Abstract
Polydimethylsiloxane (PDMS) has emerged as an extremely useful polymer for various biological applications. The conjugation of PDMS with bioactive molecules to create functional surfaces is feasible, yet limited to single molecule display with imprecise localization of the molecules on PDMS. Here we report a robust technique that can transfer and print the membrane surface of glutaraldehyde-fixed stromal cells intact to a PDMS substrate using an intermediate polyvinylalcohol (PVA) film as a transporter system. The cell-PVA film capturing the entirety of surface molecules can be peeled off and subsequently printed onto PDMS while maintaining the spatial display of the original cell surface molecules. Proof-of-concept studies are described using human bone marrow stromal cell membranes, including the demonstration of bioactivity of transferred membranes to capture and adhere hematopoietic cells. The presented process is applicable to virtually any adherent cell and can broaden the functional display of biomolecules on PDMS for biotechnology applications.
INTRODUCTION
Polydimethylsiloxane (PDMS) has many salient features in biomedical applications including biocompatibility, optical transparency, gas-permeability, elastomeric properties, low cost, ease and versatility of fabrication1. PDMS can be replica molded to existing structures with nanoscale resolution2 and also readily tailored to desired shapes and sizes by precision cutting methods3. Mechanical properties of PDMS can be readily tunable by blending different mechanical strength of elastomers or adjusting the ratio of curing agent4. Oxygen plasma treated PDMS surfaces can form covalent bonds to glass or another PDMS surface creating enclosed chambers commonly used for microfluidic applications5. The surface of PDMS can be further modified with distinct molecules via various processes such as plasma treatment, ultraviolet irradiation, chemical vapor deposition, silanization, sol-gel coating, and layer-by-layer deposition6. Yet, these functionalization methods of PDMS surface have been limited to mostly single molecules with little opportunity for creating complex surfaces with molecular displays that have relevant biological length scales.
A cell membrane represents a complex surface with intrinsic bioactivity. The surfaces of cells are composed of a distinct set of membrane molecules that have a concentration and spatial arrangement that regulates many fundamental biological processes including cell survival, proliferation, differentiation, communication and trafficking7. Cell surfaces, in particular fibroblastic stromal cell surfaces, have been used to recreate in vivo matrices for the co-culture of hematopoietic, epithelial, or stem cells in an in vitro setting. For instance, bone marrow stromal cells (BMSCs) promote the expansion and differentiation of hematopoietic progenitor cells8, 9, embryonic fibroblasts maintain embryonic stem cells in an undifferentiated state10, notch-ligand expressing stromal cells induce T-cell lineage commitment of prelymphocytes11, and fibroblasts maintain functional phenotypes of primary hepatocytes12. The preparation of feeder layers, however, is laborious with significant variability from batch-to-batch preparation that can affect experimental reproducibility. In addition, the separation of target cells from feeder layer cells is technically challenging, but essential for distinguishing the biological effects of co-culture on each individual cell type as well as for the therapeutic use of ex vivo-expanded cells that require stringent levels of purity before administration. One alternative approach to elude these issues while harnessing biological effectiveness of stromal cells is to capture and imprint cell surface molecules on PDMS materials considering the cellular membrane as a biological soft material.
Here we report a technique that transfers the complex membrane surface of glutaraldehyde-fixed human BMSCs intact to a PDMS substrate using an intermediate polyvinylalcohol (PVA) film as a transporter system and demonstrate bioactivity of transferred membranes. The PDMS display of cell membranes was indistinguishable in terms of morphology and biological activity in supporting human leukemic cell adhesion when compared to fixed BMSCs. The presented process is simple and applicable to virtually any adherent cell. The same principle and approach can also be readily extended to a broad range of nano- and micro-scale biological materials, thereby creating the opportunity for a new set of PDMS surfaces having tremendous diversity.
