Abstract
Recently-published information on the role of IFT polypeptides in vesicle exocytosis is reviewed, describing the formation of the immune synapse in non -ciliated cells as an example. An hypothesis is detailed suggesting that all polypeptides which enter the cilium, both membrane and axonemal, do so in association, first, with cytoplasmic vesicles which exocytose adjacent to the ciliary basal body, and then with the ciliary membrane. Axonemal proteins are moved to the ciliary tip by peripheral association with the inner aspects of the ciliary membrane by cannonical ciliary IFT. At the tip, some polypeptides are released for axonemal assembly, and others are budded off as part of vesicular exosomes into the environment. It is proposed that the cilium, in addition to being a sensory and motile organelle, is also a secretory organelle.
Introduction
It has now been almost 16 years since the discovery of Intraflagellar Transport (IFT) in the biflagellate alga Chlamydomonas by Kozminski et al. [1]. Since that time, investigation of the basic cell and molecular biology underlying the IFT process in the cilia and flagella of various organisms has led to an expanding literature on the role of cilia in disease [2-4]. These diseases have been grouped together and called “ciliopathies,” [5] or diseases related directly to defects in the assembly and/or function of cilia. Many of these ciliopathies have been related to “primary” non-motile (9 + 0) cilia, one of which is present on most non-dividing cells in our bodies, and which are involved in sensing the environment by specialized receptors concentrated on the ciliary membrane. Some of these ciliopathies were discovered by knocking out or down IFT polypeptides, thereby causing defects in ciliogenesis, in a variety of organisms, and observing the resulting tissue pathologies. However, as will be described in more detail below, it now appears that the IFT genes may be involved in more than ciliogenesis, and that one may be misled by assuming that the pathology resulting from knocking out or down an IFT gene product is due primarily to a ciliary defect.
Investigation of IFT in cilia assembly and function has also stimulated new interest in the role of motile cilia in disease. The role of ciliary motility in fertility and sterility has been well-researched, but new insights into the role of cilia motility in conditions such as situs inversus are just now being obtained [6,7]. The recent interest in ciliopathies has also shown relationships of ciliary motility in brain ventricles to pathologies such as hydrocelphalus [8].
It is well-known the that (9 + 2) motile cilia have similar sensory functions to the nonmotile primary cilia in addition to their mechanical function of moving cells or fluids over surfaces. The basic biological rule of ciliary function, most simply stated, is that “all cilia, motile or not, have a sensory function.” Recent papers which ascribe sensory functions to motile cilia as being “new findings” therefore are not correct [9]; indeed, the sensory function of motile cilia was noted long ago [10] and has been studied for decades (e.g. see reviews on Paramecium [11] and Chlamydomonas [12]), and, recently, sensory proteins have been identified in other motile cilia [13-15].
Because the role of IFT in cilia assembly and, thereby, its role in a great variety of diseases, has already been adequately reviewed, it will not be the purpose of this brief article to go over this literature again. Indeed, an entire volume has recently been published, exhaustively describing the role of cilia and IFT in vertebrate pathologies [16]. Rather, we will use this opportunity to examine some recent findings on new functions for IFT and cilia and to formally detail a hypothesis on how all ciliary proteins, in the ciliary membrane and the axoneme, may be moved into and out of the cilium from the cytoplasm.
Are the IFT Polypeptides Involved Only in Cilia Assembly/Disassembly and Function?
