH response to metyrapone was significantly lower in subjects with the 118G allele than in those with the prototype AA genotype (main effect of genotype, F1,44 = 4.90, P < 0.05); again gender has been found to have no effect (F1,44 < 1.00).
Figure 2.
AUC for plasma ACTH (±SEM) from 0900 to 1700 hours in OPRM1 118A/G genotype normal volunteers
Effect of metyrapone on cortisol
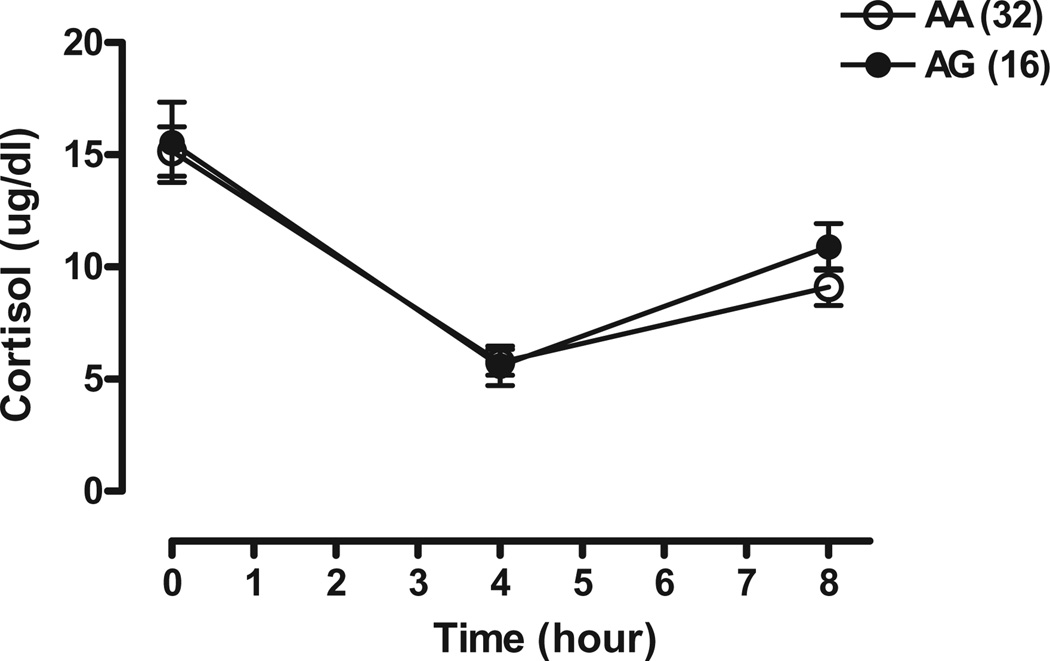
Cortisol levels are shown in Fig. 3. As expected, cortisol levels were lower following metyrapone in all subjects of both genotypes.
Figure 3.
Mean plasma cortisol levels (±SEM) at 0900 hours (just prior to metyrapone administration) and at 1300 and 1700 hours in OPRM1 118A/G genotype normal volunteers
DISCUSSION
The HPA axis has been widely studied in humans and it is now well understood that two major and separate components modulate this system. The earliest recognized is the circadian pattern of alternating inhibition and non-inhibition of the hypothalamic and pituitary sites of HPA action by glucocorticoids and cortisol in humans (Weitzman et al. 1971). An initial observation by Volavka followed by work of many other laboratories has shown that the mu-opioid receptor system tonically inhibits the HPA axis (Volavka et al. 1979; Volavka 1980). In fact, studies in healthy volunteers have shown that the inhibition through mu receptor sites, presumably in the hypothalamus and anterior pituitary, is comparable to that of glucocorticoid inhibition at these sites (Schluger et al. 1998).
Four major studies have found that blocking the endogenous ligand of the MOP-r with an opiate antagonist causes greater HPA activation in healthy, normal subjects with the 118G allelic variant than in those with the 118A prototype (Wand et al. 2002; Chong et al. 2006; Hernandez-Avila et al. 2003, 2007), possibly due to tighter binding of beta-endorphin in the presence of the variant 118G allele reported by our group (Bond et al. 1998). We would then expect the opposite to occur when subjects with the 118AG genotype are administered metyrapone. With the transient removal of glucocorticoid negative feedback, one would anticipate the ACTH levels to have a more gradual and lower rise due to greater tonic inhibition through the MOP-r in those with the variant 118G allele. This is indeed what we have found in healthy volunteers in this study.
We did not find differences in basal cortisol levels by genotype, in contrast to the study of Bart et al. (2006) in which multiple ethnic groups were included, and to the smaller Hernandez-Avila et al. (2007) study in which a European American cohort was used. This may be due to methodological differences: the current study design used a single timepoint (0) just prior to the administration of metyrapone capsules for basal cortisol determination, whereas the study of Bart et al. measured cortisol AUC for nine timepoints obtained from 0–90 minutes after an intravenous placebo injection.