EXPERIMENTAL SECTION
Preparation of fixed and PDMS transferred stromal cells
Primary human BMSCs were isolated and expanded with medium composed of 15% (v/v) FBS, 100U/mL penicillin, 100μg/mL streptomycin, 20mg/L gentamicin, 1ng/L fibroblast growth factor, and 3g/L sodium bicarbonate in alpha-MEM media13. Human skin fibroblasts, MDA-MB-231, PC-3 and DU-145 cells were cultured in DMEM media supplemented with 10% FBS, 100U/mL penicillin and 100μg/mL streptomycin. Human BMSCs or cancer cells were cultured in 10 or 15cm diameter of tissue culture dishes. At 80% confluence, cells were fixed with pre-warmed 1% (v/v) glutaraldehyde solution in PBS for 10min. After washing three times with DI-water, 5% (w/v) PVA (Sigma, 363170) solution was poured in a culture dish. As water evaporated, the remaining PVA formed a thin, transparent and mechanically durable film on top of cells. The circumference of a PVA film on a dish was carved out and the film was peeled off using serrated tip forceps. The peeled off cell-PVA film was attached on a 10cm Petri dish using a double-sided tape and 10g of pre-PDMS in 10:1 (base: curing agent) (Sylgard 184, Dow Corning) was layered. After removing entrapped air bubbles under vacuum, PDMS was cured at 70°C for 4 hours. Solidified PDMS-cell-PVA film was immersed in a DI-water containing beaker, microwaved 3 times for 2min each, and incubated overnight at 37°C that gently and completely dissolved PVA. For micro-patterned culture, 500μm diameter circular patterns were fabricated on a 250μm thick PDMS sheet (Rogers Corporation) using a laser-cutter. The prepared PDMS stencils were subsequently attached on a tissue culture dish and then MDA-MB-231 cells were seeded. Once cells reached confluence, the PDMS stencils were removed and patterned culture was fixed and PDMS transferred.
Analysis of Nalm-6-Luc leukemic cell adhesion on fixed-PDMS transferred BMSCs
Nalm-6 human leukemic cells were cultured in RPMI-1640 media supplemented with 10% FBS, 100U/mL penicillin, and 100μg/mL streptomycin. Luciferase gene transduced Nalm-6 cells (Nalm-6-Luc) were generated using a third generation bidirectional lentivirus (pCCL.sin.cPPT.polyA.CTE.eGFP. minhCMV.hPGK.Luc.WPRE) that simultaneously expresses GFP and luciferase14, 15. Nalm-6 cells (1×106) were transduced with the virus along with 8mg/mL polybrene (Sigma) and medium was changed 12 hours after infection. The fixed-PDMS transferred BMSCs were cut out as 15mm diameter discs that exactly fit into a 24-well plate such that the discs did not float after insertion into individual wells. Fixed-PDMS BMSC discs were sterilized with 70% isopropanol for 5min and further exposed to UV light for 1 hour. 1×106 Nalm-6-Luc cells were loaded in each well with 1mL medium. Empty wells and live or glutaraldehyde fixed BMSCs were used as negative and positive controls, respectively. After 2, 4 and 24 hours culture, the plates were shaken uniformly on an orbital shaker for 10min and washed with PBS 3 times to get rid of non-adhered Nalm-6 cells. Remaining adhered Nalm-6 cells were lysed and stored at −20°C. After collecting all the samples, 50μL of lysed cell suspension was mixed with 100μL luciferase substrate (Luciferase assay kit, Promega) in a 96-well black plate and its bioluminescent signal was immediately measured with a microplate reader (Synergy2, BioTek). The bioluminescent reading results were normalized by baseline BMSCs seeding quantity that was determined by a crystal violet assay of an identical set of 24-well plates seeded with live, fixed and fixed-PDMS transferred BMSCs having the same cellular density as the luciferase assay plates. Each group had 4 replicates and statistical comparison was performed using SPSS ver. 17 applying Mann-Whitney tests.
Imaging
Optical images of cells and fixed-PDMS transferred cell surfaces were taken using Zeiss200 inverted microscope with 10× objective lenses. For colorimetric staining of cellular proteins, fixed-PDMS transferred cells were stained with total protein (Bio Rad), crystal violet (Sigma), a plasma membrane dye (CellMask plasma membrane stain, Invitrogen), or a nucleic acid dye (DAPI, Invitrogen) and then imaged under Leica SP5 confocal laser scanning microscope with 10× objective lenses. For SEM imaging, cells were fixed with 2% glutaraldehyde, serially dehydrated with 20, 50, 70, 90, 95 and 100% ethanol solutions and further dried using a lyophilizer for overnight. Subsequently, samples were deposited on a thin Au film using a sputter coating machine (208HR, Cressington) and imaged under FESEM Ultra55 (Zeiss).
