Abstract
A series of O-aryl- and alkyl-substituted phosphorodithioates were designed and synthesized as hydrogen sulfide (H2S) donors. H2S released capability of these compounds was evaluated by fluorescence methods. O-aryl substituted donors showed slow and sustained H2S release while O-alkylated compounds showed very weak H2S release capability. We also evaluated donors’ protective effects against hydrogen peroxide (H2O2)-induced oxidative damage in myocytes and donors’ toxicity toward B16BL6 mouse melanoma cells.
Hydrogen sulfide (H2S) was known as a toxic pollutant for years. However, since 2000 this gaseous molecule has been classified as an important cell signaling molecule, much like nitric oxide (NO). Literature published in the past few years increasingly suggests that H2S is a mediator of many physiological and/or pathological processes.1–5 The production of H2S in mammalian systems has been attributed to at least three endogenous enzymes:6–9 cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfur-transferase. These enzymes use cysteine or cysteine derivatives as substrates and convert them into H2S within different organs and tissues. In addition to these enzymatic pathways, there are also a range of comparably simple chemical events which may liberate H2S from the intracellular pool of ‘labile’ sulfur, for instance from the ‘sulfane sulfur’ pool (compounds containing sulfur atoms bound only to other sulfur atoms).10 While the exact mechanisms of action of H2S are still under investigation, some chemical and biochemical catabolic reactions of H2S have been disclosed that may be responsible for its biological functions. For example, H2S reacts readily with methemoglobin to form sulfhemoglobin, which might act as a metabolic sink for H2S. H2S is a powerful reducing agent and is likely to be consumed by endogenous oxidant species, such as peroxynitrite, superoxide, and hydrogen peroxide.11–13 H2S can also promote protein S-sulfhydration providing a possible mechanism whereby H2S alters the functions of a wide range of proteins and modulates signaling.14–16 It is likely that many more important reactions of H2S are to be discovered. Nevertheless, the production of endogenous H2S and the exogenous administration of H2S have been demonstrated to exert protective effects in many pathologies.1–5 For example, H2S has been shown to relax vascular smooth muscle, induce vasodilation of isolated blood vessels, and reduce blood pressure. H2S can also inhibit leukocyte adherence in mesenteric microcirculation during vascular inflammation in rats, suggesting H2S is a potent anti-inflammatory molecule. Additionally, it has become evident that H2S is a potent antioxidant and, under chronic conditions, can up-regulate antioxidant defense. These results strongly suggest that modulation of H2S levels could have potential therapeutic values.
In H2S research, researchers typically use sulfide salts (NaHS or Na2S) as H2S source. The pKa values for the first and second dissociation steps of H2S are 7.0 and >12.0, respectively.17–18 In aqueous state under the physiological pH of 7.4, the ratio of HS−/H2S is ~3:1 as long as sulfide salt solution is prepared. Sulfide salts are therefore considered as short-lasting H2S donors as they release H2S quickly. The rapid release of H2S may cause acute changes in blood pressure and may exert toxic actions. In addition, sulfide concentrations in aqueous solution can rapidly decrease due to volatilization,19 thus significantly limiting the utility of these chemical precursors.
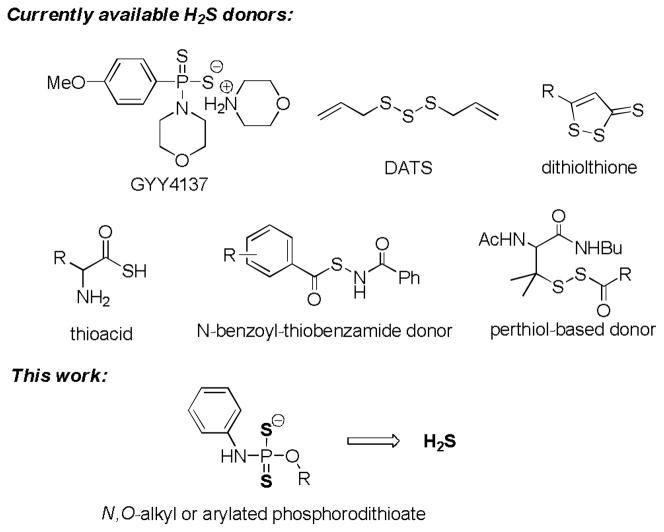
Due to these considerations, researchers have started to use synthetic H2S-releasing agents (i.e., H2S donors) to explore the biological functions of H2S.20–22 Currently there are six types of H2S donors known in literature (Scheme 1): 1) a Lawesson’s reagent derivative named GYY4137;23 2) garlic-derived organic polysulfides such as diallyl trisulfide (DATS),24 3) the dithiolthione moiety25 4) a series of N-(benzoyl)-thiobenzamide derivatives as thiol-activated H2S donors,26 5) S-acylated perthiol based donors,27 6) amino acid-based thioacids in the presence of bicarbonate buffers.28 Among these donors, GYY4137 is probably the most well-known donor. GYY4137 has a phosphorodithioate core structure and H2S release from this compound is due to hydrolysis. It is considered as a slow-release donor. GYY4137 has shown some H2S-relevant biological activities. For example, it relaxes arotas, vasodilates the preconstricted kidney, and exhibits antihypertensive activity in rats. It can also stimulate heart contraction by interaction with endogenous NO generation.29 Although well-applied in biological studies, only one donor (i.e. GYY4137) with fixed H2S release capability may not fulfill the requirements of different biological applications. We envisioned that the phosphorodithioate template have rich chemistry related to H2S to be explored. Structure modifications on phosphorodithioate may lead to H2S release capability change and in turn lead to their biological activity changes. Herein we report the synthesis and evaluation of a series of O-substituted phosphorodithioate-based H2S donors.
