Abstract
Ebola virus causes severe hemorrhagic fever in susceptible hosts. Currently, no licensed vaccines or treatments are available; however, several experimental vaccines have been successful in protecting rodents and nonhuman primates (NHPs) from the lethal Zaire ebolavirus (ZEBOV) infection. The objective of this study was to evaluate immune responses correlating with survival in these animals after lethal challenge with ZEBOV. Knockout mice with impaired ability to generate normal T and/or B cell responses were vaccinated and challenged with ZEBOV. Vaccine-induced protection in mice was mainly mediated by B cells and CD4+ T cells. Vaccinated, outbred guinea pigs and NHPs demonstrated the highest correlation between survival and levels of total immunoglobulin G (IgG) specific to the ZEBOV glycoprotein (ZGP). These results highlight the relevance of total ZGP-specific IgG levels as a meaningful correlate of protection against ZEBOV exposure.
Introduction
Ebola virus (EBOV) is one of the most deadly human pathogens, with Zaire ebolavirus (ZEBOV) causing up to 90% mortality among infected individuals (1). There are currently no licensed EBOV treatments available, but several promising viral vector–based vaccine candidates expressing EBOV genes have been shown to protect nonhuman primates (NHPs) from EBOV infection. The EBOV glycoprotein (GP) has been the focus antigen in many EBOV vaccine strategies because it can induce both antigen-specific cellular and humoral immune responses (2). Vectors tested include recombinant adenovirus (3, 4), parainfluenza virus (5–7), and vesicular stomatitis virus (VSV) (8, 9). Of these, adenoviral-based vectors were among the first vaccine platforms capable of protecting NHPs against an otherwise lethal dose of EBOV (3, 4). Replication-deficient human adenovirus serotype 5 (AdHu5)– based platforms generate strong T and B cell responses to both the adenoviral capsid proteins and the transgene product (10–12). However, the nature of these protective immune responses is still not fully understood and is currently a matter of debate. For vaccine licensing and quality assurance purposes, it is crucial to elucidate the mechanism of immune response correlating with protection from EBOV to ensure consistency of vaccine-based immune protection.
Using the DNA-based and Venezuelan equine encephalitis virus vaccine platforms, EBOV-specific immunoglobulin G (IgG) has previously been suggested as a quantifiable correlate of protection against EBOV infection in the mouse and guinea pig animal models (13–15). Additionally, passive transfer of immune serum into naïve and severe combined immunodeficient mice resulted in complete protection and a delay in the peak level of viral replication, suggesting that the EBOV-specific IgG is linked to protection in mice (16). Vaccine-induced antigen-specific antibodies were reported in surviving NHPs immunized with VSV-based and parainfluenza virus–based EBOV vaccines, in addition to treatment with EBOV-like particles before lethal EBOV challenge (5, 8, 17).
An antigen-specific cellular and humoral immune response has also been suggested to contribute to immunity against EBOV infection in NHPs vaccinated with AdHu5 expressing GP (3, 4). A follow-up study concluded that the role of humoral immunity is limited because passive transfer of polyclonal antibodies from vaccinated to naïve NHPs induced only partial survival. Instead, it was concluded that the main immune parameter correlating with protection after adenovirus-based vaccination consisted of activated CD8+ T cells: There was no protection in vaccinated CD3+ T cell–depleted NHPs, and four of five NHPs depleted of CD8+ cells were not protected from EBOV challenge (18). A recent study, however, reported complete protection against EBOV and Marburg viruses after passive transfer of virus-specific immune sera in NHPs, contradicting the first assessment (19). In addition, passive transfer of monoclonal antibodies resulted in complete survival of lethally infected NHPs when administered 24 hours after infection with EBOV (20).
The present study evaluates specific T and B cell responses to ZEBOV glycoprotein (ZGP) after immunization with an AdHu5-based vaccine expressing ZGP (Ad-CAGoptZGP) or VSV-based vaccine expressing ZGP (VSV-ZGP) and their relative importance to survival against a lethal challenge with ZEBOV in mice, guinea pigs, and NHPs. Several strains of inbred knockout mice with genetic ablation in various portions of the adaptive immune system [CD8+ T cell, CD4+ T cell, interferon-γ (IFN-γ), B cell, and double T and B cell knockout] were vaccinated with Ad-CAGoptZGP, and immune responses essential for protection against ZEBOV challenge were characterized. Specific T and B cell responses were further investigated in the outbred guinea pig and NHP animal models where possible.