RESULTS AND DISCUSSION
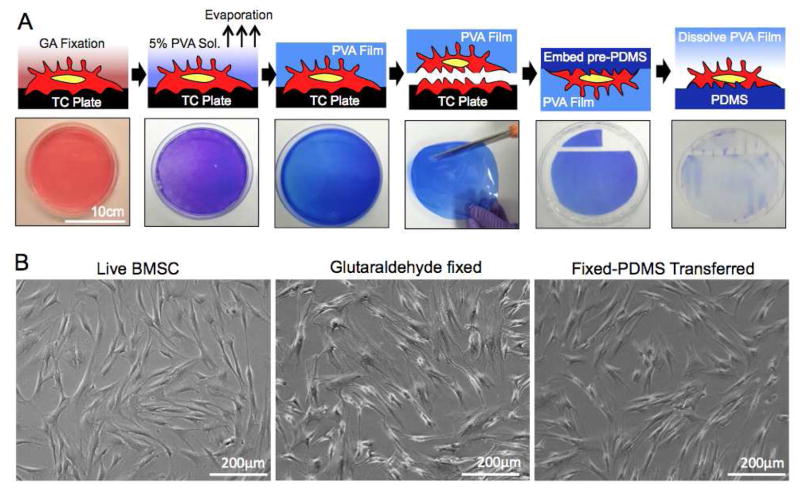
The process used to prepare fixed stromal cell membranes displayed on a PDMS surface is shown in Figure 1A. We first prepared a fixed human BMSC culture with a 1% glutaraldehyde solution and then overlayed a 5% PVA solution on the stromal cell surface. Water-soluble PVA polymer, a commonly used surfactant for stabilizing oil-in-water emulsions, was effectively deposited at the interface of lipid bilayer based cell membranes and, once the water evaporated, formed a film on top of the fixed stromal cells. A deposited PVA film captured the entire fixed cell surface membrane and conserved the cellular morphology for more than 6 months when stored in a humidified 4°C environment. The PVA film was mechanically durable, flexible, and could be easily peeled off from the culture plate. The captured adherent cell surface was maintained intact while the residual cell material that was adherent to the tissue culture plate remained. The captured cell surface qualitatively had a submicron scale roughness. A PDMS pre-polymer was casted to mold to the rough surface under vacuum. After curing PDMS, the PVA captured features were transferred onto the PDMS substrate. Finally, the PVA film was dissolved to expose the captured cell surface on PDMS in its natural polarity. The morphology of BMSCs after this molding process was indistinguishable from their live and fixed counterparts under optical microscopy (Figure 1B). We further determined whether the process of PDMS display of fixed cell surfaces is applicable to other adherent cell types with human dermal fibroblasts, breast and prostate cancer cells (Supporting Figure 1). The process was virtually applicable for any adherent cell and can be scaled up by simply increasing the surface area of the culture substrate.
Figure 1. Fixed stromal cells transfer process.
(A) Schematic of a fixed-PDMS transfer of stromal cells with corresponding images. Adherent cells were cultured in a 15cm diameter of Petri dish and stained with crystal violet dye in order to visualize the process. (B) Morphological comparison between live, fixed and fixed-PDMS transferred BMSCs under optical microscope.
We next characterized the structural composition of the cellular surface at each stage of the capture and display process under scanning electron microscope (SEM). Cross-sectional images revealed the tight integration of the cellular surface with a PVA film that made the interface between the two surfaces indistinguishable. The bottom side of the cell-PVA film showed a distinct submicron scale rough topology as a result of split cellular components (Figure 2A). Significant cellular mass remained on the tissue culture plate that clearly exhibited disrupted intracellular organelles such as the nucleus and Golgi apparatus (Figure 2B, Supporting Figure 2). The surface morphology of fixed-PDMS transferred stromal cells was strikingly similar to their original counterpart without any residual PVA polymer. We confirmed that the cellular features were well transferred to PDMS even at a nanoscale resolution (Figure 2C). These results indicate that: (i) the PVA polymer can effectively deposit on cell membranes and capture surface components, (ii) stripping of the film splits cells into two distinct parts (i.e. captured within a PVA film and anchored on tissue culture plastic), (iii) PDMS is capable of stably transferring the PVA film captured cell surface components once solidified, and (iv) the intermediate PVA film is completely dissolved.