There has been only one study that reports differences in response to naloxone challenge between European American and Asian subjects with the 118G variant (Hernandez-Avila et al. 2007). We cannot compare our study to that one because we did not recruit by genotype and our demographics within the 118G group reflect a higher percentage of Caucasians and Hispanics than African Americans and there were no Asians. A functional polymorphism, however, would be expected to have similar effects in different ethnic groups and we have indeed found an effect of the 118G allele in this group of mixed ethnicity.
Addiction is characterized by alterations in the stress-responsive HPA axis, primarily due to the pharmacological effects of abused drugs, but also influenced by developmental, environmental and genetic factors (Kreek 1992). The HPA axis responds to physiological and psychosocial stressors, and chronic HPA axis dysregulation has been shown to contribute to numerous medical disorders (e.g. Chrousos 2009). Atypical stress responsivity has been hypothesized and shown by our group and others to contribute to the persistence of and relapse to specific addictive diseases. Studies using rodent model and human subjects have shown that both alcohol and cocaine activate the HPA axis, but that adaptation or tolerance develops to these effects (e.g. Zhou et al. 1996, 2000). Further, several self-administration studies in rodents have suggested that modest activation of the HPA axis is sought as rats increase self-administration of cocaine in a setting of decreasing corticosterone levels (e.g. Mantsch et al. 2000).
In a collaborative study, we found that the opioid-antagonist naltrexone decreased craving and alcohol self-administration in alcohol-dependent subjects, concomitant with HPA axis activation (O’Malley et al. 2002). A subsequent study found a negative correlation between a blunted HPA axis response (as measured by cortisol levels) and increased alcohol consumption in response to stress in a cohort of alcohol-dependent volunteers (Pratt & Davidson 2009). Further, studies have shown that naltrexone pharmacotherapy in alcohol-dependent persons is more likely to be effective in persons with the 118G variant allele (Oslin et al. 2003; Anton et al. 2008) A recent study identified an increase in the neurosteroid allopregnanolone after naltrexone administration in subjects with one or two copies of the G allele, suggesting this may contribute to naltrexone’s efficacy in individuals with the 118G variant (Ray et al. 2010).
In the study of Kakko et al. (2008), former heroin-dependent subjects who have the 118G allele treated with buprenorphine showed a greatly blunted or absent ACTH response to metyrapone. Our study shows for the first time a significantly lower ACTH response in healthy volunteers with no problem alcohol use or addiction history who possess the 118G allele.
In certain individuals, atypical stress responsivity may precede self-exposure to illicit drugs, which may be due in part to specific gene variants; such effects of a gene variant have now been documented. Individuals with a genetic variant that increases endogenous inhibitory tone may not show HPA axis differences under normal conditions. However, when provoked by a stressor, i.e. metyrapone, an atypical HPA-axis response results. Thus, the 118G variant of the MOP-r gene may confer increased risk to addiction in concert with exposure to a drug or an environment that chronically induces HPA-axis stress response.
In conclusion, we found a functional effect of the 118G allele on HPA axis stress responsivity in healthy normal volunteers. The functional HPA-axis response of this genetic variant may help us understand its role in vulnerability to addiction through differential modulation of the HPA axis, as well as its role in the positive response to opioid-antagonist pharmacotherapy in the treatment of alcoholism.
Acknowledgements
This work was supported by funding received from the following National Institutes of Health (NIH) grants: NIH-NIDA P60-DA05130 (M. J. Kreek) the NIH-National Center for Research Resources (NCRR) Center for Clinical and Translational Science Award (CTSA) (UL1-RR024143) (B. Coller), and the Adelson Research Foundation. All subjects were studied on the inpatient unit of the NIH-supported Center for Clinical and Translational Science (CCTS) at The Rockefeller University Hospital. We would like to thank the following members of the Laboratory of the Biology of Addictive Diseases for their assistance: Lisa Borg, MD, contributed to the acquisition of subject data; Colin Jackson assisted with the preparation of data for analysis; Matthew Randesi performed genotyping on subject samples; and Susan Russo provided assistance in editing the manuscript.