The IFT gene analyses of Jekely and Arendt [17] and Avidor-Reiss [18] suggested that the IFT polypeptides have protein-protein interaction motifs arranged similarly to those in some of the clathrin/COP1 polypeptides involved in the exocytosis process, in which Golgi-derived cytoplasmic coated vesicles fuse with the cell membrane. They speculated that the exocytotic system in early non-ciliated cells gave rise to a specialized plasma membrane domain, and, ultimately, this domain and the co-evolution of the now-classical ciliary IFT system from the COP1/clathrin exocytosis polypeptides gave rise to the development of the eukaryotic cilium. The publication of these reports helped stimulate research directed at determining if IFT polypeptides could, indeed, be found on the cytoplasmic vesicle pathway between the Golgi and vesicle fusion at the cell membrane. The first suggestion that IFT polypeptides might be involved in functions other than ciliogenesis came from studies on the immuno-localization of IFT polypeptides in vertebrate rod cells of the retina. In these cells, the IFT polypeptides were concentrated, as usual, around the basal body/centriole of the connecting cilium and in the connecting cilium itself [19]. However, IFT antibody fluorescence was also noted at the other end of the rod cell where synapses are present between the rod cell and neurons heading back to the CNS, and it is here at the presynaptic ending that synaptic vesicle exocytosis takes place. The authors of the report do not comment extensively on this synaptic localization of IFT polypeptides and may even have regarded it as an artifact. The first detailed report showing that at least one IFT polypeptide might be involved in vesicle trafficking and exocytosis indicated that IFT20 is localized at the Golgi complex, is anchored there by the Golgi polypeptide, GMSAP210, and moved between the Golgi and the cilium. Other IFT polypeptides were not found in this pathway [20,21]. Like other IFT polypeptides, IFT20 can also be found closely associated with the ciliary basal body/centriole, and, of course, the cilium itself where it is a member of the Complex B subunit of the IFT particle [22]. Since IFT20 has been shown to be on the “outside” of the Complex B IFT sub-particle [23], its association with cytoplasmic vesicles may be maintained even after these vesicles fuse with the cell membrane, adjacent to the ciliary basal body/centriole. A localization on the outside of the cytoplasmic vesicle would, therefore, place it on the inner surface of the cell or ciliary membrane following exocytosis. Almost certainly the other IFT polypeptides, largely localized around the basal body/centriole, join the membrane-associated IFT20 at the time of exocytosis to facilitate the exocytotic process itself and, secondly, to participate in the assembly of the intact IFT particles. The IFT particles, now in “trains” [24] continue into the flagellum, associated with the membrane. The model that presents itself (Figure 1), therefore, is one in which IFT 20 accompanies Golgi-derived vesicles to the point of exocytosis near the basal bodies where the other IFT polypeptides are present, and where the intact IFT particle is assembled in association with the inner surface of the membrane, followed by the passage of the IFT complex through the flagellar pore recognition site at the transition region [25] and into the ciliary compartment. As described below, this vesicle trafficking pathway may also be the path that all cilia polypeptides, both membrane and axonemal, take if they are destined to enter the cilium.
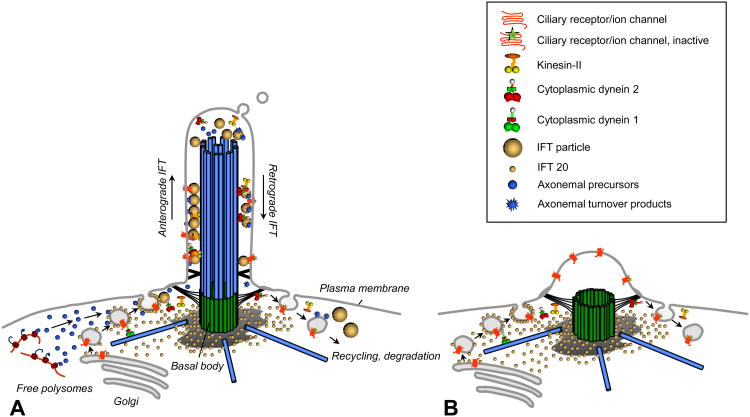
Figure 1.
These diagrams illustrate similarities between (A) cilia and the (B) immune synapse. IFT20 is associated with the Golgi and vesicles destined for the cilia and is also involved in formation of the immune synapse. Although the entire complement of IFT proteins has not been examined, at least IFT57, IFT88 and the IFT motor Kif3a are also involved in formation of the immune synapse [26]. The centriole, which nucleates the microtubules of the cilium also moves near the cell surface during formation of the immune synapse, positioned between Golgi and the plasma membrane, which sometimes is seen to form a bulge in this area. Figure modified after [53].