Figure 2. Morphological characterization of fixed BMSC transfer onto PDMS under SEM.
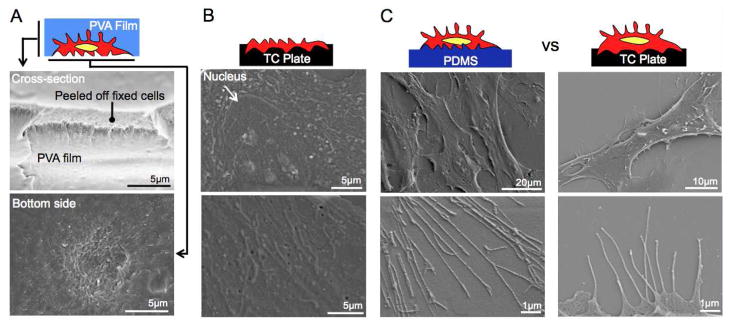
(A) A cross-sectional image of stripped Cell-PVA film indicates complete integration of fixed stromal and PVA film without any gap. This image shows a cross-section of layered stromal cells. The bottom side Cell-PVA film shows submicron scale rough surface as a result of torn intracellular components. (B) A tissue culture plate after being stripped off a cell-PVA film shows intracellular components of remaining cellular mass that formed firm attachment on substrate. (C) Morphological comparison of fixed-PDMS transferred BMSCs and fixed BMSCs shows integrally transferred fixed BMSCs surface on PDMS membrane even in nanoscale resolution.
PDMS molds displaying BMSC membranes could be readily cut into desired shapes and sizes and retained intra-cellular components as confirmed by crystal violet staining (Figure 3A). We also examined whether nuclear and plasma membrane proteins were detectable after the transfer process. Fixed-PDMS transferred cells reacted to nuclear and membrane staining in an analogous pattern to their fixed counterparts (Figure 3B, Supporting Figure 3). The captured cytoplasmic and nuclear material is inferred to be localized beneath the displayed cell surface and therefore compartmentalized from surface reactions. It is highly unlikely that the nuclear material, particularly nucleic acids, are functional yet further studies are required to understand if the presence of cytoplasmic and nuclear material could be harmful, beneficial or unimportant to the use of these materials for a given application. These molecular and morphological analyses imply that PDMS displayed BMSC surfaces is capable of preserving an entire stromal cell membrane surface and the spatial arrangement of surface proteins as a replica mold of fixed BMSCs. The bio-process was further applicable to transferring and displaying micro-patterned cell culture to a PDMS substrate maintaining cellular pattern and morphology (Supporting Figure 4).
Figure 3. Nucleus and plasma membrane staining of fixed-PDMS transferred BMSCs.
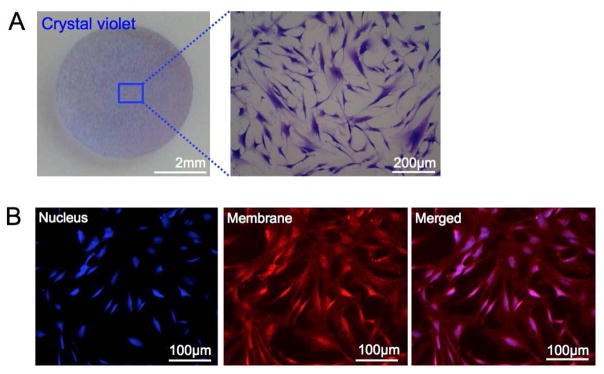
(A) Optical images at different magnifications of fixed-PDMS transferred BMSC disc after crystal violet staining. (B) Fluorescent images of nucleus DAPI stained, plasma membrane stained, and merged fixed-PDMS transferred BMSCs.