Scheme 1.
Structures of H2S donors.
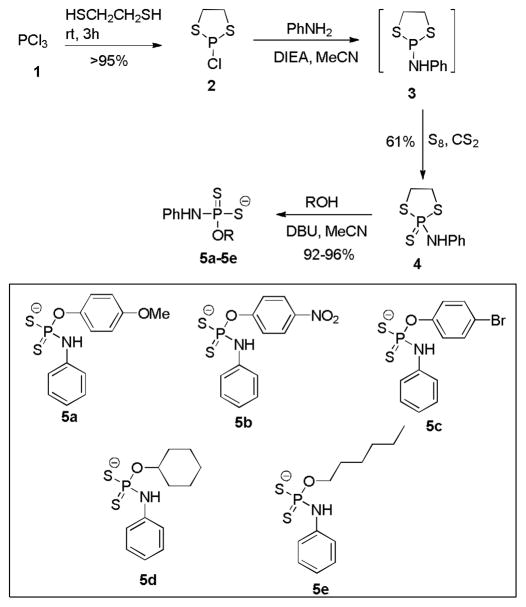
In the structure of GYY4137, there is a phenyl-phosphorus linkage via a C-P bond. We decided to replace the C-P bond with O-substitution and to explore H2S production from the resulted analogs. To this end, a four-step synthesis was developed (Scheme 2). Freshly distilled trichlorophosphine 1 was treated with 1, 2-dithioethane in 3:1 ratio in the absence of solvent and base. The desired product 2-chloro-1,3,2-dithiaphospholane 2 was obtained in almost quantitative yield by simply removing excess 1 upon distillation. Without further purification, compound 2 was treated with aniline followed by sulfurization with elemental sulfur to provide the key intermediate: 2-amine-1,3,2-dithiaphospholan-2-sulfide 4. Finally compound 4 was reacted with different phenols and alcohols in the presence of 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) to afford a series of phosphorodithioate analogs 5 as DBU salts. In all, five O-substituted compounds 5a–5e were prepared and their structures are shown in Scheme 2.
Scheme 2.
Synthesis of phosphorodithioate-based H2S donors.