IFT is Associated with Exocytosis Even in Cells Without Cilia
Recently, the IFT system has been found in non-ciliated cells, associated with exocytosis. In this work IFT poypeptides and the kinesin-2 motor were shown to be present in T lymphocytes and to be required for T-cell interaction with antigen-presenting cells bearing specific ligands on their surface [26]. This interaction promotes the rapid redistribution of proteins and lipids on the T lymphocyte plasma membrane, which results in the transient assembly of a highly specialized membrane patch at the interface with the antigen presenting cell, known as the immune synapse, which acts as a signaling platform [27]. Both the centrosome and the Golgi apparatus polarize to the immune synapse, thereby assisting targeted delivery to this location of the various components required for synapse formation [28,29]. Of considerable interest from the standpoint of the evolution of the eukaryotic cilium [30] is the fact that when the immune synapse forms between cytotoxic T cells and target cells, a small ‘bulge’ appears above the centrosome of the T cell (Fig. 1B), as if a cilium is about to be assembled on the centriole [31]. It is to this membrane ‘bulge’ that the lytic granules are directed for polarized delivery onto the target cell.
The recent report [26], which clearly shows that T lymphocytes express a number of IFT polypeptides, as well as the IFT-dependent motor, kinesin-2, is a striking example of the function of the IFT system in the process of exocytosis. It is especially important since these cells do not have, or never will have, cilia. In these cells, IFT20 and other IFT polypeptides associate with both the centrosome and the Golgi, similar to the localization of IFT20 in ciliated cells [20]; IFT20 co-localizes, moreover, with post-Golgi membrane compartments, i.e. trans-Golgi network, recycling endosomes, early endosomes. When T lymphocytes are engaged by antigen-presenting cells bearing specific ligands, IFT20 is recruited together with other IFT polypeptides to the immune synapse. Importantly, RNAi knockdown of either the kinesin which powers ciliary IFT or other IFT polypeptides was found to interfere with immune synapse formation and downstream signaling. The defect was traced to a defective polarization of recycling endosomes to the immune synapse, a process which is crucial for targeted delivery of the T-cell receptors to the synapse [32]. Targeting of the endosomal vesicles involves the transient assembly of an IFT polypeptide complex onto the T-cell antigen receptors primed by IFT20. Interestingly, IFT polypeptides did not appear to be involved in reclaiming the membrane moieties of the immune synapse back into the cell by endocytosis, and this process occurred normally in the IFT knockdown cells. These data are some of the first to illustrate that the predicted homology between IFT polypeptides and the COPI/clathrin polypeptides involved in exocytosis [17,18] is functional, and that IFT polypeptides will probably be observed in many systems in which exocytosis is occurring, e.g. neuronal synapses and, generally, exocytosis of vesicles containing secretory proteins in addition to the targeting of signaling receptors, ion channels, and other polypeptides to specific membrane locations.
The work described above on the role of IFT polypeptides in immune synapse formation also provides a cautionary note to those researchers who, having knocked out, or down, IFT or IFT motor polypeptides, ascribe the resulting phenotype or pathology to a ciliary defect, labeling it a “ciliopathy.” The primary defect in some of these observed ciliopathies may be at the level of the Golgi-secretory vesicle pathway in spite of the fact that ciliogenesis is ultimately affected. At the very least, knocking IFT gene products down or out means doing a thorough investigation of both exocytosis and ciliogenesis in the system being investigated.
Are there other cell biological processes that require IFT? Recently we published a report [33] that showed that one of the IFT polypeptides, IFT27, a small G-protein, which, when knocked down by RNAi, appeared to be lethal, unlike other IFT Complex B polypeptides whose knockout/down generally had little or no effect on the cell cycle, even though their knockdown affected, to some degree, ciliogenesis. The lethality appeared to be associated with an inhibition of cytokinesis. Since it is also known that cilia loss or resorption normally occurs prior to the S phase of the cell cycle, before cytokinesis, we first assumed that the lethality after IFT27 RNAi knockdown was somehow related to its effect on ciliogenesis. However, in light of the reports described above on the role of certain IFT polypeptides in exocytosis, we now believe that this lethality, following loss of the G-protein IFT27, is probably due to its direct (non-ciliary) effect on the process of vesicle fusion and exocytosis at the forming cleavage furrow. Indeed, in following the localization of IFT27 during the cell cycle in Chlamydomonas we find highly specific localization of IFT27 on the vesicles associated with cytokinetic furrow formation (Z. Wang et al., abstract in Mol. Biol. Cell suppl (2008) 19 M-L2 (CD-ROM), and C. Wood, Z. Wang, J. Umen, J. Rosenbaum, unpublished). This IFT27 RNAi knockdown effect on cytokinesis is, therefore, still another example of an IFT polypeptide probably having its primary effect on a process other than ciliogenesis; once again on exocytosis.