As a next step we measured the bioactivity of the PDMS membrane display to verify if the surface was functional. Previous studies have shown that human leukemic B cells (Nalm-6) actively adhere to BMSCs via surface ligand-receptor interactions between leukemic and stromal cells16. We used this phenomenon as an adhesion assay to quantify the biological activity of captured and displayed membranes on PDMS as compared to live and fixed counterparts. PDMS displayed cell surfaces supported equivalent Nalm-6 cell adhesion to fixed and live BMSCs (Figure 4A). SEM studies revealed that Nalm-6 cells physically contacted fixed and PDMS displayed BMSCs comparable to live BMSCs (Figure 4B). We quantified these results using an engineered Nalm-6 cell population that expressed firefly luciferase. At three different time-points within 24 hours, the co-culture was washed vigorously and the bioluminescent signal from Nalm-6 cells was immediately measured after adding luciferin substrate. Fixed and fixed-PDMS BMSCs supported a steady-state level of Nalm-6 cell adhesion, whereas live BMSCs showed slightly increased Nalm-6 cell adhesion over time. In order to exclude any effect of BMSC proliferation on Nalm-6 cell adhesion, we stopped the experiment after 24 hours. PDMS displayed BMSC surfaces showed comparable degree of Nalm-6 cell adhesion to fixed BMSCs that was approximately 75% of leukemic cell adhesion observed on live BMSCs (Figure 4C). Our results indicate that our bioprocess to capture and display stromal cell surfaces preserved a considerable level of biological activity that can be useful, in this example, for short-term leukemic cell binding assays.
Figure 4. Bioactivity testing of fixed-PDMS transferred BMSC layer for supporting Nalm-6 human leukemic cell adhesion.
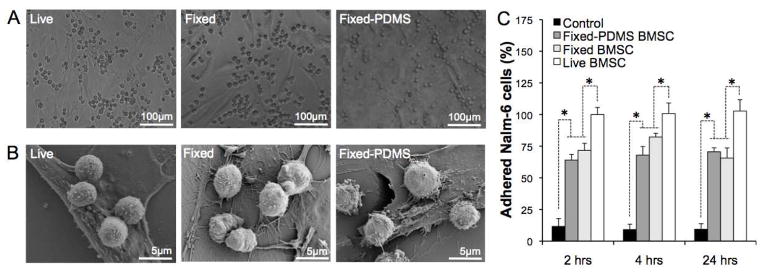
(A) Optical images of Nalm-6 cell adhesion on three different BMSC stromal layers after 24 hours incubation, (B) SEM images of cellular interaction between Nalm-6 cells and different BMSC stromal layers, (C) Quantitative characterization of adhered Nalm-6 cells on different stromal layers. (N=4, * P<0.05)
Our results demonstrate a new process for the transfer and preservation of fixed stromal cell surface to PDMS membranes using PVA as an intermediate carrier film. Several techniques have been introduced to create biomimetic surfaces including varying substrate rigidity17, 18 and surface topology19, coating biological20–22 and synthetic23, 24 molecules, as well as bioimprinting25, 26 and replicating27 cellular morphology. While retaining bioactivity, these methods do not recapitulate the complexity of a biological surface that cells encounter in vivo. The goal of this work was to develop a biological surface that mirrors a cell’s membrane morphology and biochemistry including an array of surface ligands in their native spatial organization.
Recent studies have demonstrated that glutaraldehyde fixed human BMSCs conserve the biological activity of stromal cells with respect to the display of surface adhesion and extracellular matrix proteins28–30 and their ability to support the ex vivo expansion of hematopoietic or embryonic stem cell populations31–33. Fixation can also preserve the biological activity of stromal cells for an extended period of time to improve experimental scale and reproducibility33. Glutaraldehyde is a strong cross-linking agent that rapidly develops a chemical mask on a cell surface and inevitably alters the physical, chemical and mechanical properties of surface proteins. This chemical fixation can inactivate some of the functionality of these molecules, which is a limitation of this approach. The benefit, however, is that glutaraldehyde fixation can protect the integrity of membrane proteins in their fixed configuration during some of the caustic downstream conditions of the process such as high PDMS curing temperature, microwaving, and ultraviolet sterilization. We used microwave energy to retrieve antigens, a technique commonly performed in immunohistochemistry to break covalent bonds made during fixation and restore protein structures for antibody recognition34.