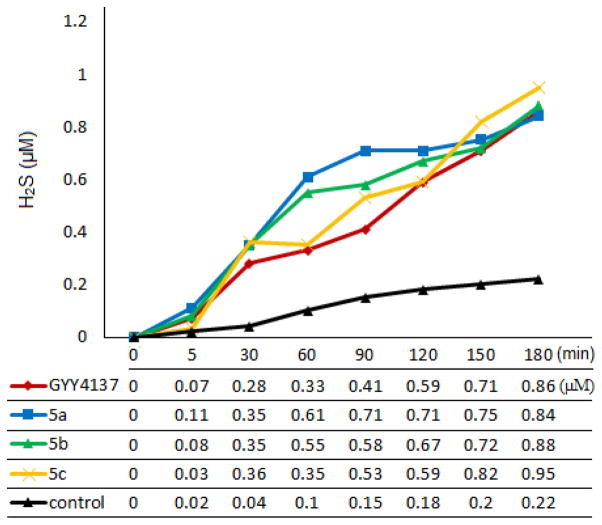
With these compounds in hand, we turned to test their H2S release capabilities. In comparison GYY4137 was also tested. Previously the measurement of H2S release from GYY4137 was mainly done by using the standard methylene blue method.23,30 However strong acidic conditions were involved in the methylene blue method. It is known that the hydrolysis of phosphorodithioates is affected by pH changes and hydrolysis under highly acidic conditions is much faster than under neutral pH.31 Therefore methylene blue method is not appropriate for evaluating phosphorodithioate-based donors. Recently a number of fluorescent probes have been developed for H2S detection32–34 and these probes are used under neutral pH. We envisioned that such probes could be useful for evaluating H2S donors. Dansyl azide (DNS-Az), a fluorescent probe developed by the Wang group,35 was selected in this study due to its fast reaction rate and easy of synthesis. In our experiments, 100 μM of each donor was dissolved in a mixed MeCN/phosphate buffer solution (1:1 v/v, pH 7.4) containing DNS-Az (200 μM). The changes of fluorescence emission spectra at 535 nm (λex = 340 nm) were then monitored for three hours at room temperature. Finally the fluorescence signals were converted to H2S concentrations based on a reference curve obtained with a series of Na2S standard solutions. Our results are summarized in Figure 1. GYY4137 was first tested as a reference compound. In consistent with previous reports, this donor showed slow and low H2S release. After 3 h GYY4137 produced approximately 600 nM H2S, which counted for ~0.6% of initial material. Using the same protocol the newly synthesized donors 5a–5e were also tested. Three compounds 5a–5c showed similar activities as GYY4137. They released H2S in a slow and sustain fashion and the amounts of H2S production were also similar to that of GYY4137. Compounds 5d and 5e only released trace amount of H2S (data not shown). Presumably the O-alkyl substitutions led to increased stability of phosphorodithioates and therefore decrease the tendency of the hydrolysis to generate H2S.
Figure 1.
Time dependent H2S releasing profiles of donors 5a–5c and GYY4137. Donors 5d and 5e released trace amounts of H2S (data not shown).
Having demonstrated H2S release from phosphorodithioate-based donors in buffers, we wondered whether they could produce H2S in cells. To address this question, we conducted cell image experiments using H9c2 myocytes. One example using donor 5a is shown in Figure 2. H9c2 cells were incubated with donor 5a under different concentrations (0, 100, and 200 μM) for 24 h. Then a H2S-specific fluorescent probe, WSP-1,36 was applied into the cells to monitor the production of H2S. As expected, donor-treated cells (Figure 2B and 2C) shown much enhanced fluorescent signals compared to vehicle treated cells (Figure 2A). These results suggest that phosphorodithioate-based donors indeed can sustainably release H2S in cells.
Figure 2.
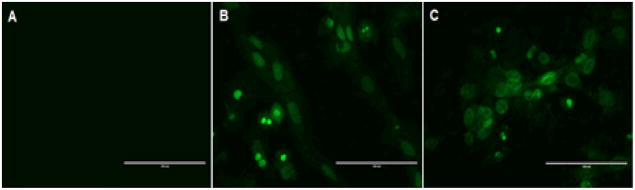
H2S production of donor 5a in H9c2 cells. Cells were incubated with vehicle (A), 100 μM 5a (B), and 200 μM 5a (C) for 24 h. After removal of excess donor, 100 μM of a H2S fluorescent probe (WSP-1) was added. Images were taken afterwards.
In our imaging experiments, we did not observe shape changes of the cells after the treatment with donors, which indicates the donors may not be toxic under the doses we used. To further understand phosphorodithioate-based donors’ cytotoxicity, we tested H9c2 cell viability upon treating with donors 5a–5c. NaHS and GYY4137 were also tested as controls. The cells were treated with 50, 100, and 200 μM donors for 24 h and then cell viability was determined using cell counter kit (CCK)-8 assay. As shown in Figure 3, 5a, 5b and NaHS did not show significant toxicity to H9c2 cells under these doses. 5c exhibited some toxicity when concentration increased. Interestingly GYY4137 was safe at 50 and 100 μM, but showed strongest toxicity at 200 μM.
Figure 3.