Results
Immune responses, weight loss, and survival in knockout mice
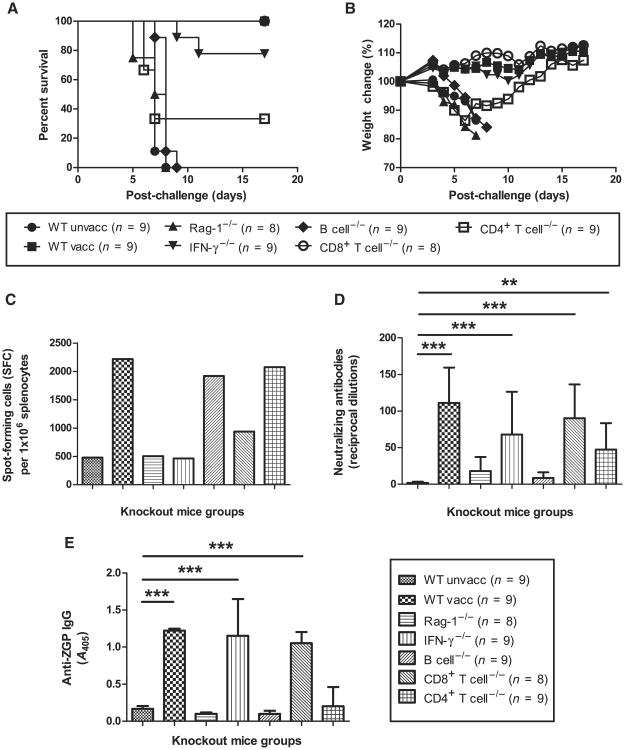
The Ad-CAGoptZGP vaccine was tested in C57BL/6J wild-type and various knockout mice of the same background for both protective efficacy and the induction of specific T and B cell immune responses, and then challenged with mouse-adapted ZEBOV (MA-ZEBOV) 28 days after vaccination. Infection of unvaccinated wild-type mice resulted in 100% mortality between days 5 and 8 after challenge, whereas complete protection was observed in vaccinated wild-type mice (Fig. 1A). Vaccinated Rag-1−/− mice failed to protect against MA-ZEBOV challenge. Complete protection was achieved in CD8+ T cell−/− mice. Vaccination of B cell−/− mice was ineffective in protection against ZEBOV with 100% mortality and rapid weight loss after challenge (Fig. 1, A and B). Vaccinated IFN-γ −/− and CD4+ T cell−/− mice were able to mount a partial protective immune response, with seven of nine mice and three of nine mice surviving the lethal challenge, respectively.
Fig. 1.
Survival and weight loss after challenge, and cellular and humoral immune response after vaccination in mice. (A and B) Protective efficacy (A) and weight loss (B) of vaccinated wild-type (WT) and knockout mice represented as percentage survival and weight change, respectively. (C) IFN-γ response. The number of IFN-γ–secreting cells is expressed as SFCs per million splenocytes. (D) Serum NAb levels. Antibody levels are reported as reciprocal dilutions. (E) Total serum IgG levels. Antibody levels are reported as A405. Error bars represent means ± SD.
Ten days after vaccination, mouse splenocytes were harvested and stimulated ex vivo with a ZGP peptide library. The numbers of ZGP-specific IFN-γ–positive cells were measured by enzyme-linked immunospot (ELISPOT) assay. Background T cell responses were detected for unvaccinated wild-type, Rag-1−/−, and IFN-γ−/− mice. ZGP-specific IFN-γ–secreting cells were observed in vaccinated wild-type, B cell−/−, CD8+ T cell−/−, and CD4+ T cell−/− mice with 2218, 1920, 936, and 2172 spot-forming cells (SFCs) per million splenocytes, respectively (Fig. 1C).
Humoral immune responses were quantified for neutralizing antibody (NAb) and total anti-ZGP IgG on sera harvested 28 days after vaccination using NAb assays and enzyme-linked immunosorbent assay (ELISA), respectively. The limit of detection was set at 10 reciprocal dilutions, and background NAb titers were observed for unvaccinated wild-type, Rag-1−/−, and B cell−/− mice (Fig. 1D). Average NAb titers detected in vaccinated wild-type, CD8+ T cell−/−, IFN-γ−/−, and CD4+ T cell−/− mice were 111 ± 48 (P = 0.0003), 90 ± 47 (P = 0.0005), 68 ± 58 (P = 0.0007), and 47 ± 36 (P = 0.0034), respectively. Background total anti-ZGP levels were observed for unvaccinated wild-type mice with an average absorbance at 405 nm (A405) of 0.17 ± 0.04 (Fig. 1E). Nonzero optical density readings were observed for Rag-1−/− and B cell−/− mice. Increased total anti-ZGP IgG levels were detected for vaccinated wild-type, CD8+ T cell−/−, and IFN-γ−/− mice, with corresponding A405 values of 1.22 ± 0.02, 1.05 ± 0.15, and 1.15 ± 0.50, respectively (all P < 0.0001). The two nonsurviving IFN-γ−/− mice had lowered levels of anti-ZGP IgG compared to the survivors. CD4+ T cell−/− mice had an average A405 value of 0.20 ± 0.26, where only two survivors from a total of nine mice tested positive for anti-ZGP IgG. This difference as a group was not statistically significant compared to unvaccinated wild-type mice (P > 0.05). Cumulatively, these results suggest that among the parameters monitored, IFN-γ, CD4+ T cell, and, most importantly, the B cell response play essential roles in the development of a protective immune response against MA-ZEBOV in mice after vaccination with Ad-CAGoptZGP.