Using PVA as an intermediate carrier film is the key inventive step of our approach. PVA has been used in various applications including emulsion polymerization, film and fiber production, and as a medical reagent because of its water-soluble, biocompatible, and film- forming properties35. After transferring membrane structures onto a new substrate (e.g. PDMS), the PVA film can be completely dissolved away without damaging surface structure as confirmed by SEM. Our cell surface transfer is an entirely physical process that does not involve any chemical reaction, which is beneficial to preserve biological activity of membrane molecules and also can be applied to virtually any hydrophilic molecules for immobilization on hydrophobic surfaces.
The fabrication process of PDMS displaying cell surfaces is flexible and scalable based on the area of BMSC culture that can improve the reproducibility of feeder layer-based experiments and can be designed into miniaturized, high throughput platforms for screening. For instance, more than 40 units of fixed-PDMS transferred BMSC discs having the same diameter of a 24-well plate (D-15mm) were obtained from a large surface area of BMSC culture (D=100mm). We have demonstrated that, by transferring high quality fixed stromal layers prepared on a tissue culture plate to functionally adaptive PDMS membranes, we can study apply fixed stromal layers on new substrates.
There are several characteristics of this bioprocess that are beneficial to new applications in cell surface capture and display technology. As proof-of-concept, we demonstrated the bioactivity of PDMS display stroma membranes using a short-term binding assay of human leukemic cells. Fixed-PDMS transferred BMSCs supported a similar level of leukemic cell adhesion to fixed BMSCs. One direct application of this short-term binding assay would be to separate live-dead leukemic cells based on their binding activity to fixed BMSCs. It should also be possible to integrate a PDMS based fluidic system to explore BMSC-leukemic cell interaction under fluidic environments. Accompanied with defined soluble factors, this approach potentially enables reconstructing more complete, controlled and standardized in vivo like soluble and insoluble microenvironments that can be used for long-term hematopoietic and embryonic stem cell studies.
CONCLUSION
We developed a simple and scalable technique for the transfer and preservation of fixed stromal cell surface molecules to PDMS membrane using PVA as an intermediate carrier film. In a broader sense, PVA can capture and transfer arrays of hydrophilic biomolecules e.g. proteins, bio-particles, or viruses as well as insoluble micro-/nano-particles. This new surface modification technique can spur the practical application of fixed cellular surfaces to be presented on a broad spectrum of materials to create a new class of biomaterial-cell membrane conjugates.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01EB012521 and K01DK087770) and postdoctoral fellowships from the Shriners Hospitals for Children and National Cancer Institute (1K99CA163671-01A1).
Footnotes
The authors declare no competing financial interest.
Characterization of fixed and PDMS transferred human skin fibroblasts, breast and prostate tumor cells. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003;24 (21):3563–76. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 2.Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 3.Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Microfabricated elastomeric stencils for micropatterning cell cultures. J Biomed Mater Res. 2000;52 (2):346–53. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Palchesko RN, Zhang L, Sun Y, Feinberg AW. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One. 2012;7(12):e51499. doi: 10.1371/journal.pone.0051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21 (1):27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Ellis AV, Voelcker NH. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis. 2010;31 (1):2–16. doi: 10.1002/elps.200900475. [DOI] [PubMed] [Google Scholar]
- 7.Mager MD, LaPointe V, Stevens MM. Exploring and exploiting chemistry at the cell surface. Nat Chem. 2011;3 (8):582–9. doi: 10.1038/nchem.1090. [DOI] [PubMed] [Google Scholar]
- 8.Nichols JE, Cortiella J, Lee J, Niles JA, Cuddihy M, Wang S, Bielitzki J, Cantu A, Mlcak R, Valdivia E, Yancy R, McClure ML, Kotov NA. In vitro analog of human bone marrow from 3D scaffolds with biomimetic inverted colloidal crystal geometry. Biomaterials. 2009;30 (6):1071–9. doi: 10.1016/j.biomaterials.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89 (12):4337–47. [PubMed] [Google Scholar]