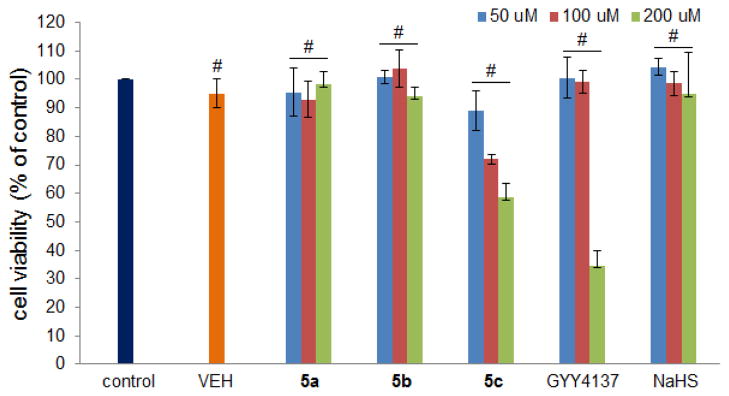
Effects of H2S donors on cell viability. H9c2 cells were treated with different concentrations of 5a–5c, GYY4137, and NaHS for 24 h. CCK-8 assay was performed to measure cell viability. Data were shown as mean ± SD (n = 4). #P < 0.01 versus control group.
Figure 5.
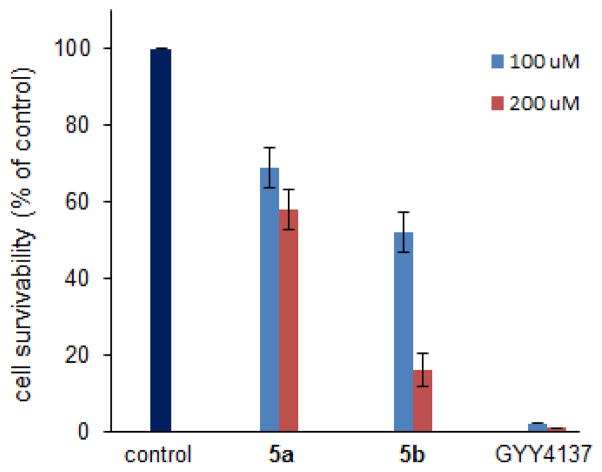
Mouse melanoma B16BL6 cells were treated with indicated donors in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin, respectively. Cells were cultured for 4 days and analyzed by a cell viability analyzer.
It is known that H2S exhibits dose-dependent improvement of cell viability following oxidative injury.37 We hypothesized that phosphorodithioate-based H2S donors could exert similar protective effects. 5a and 5b were then selected to explore their protective effects against H2O2-induced oxidative damage in H9c2 cells. Again GYY4137 and NaHS were tested as controls. Three concentrations (50, 100, and 200 μM) for each donors were used. For GYY4137 only two concentrations (50 and 100 μM) were tested because of its strong toxicity at 200 μM. In these experiments, cells were incubated with each donor for 24 h and then challenged with 150 μM H2O2 and cultured for 5 h. After that cell viability was determined by CCK-8 assay. As shown in Figure 4, cell viability dropped to about 65% if H2S donors were absent. In the presence of donors, however, cell viability increased significantly. These results suggest that H2S donors indeed have some protective effects against oxidative injury. It should be noted that we did not observe significant protective effects when donors were used at lower concentrations (Figure S4 in supporting information).
Figure 4.
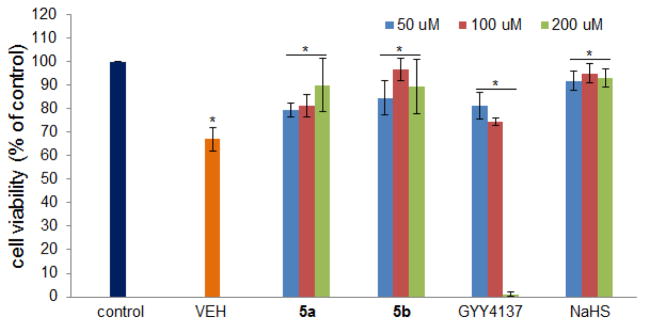
H9c2 cells viability after oxidative injury by H2O2 in the presence of donors 5a, 5b, GYY4137, and NaHS. Data were shown as mean ± SD (n = 4). *P < 0.01 versus control.
In 2011 Moore and co-workers showed that the slow-releasing donor GYY4137 exhibited some interesting anti-cancer activities in cells and mice xenograft.30 We wondered if our donors would show similar activity. Therefore we tested the effects of 5a, 5b, and GYY4137 on cell growth and viability of mouse melanoma B16BL6 cell line. Two different concentrations (100 μM and 200 μM) were applied. Cell growth inhibitory effects were monitored for a 4-day culture period. As shown in Figure 5, 5a showed moderate toxicity at both concentrations while 5b showed relatively strong toxicity at 200 μM. Interestingly, GYY4137 showed the strongest activity to this cell line, which may suggest the C-P linkage is crucial for potency. However, at this moment it is still uncertain if these donors’ inhibitory effects on B16BL6 cells are associated with their H2S production. Since H2S generation is a very slow process, the inhibition may come from donor compounds themselves. Nevertheless more studies are needed to elucidate the detail mechanism.