Delivery of Axonemal Polypeptides into the Flagellar Compartment
Since certain IFT polypeptides appear to be involved in the targeted delivery of Golgi-derived vesicles and their accompanying polypeptides to specific vesicle fusion and exocytosis points on the cell surface, it is possible that these same vesicles are also carrying additional proteins, peripherally-associated with the outside of the vesicles, to the same membrane location. These proteins, unlike the integral vesicle proteins themselves, are hypothesized to be synthesized on free, rather than membrane-bound, polysomes.
In the specific case of the cilium, it is our hypothesis [34] that vesicle targeting to a point adjacent to the ciliary basal body/centriole, a place where ciliary membrane glycoprotein targeting has already been clearly observed by electron microscopy [35], may also be used for the targeting of axonemal proteins to the cilium. We suggest, therefore, that not only ciliary membrane but also axonemal polypeptides are targeted to the cilium by association with vesicles of the Golgi-exocytosis pathway. Probably one of the best examples of the delivery of an axonemal protein would be the targeting of the tubulin dimer to the cilium. Alpha/beta tubulin dimer is synthesized on free polysomes in the cytoplasm, and there is no clear indication, at this point, as to how this major axonemal structural protein enters the ciliary compartment. It is our contention that, following synthesis, tubulin becomes peripherally associated with the outside of vesicles being targeted by exocytosis to the ciliary membrane. It is already quite clear that tubulin is associated with isolated flagellar membranes [36,37], and recent work (K. Huang et al., Mol. Biol Cell 16a, 2005, abst. #1299 and K. Huang, C. Wood, and J. Rosenbaum, unpublished) shows that tubulin is also associated with vesicles purified from the cytoplasm that are destined for the cilium. A similar example of this type of targeting of soluble proteins synthesized on free polysomes to a specific membrane compartment is the recent work showing the association of actin filaments with Golgi-derived vesicles which are being targeted to the furrow being formed by exocytosis during cytokinesis. The actin attaches peripherally to the outside of the targeted endosomal vesicles and, following exocytosis, ends up in the correct position on the inside of the furrow ready to take part in cytokinesis [38]. Likewise, tubulin attached to the outside of vesicles on their way to exocytosis adjacent to the basal body/centriole, would end up inside the cell membrane after exocytosis, close to the IFT system surrounding the centriole, and, while still associated with the membrane, would be moved into the flagellum by the now-intact IFT system. This would require that axonemal proteins have sequences that specifically target them to the outside of the vesicle surface prior to exocytosis. It is now known that axonemal dynein, which is prefabricated in the cytoplasm [39], like the radial spokes [40], is attached to an adaptor protein which is required for its transport into the flagellum [41] but it is not known if this adaptor-dynein complex is associated with cytoplasmic vesicles. It is already established that the IFT particles are not only closely associated with the inner surface of the flagellar membrane [24] but that they are also involved in the movement of integral flagellar membrane polypeptide channels up and down the length of the flagellum within the plane of the flagellar membrane bilayer [42,43]. This means of transport of axonemal polypeptides such as tubulin to the flagellar tip assembly site in association with the ciliary membrane has already been suggested by Stephens from his studies on tubulin in the cilia membranes of regenerating cilia of the sea urchin blastula [44].
Is the Cilium a Secretory Organelle?
It has been observed by thin sectioning and electron microscopy of Chlamydomonas cells that vesicles (exosomes) pinch off from the tip of the flagellum (K. Huang et al., Mol. Biol Cell 16a, 2005, abst. #1299). These vesicles, which can be isolated and purified from the medium [45-47], are now shown to contain a subset of the polypeptides one finds in membranes obtained from isolated, intact flagella, i.e. there are both quantitative and qualitative differences between the polypeptides of isolated flagellar membranes and the flagellar membrane vesicles isolated from the medium. Although tubulin is present in both membrane preparations, there is much more in the flagellar membrane preparation. This is what one might expect if macromolecules were being delivered to the flagellar tip in association with the ciliary membrane for assembly and turnover of the axoneme at its distal end.