- 10.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292 (5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 11.de Pooter R, Zuniga-Pflucker JC. T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol. 2007;19 (2):163–8. doi: 10.1016/j.coi.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13 (14):1883–900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 13.Parekkadan B, Tilles AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26 (7):1913–9. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- 14.Miretti S, Roato I, Taulli R, Ponzetto C, Cilli M, Olivero M, Di Renzo MF, Godio L, Albini A, Buracco P, Ferracini R. A mouse model of pulmonary metastasis from spontaneous osteosarcoma monitored in vivo by Luciferase imaging. PLoS One. 2008;3 (3):e1828. doi: 10.1371/journal.pone.0001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taulli R, Scuoppo C, Bersani F, Accornero P, Forni P, Miretti S, Grinza A, Allegra P, Schmitt-Ney M, Crepalidi T, Ponzetto C. Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res. 2006;66 (9):4742–4249. doi: 10.1158/0008-5472.CAN-05-4292. [DOI] [PubMed] [Google Scholar]
- 16.Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ. Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia. 2000;14 (5):882–8. doi: 10.1038/sj.leu.2401729. [DOI] [PubMed] [Google Scholar]
- 17.Fuard D, Tzvetkova-Chevolleau T, Decossas S, Tracqui P, Schiavone P. Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectronic Engineering. 2008;85 (5–6):1289–1293. [Google Scholar]
- 18.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28 (10):1123–8. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 19.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo RO, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10 (8):637–44. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Chen JX, Wang JHC. RGD peptide-conjugated poly(dimethylsiloxane) promotes adhesion, proliferation, and collagen secretion of human fibroblasts. Journal of Biomedical Materials Research Part A. 2006;79A(4):989–998. doi: 10.1002/jbm.a.30847. [DOI] [PubMed] [Google Scholar]
- 21.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28 (6):611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Kotov NA. Notch ligand presenting acellular 3D microenvironments for ex vivo human hematopoietic stem-cell culture made by layer-by-layer assembly. Small. 2009;5(9):1008–13. doi: 10.1002/smll.200801242. [DOI] [PubMed] [Google Scholar]
- 23.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, Shogbon C, Martin AW, Nelson J, West P, Beltzer JP, Pal S, Brandenberger R. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28 (6):606–10. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 24.Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O’Shea KS, Lahann J, Smith GD. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28 (6):581–3. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samsuri F, Mitchell JS, Alkaisi MM, Evans JJ. Formation of Nanoscale Bioimprints of Muscle Cells Using UV-Cured Spin-Coated Polymers. J Nanotech. 2009:Article ID 593410. [Google Scholar]
- 26.Tong WY, Shen W, Yeung CW, Zhao Y, Cheng SH, Chu PK, Chan D, Chan GC, Cheung KM, Yeung KW, Lam YW. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33 (31):7686–98. doi: 10.1016/j.biomaterials.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Bruder JM, Monu NC, Harrison MW, Hoffman-Kim D. Fabrication of polymeric replicas of cell surfaces with nanoscale resolution. Langmuir. 2006;22 (20):8266–8270. doi: 10.1021/la0608563. [DOI] [PubMed] [Google Scholar]
- 28.Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128 (5):929–38. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurley RW, McCarthy JB, Verfaillie CM. Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J Clin Invest. 1995;96 (1):511–9. doi: 10.1172/JCI118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy V, Verfaillie CM. Soluble factor(s) produced by adult bone marrow stroma inhibit in vitro proliferation and differentiation of fetal liver BFU-E by inducing apoptosis. J Clin Invest. 1997;100 (4):912–20. doi: 10.1172/JCI119607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito Y, Kawamorita M, Yamabe T, Kiyono T, Miyamoto K. Chemically fixed nurse cells for culturing murine or primate embryonic stem cells. J Biosci Bioeng. 2007;103 (2):113–21. doi: 10.1263/jbb.103.113. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Hasauda H, Kitajima T, Kiyono T. Ex vivo expansion of human cord blood hematopoietic progenitor cells using glutaraldehyde-fixed human bone marrow stromal cells. J Biosci Bioeng. 2006;102 (5):467–9. doi: 10.1263/jbb.102.467. [DOI] [PubMed] [Google Scholar]
- 33.Yue XS, Fujishiro M, Nishioka C, Arai T, Takahashi E, Gong JS, Akaike T, Ito Y. Feeder cells support the culture of induced pluripotent stem cells even after chemical fixation. PLoS One. 2012;7(3):e32707. doi: 10.1371/journal.pone.0032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Amico F, Skarmoutsou E, Stivala F. State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods. 2009;341 (1–2):1–18. doi: 10.1016/j.jim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res B Appl Biomater. 2012;100 (5):1451–7. doi: 10.1002/jbm.b.32694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.