In conclusion, here we report the design and synthesis of a series of O-arylated and alkylated phosphorodithioate-based H2S donors. Their H2S releasing capabilities were evaluated by fluorescence methods. N,O-Diarylated donors showed slow and sustainable H2S generation which is similar to the well-known donor GYY4137, whereas O-alkylated donors exhibited very weak H2S production. In addition, we explored some H2S relevant activities of the donors. Compounds 5a and 5b exhibited notable protective effects against H2O2-induced oxidative damage in H9c2 myocytes. These donors also showed inhibitory effects against B16BL6 cancer cell proliferation but detailed mechanisms are to be explored. We are now actively studying other phosphorodithioates-based donors and their applications in H2S research.
Supplementary Material
Acknowledgments
This work is supported by an American Chemical Society-Teva USA Scholar Grant and NIH (R01GM088226).
Footnotes
Electronic Supplementary Information (ESI) available: experimental details, supporting figures, and characterizations. See DOI: 10.1039/b000000x/
Notes and References
- 1.Li L, Rose P, Moore PK. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 2.Wang R. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 3.Olson KR, Donald JA, Dombkowski RA, Perry SF. Respir Physiol Neurobiol. 2012;184:117–129. doi: 10.1016/j.resp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Chem Res Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavu M, Bhushan S, Lefer DJ. Clin Sci. 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H, Shibuya N, Kimura Y. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson KR. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Predmore BL, Lefer DJ, Gojon G. Antioxid Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob C, Anwar A, Burkholz T. Planta Med. 2008;74:1580–1592. doi: 10.1055/s-0028-1088299. [DOI] [PubMed] [Google Scholar]
- 11.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Amino Acids. 2008;34:573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 13.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. Biochem Biophys Res Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan N, Fu C, Pappin DJ, Tonks NK. Sci Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangerman A. J Chromatogr B: Anal Technol Biomed Life Sci. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Kabil O, Banerjee R. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLeon ER, Stoy GF, Olson KR. Anal Biochem. 2012;421:203–207. doi: 10.1016/j.ab.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Hughes MN, Centelles MN, Moore KP. Free Radical Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Kashfi K, Olson KR. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caliendo G, Cirino G, Santagada V, Wallace JL. J Med Chem. 2010;53:6275–6286. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 24.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Proc Natl Acad Sci U S A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Wang H, Xian M. J Am Chem Soc. 2011;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Bhushan S, Yang C, Otsuka H, Stein JD, Pacheco A, Peng B, Devarie-Baez NO, Aguilar HC, Lefer DJ, Xian M. ACS Chem Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, von Wantoch Rekowski M, Coletta C, Szabo C, Bucci M, Cirino G, Topouzis S, Papapetropoulos A, Giannis A. Bioorg Med Chem. 2012;20:2675–2678. doi: 10.1016/j.bmc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Yong QC, Cheong JL, Hua F, Deng LW, Khoo YM, Lee HS, Perry A, Wood M, Whiteman M, Bian JS. Antioxid Redox Signal. 2011;14:2081–2091. doi: 10.1089/ars.2010.3572. [DOI] [PubMed] [Google Scholar]
- 30.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bode H, Arnswald W. Z Anal Chem. 1962;185:99. [Google Scholar]
- 32.Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973–984. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin VS, Chang CJ. Curr Opin Chem Biol. 2012;16:595–601. doi: 10.1016/j.cbpa.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xuan W, Sheng C, Cao Y, He W, Wang W. Angew Chem Int Ed. 2012;51:2282–2284. doi: 10.1002/anie.201107025. [DOI] [PubMed] [Google Scholar]
- 35.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. Angew Chem Int Ed. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Angew Chem Int Ed. 2011;50:10327–10329. doi: 10.1002/anie.201104305. The probe reported in this paper is now commercially available as ‘WSP-1’ by Cayman Chemical Co. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo G, Veres G, Radovits T, Gero D, Modis K, Miesel-Groschel C, Horkay F, Karck M, Szabo C. Nitric oxide. 2011;25:201–210. doi: 10.1016/j.niox.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.