It is not known if a similar secretion to that observed with Chlamydomonas flagella occurs from primary cilia in vertebrate tissues, although there is evidence that primary cilia on neuroepithelial cells are a source of membrane containing the somatic stem cell marker, prominin-1, found in neural tube fluid [48]. It has also been shown that vesicles isolated from urine can interact with the primary cilia of kidney tubule cells, although the exact origin of the vesicles is not known [49]. It is also possible that exosomes secreted from ciliary tips could be moved to the surface of other cells where they interact to initiate signaling pathways, analogous to that proposed for the left to right cilia-dependent movement of vesicles secreted from the tips of microvilli in the mouse embryonic node prelude to the establishment of left-right symmetry in the embryo [50]. The vesicles isolated from the medium in which sexually active gametes of one mating type of Chlamydomonas are growing are able to cause flagellar agglutination [45-47] and promote gamete activation during mating of intact gametes and this could be one of the functions of vesicle secretion from the flagellar tips, at least in Chlamydomonas.
It is not known if the primary cilia found on many non-dividing vertebrate cells can signal other cells or cilia on adjacent cells by physically interacting with these adjacent cells or cilia. Certainly one of the best examples of functional interaction between flagella on different cells is in Chlamydomonas where physical interaction between the tips of the plus and minus gametes initiates the PKD-2 channel-dependent signaling pathway resulting in the loss of the cell walls, differentiation of mating structures on the plus and minus gamete cells, and gamete cell fusion to form the zygote [12,43]. In theory, similar interactions resulting in the initiation of signaling pathways could take place between primary cilia on adjacent vertebrate cells, particularly during embryonic tissue differentiation.
The above suggests that the ciliary membrane is just one more membrane compartment in a pathway starting in the ER and Golgi providing channels, receptors and other signaling components to the cilium, and possibly as a source of exosomes secreted into the medium. The study of this pathway in a model genetic system is, currently, best done in the bi-flagellate alga Chlamydomonas, where all the compartments can be isolated, i.e. cytoplasmic vesicles, cell membranes, flagellar membranes, and vesicles pinched off from the cilium into the medium. There is little or no cellular contamination of the vesicle fraction isolated from the medium because wild type cells are surrounded by a cell wall, the flagellar membrane being the only “naked” membrane of the cell. If this is to be investigated in cells in culture or tissues by means other than, or in addition to, fluorescence microscopy, then it would be helpful to have efficient methods for the isolation of primary cilia and, in turn, ciliary membranes from these cells. Although methods for doing this have been published, it is technically demanding, and only small amounts of cilia are obtained [51,52].
In this brief ‘Opinions’ report we have hi-lighted some recent work on IFT and cilia, especially as related to processes other than ciliogenesis. We have also formally presented a hypothesis for the movement of polypeptides of the ciliary axoneme into the cilium as well as for the secretion of membrane vesicles from the ciliary tips into the environment. The technology and biological material for testing these hypotheses are available.
Acknowledgments
Thanks to Dennis Diener, Rosenbaum Laboratory, for help in preparing the manuscript and Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cosima T. Baldari, Email: Baldari@unisi.it, Department of Evolutionary Biology, University of Siena, Siena 13200, Italy.
Joel L. Rosenbaum, Email: Joel.Rosenbaum@yale.edu, Department of Molecular, Cellular and Developmental Biology, Yale University, New Haven, Ct. 06520-8103.