Footnotes
Disclosure/Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Authors Contribution
ED assisted with data analysis, interpretation of findings and in preparation of the manuscript. MJK was responsible for study concept and design and provided critical interpretation of findings. AH performed statistical analysis, assisted in the interpretation of findings and in preparation of the manuscript. GB, ED and BR contributed to the acquisition of subject data. YU and JV assisted in the preparation of data for analysis. MJK, GB and AH provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Anton R, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of μ-opioid receptor (OPRM) as a predictor of naltrexone response in the treatment of alcohol dependence. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Heilig M, LaForge KS, Pollak L, Leal S, Ott J, Kreek MJ. Substantial attributable risk relative to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional μ-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreak MJ. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of themu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong R, Oswald L, Yang X, Uhart M, Lin P, Wand G. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Culpepper-Morgan JA, Kreek MJ. Hypothalamicpituitary-adrenal axis hypersensitivity to naloxone in opioid dependence: a case of naloxone-induced withdrawal. Metabolism. 1997;46:130–134. doi: 10.1016/s0026-0495(97)90289-4. [DOI] [PubMed] [Google Scholar]
- Cushman P, Kreek MJ. Some endocrinologic observations in narcotic addicts. In: Zimmerman E, George R, editors. Narcotics and the Hypothalamus. NewYork: Raven Press; 1974. pp. 161–173. [Google Scholar]
- Hernandez-Avila C, Covault J, Wand G, Zhang H, Gelernter J, Kranzler H. Population-specific effects of the Asn40Asp polymorphism at the mu-opiod receptor gene (OPRM1) on HPA-axis activation. Pharmacogenet Genomics. 2007;17:1031–1038. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila C, Wand G, Luo X, Gelernter J, Kranzler H. Association between the cortisol response to opioid blockade and the Asn40Asp Polymorphism at the μ-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Kakko J, Wachenfeldt J, Svanborg K, Lidstrom J, Barr C, Heilig M. Mood and neuroendocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biol Psychiatry. 2008;63:172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kapur S, Sharad S, Singh R, Gupta A. A118G polymorphism in mu-opioid receptor gene (OPRM1): association with opiate addiction in subjects of Indian origin. J Integr Neurosci. 2007;6:511–522. doi: 10.1142/s0219635207001635. [DOI] [PubMed] [Google Scholar]
- Kosten T, Morgan C, Kreek MJ. Beta endorphin levels during heroin, methadone, buprenorphine, and naloxone challenges: preliminary findings. Neuropsychopharmacology. 1992;32:523–528. doi: 10.1016/0006-3223(92)90220-t. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973;223:665–668. [PubMed] [Google Scholar]
- Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. In: O’Brien CP, Jaffe JH, editors. Addictive States. Association of Research in Nervous and Mental Disease. Vol.70. New York: Raven Press; 1992. pp. 205–230. [PubMed] [Google Scholar]
- Kreek MJ. Role of a functional human gene polymorphism in stress responsivity and addictions. Clin Pharm Ther. 2007;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction and a functional variant of the human mu-opioid receptor gene. Mol Inter. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and β-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–278. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- Kroslak T, LaForge KS, Gianotti R, Ho A, Nielsen D, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the humanmu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential Implications for addictions. Eur J Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Nishizawa D, Han W, Hasegawa J, Ishida T, Numata Y, Sato T, Kawai A, Ikeda K. Association of mu-opioid receptor gene polymorphism A118G with alcohol dependence in a Japanese population. Neuropsychobiology. 2006;53:137–141. doi: 10.1159/000093099. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitray-adernocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin D, Berrettini W, Kranzler H, Pettinati H, Gelernter J, Volpicelli J, O’Brien C. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Pratt W, Davidson D. Role of the HPA axis and the A118G polymorphism of the μ-opioid receptor in stress-induced drinking behavior. Alcohol Alcohol. 2009;44:358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchinson KE, Ashenhurst JR, Morrow AL. Naltrexone selectively elevates GABAergic neuroactive steroid levels in heavy drinkers with the asp40 allele of the OPRM1 gene: a pilot investigation. Alcohol Clin Exp Res. 2010;34:1479–1487. doi: 10.1111/j.1530-0277.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Borg L, Ho A, Kreek MJ. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Szeto CT, Tang NL, Lee DT, Stadlin A. Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport. 2001;12:1103–1106. doi: 10.1097/00001756-200105080-00011. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong R, Oswald L, Lin P, Wand G. Gender differences in hypothalamic-pituitray-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Volavka J. Short-term hormonal effects of naloxone in man. Psychoneuroendocrinology. 1980;5:225–234. doi: 10.1016/0306-4530(80)90026-8. [DOI] [PubMed] [Google Scholar]
- Volavka J, Cho D, Mallya A, Bauman J. Naloxone increases ACTH and cortisol in man. N Engl J Med. 1979;300:1056–1057. doi: 10.1056/nejm197905033001817. [DOI] [PubMed] [Google Scholar]
- Wand G, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrin Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Young E, Ribeiro S. Sex differences in the ACTH response to 24 h metyrapone in depression. Brain Res. 2006;1126:148–155. doi: 10.1016/j.brainres.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression inbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J BiolChem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF-1 receptor mRNA levels after acute, but not chronic, daily binge intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24:1575–1582. [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and Type 1 cortiocotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during ‘binge’-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–358. [PubMed] [Google Scholar]