References
- 1.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazour GJ, Rosenbaum J. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 3.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma N, Berbari N, Yoder B. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 5.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of right-left asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 7.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left- right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 8.Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnamon SC, Reynolds SD. Using taste to clear the air(ways) Science. 2009;325:1081–1082. doi: 10.1126/science.1179180. [DOI] [PubMed] [Google Scholar]
- 10.Gray J. Ciliary Movement. London: Cambridge University Press; 1928. [Google Scholar]
- 11.Preston R, Saimi Y. Ca2+ and motility in Paramecium. In: Bloodgood R, editor. Ciliary and Flagellar Membranes. Plenum Press; 1990. pp. 173–200. [Google Scholar]
- 12.Pan J, Snell WJ. Signal transduction during fertilization in the unicellular green alga, Chlamydomonas. Curr Opin Microbiol. 2000;3:596–602. doi: 10.1016/s1369-5274(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 13.Huang K, Kunkel T, Beck CF. Localization of the blue-light receptor phototropin to the flagella of the green alga Chlamydomonas reinhardtii. Mol Biol Cell. 2004;15:3605–3614. doi: 10.1091/mbc.E04-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525–535. doi: 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- 15.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoder B, editor. Curr Top Dev Biol. Vol. 85. Academic Press; 2008. Ciliary Function in Mammalian Development. [DOI] [PubMed] [Google Scholar]
- 17.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 18.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 19.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008;4:e1000315. doi: 10.1371/journal.pgen.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II- dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- 24**.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener D, Rosenbaum J, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol. 2009;187:135–148. doi: 10.1083/jcb.200905103. The first high resolution electron-tomographic analysis of IFT trains in situ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 26**.Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum J, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature Cell Biol. 2009;11 doi: 10.1038/ncb1977. in press. A clear demonstration of the role of IFT polypeptides in exocytosis in a non-ciliated cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr Opin Immunol. 2006;18:512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Sancho D, Vicente-Manzanares M, Mittelbrunn M, Montoya M, Gordón-Alonso M, Serrador J, Sánchez-Madrid F. Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions. Immunol Rev. 2002;189:84–97. doi: 10.1034/j.1600-065x.2002.18908.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Cofreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordon-Alonso M, Sung CH, Alarcon B, Vazquez J, Sanchez-Madrid F. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Satir P, Mitchell DR, Jekely G. How did the cilium evolve? Curr Top Dev Biol. 2008;85:63–82. doi: 10.1016/S0070-2153(08)00803-X. A thoughtful article on the origin of the eukaryotic cilium. [DOI] [PubMed] [Google Scholar]
- 31**.Clark RH, Stinchcombe JC, Day A, Blott E, Booth S, Bossi G, Hamblin T, Davies EG, Griffiths GM. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nature Immunology. 2003;4:1111–1120. doi: 10.1038/ni1000. see comment. The cell biological bais for immune synapse formation: light and electron microscopic analyses. [DOI] [PubMed] [Google Scholar]
- 32.Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation- induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 33.Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum JL, Witman GB. Intraflagellar Transport. Nature Rev Cell Mol Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 35.Bouck GB. The structure, origin, isolation, and composition of the tubular mastigonemes of the Ochromonas flagellum. J Cell Biol. 1971;50:362–384. doi: 10.1083/jcb.50.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens RE. Major membrane protein differences in cilia and flagella: evidence for a membrane- associated tubulin. Biochemistry. 1977;16:2047–2058. doi: 10.1021/bi00629a001. [DOI] [PubMed] [Google Scholar]
- 37.Stephens R. Ciliary membrane tubulin. In: Bloodgood R, editor. Ciliary and Flagellar Membranes. Plenum Press; 1990. pp. 217–240. [Google Scholar]
- 38.Albertson R, Cao J, Hsieh TS, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181:777–790. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol. 2008;183:313–322. doi: 10.1083/jcb.200802025. An important paper showing that another polypeptide adapts the IFT system to carrying dynein arms into the flagellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 43.Huang K, Diener D, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens R. Tubulin in sea urchin embryonic cilia: characterization of the membrane-periaxonemal matrix. J Cell Sci. 1991;100:521–531. doi: 10.1242/jcs.100.3.521. [DOI] [PubMed] [Google Scholar]
- 45.McLean RJ, Laurendi CJ, Brown RM., Jr The relationship of gamone to the mating reaction in Chlamydomonas moewusii. Proc Natl Acad Sci USA. 1974;71:2610–2613. doi: 10.1073/pnas.71.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergman K, Goodenough UW, Goodenough DA, Jawitz J, Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol. 1975;67:606–622. doi: 10.1083/jcb.67.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snell WJ. Mating in Chlamydomonas: a system for the study of specific cell adhesion. I. Ultrastructural and electrophoretic analyses of flagellar surface components involved in adhesion. J Cell Biol. 1976;68:48–69. doi: 10.1083/jcb.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, et al. Characterization of PKD protein-positive exosome-like vesicles. J AmerSoc Neph. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell KA, Gallagher BC, Szabo GS. OAd: NDP kinase moves into developing primary cilia. Cell Motil Cytoskeleton. 2004;59:62–73. doi: 10.1002/cm.20025. [DOI] [PubMed] [Google Scholar]
- 52.Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]