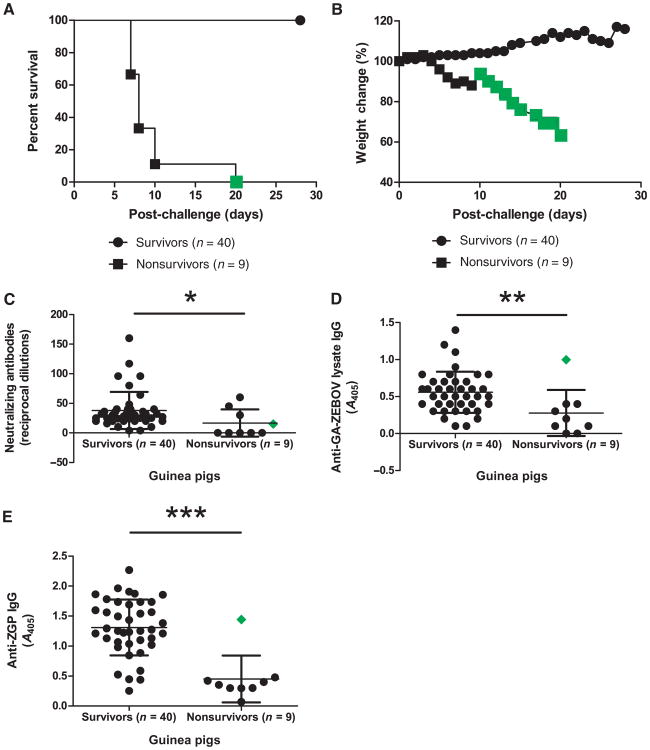
Humoral immune responses and survival in guinea pigs
Guinea pigs were used to further analyze immune markers correlating with protection against ZEBOV infection. Guinea pigs were challenged with guinea pig–adapted ZEBOV (GA-ZEBOV) 28 days after vaccination, which typically causes death within 7 to 9 days in untreated animals, but treated nonsurvivors have been observed to succumb to disease between 7 and 10 days, with one animal lasting 20 days after challenge (Fig. 2, A and B). Data generated for this animal have been highlighted in Fig. 2 for clarity. Because of the unavailability of molecular tools to evaluate T cell responses in this animal model, only antibody responses were monitored for survivor versus nonsurvivor animals 28 days after vaccination but before challenge.
Fig. 2.
Survival and weight loss after challenge, humoral immune response after vaccination in guinea pigs. (A and B) Protective efficacy (A) and weight loss (B) of survivor and nonsurvivor guinea pigs represented as percentage survival and weight change, respectively. (C) Serum NAb response. Antibody levels are reported as reciprocal dilutions. (D and E) Total serum IgG levels with GA-ZEBOV lysate (D) or His-tagged ZGP (E) as the capture antigen. Antibody levels are reported as A405. The animal that succumbed to ZEBOV infection 20 days after challenge was highlighted (green diamond). Error bars represent means ± SD.
Average levels of ZEBOV-specific NAb reached 38 ± 31 in survivors and 17 ± 23 in nonsurvivors (P = 0.0221) (Fig. 2C). Two ELISA-based approaches were used to identify circulating IgG levels. In the first method, GA-ZEBOV–infected cell lysate was used as a capture antigen to assay for the overall IgG immune response to ZEBOV. Sera IgG levels between survivors and nonsurvivors were 0.56 ± 0.28 and 0.28 ± 0.31 A405, respectively (P = 0.0054) (Fig. 2D). In the second method, recombinant ZGP protein was used as the capture antigen to assay for ZGP-specific immune responses. Using this method, surviving and nonsurviving animals had circulating IgG levels of 1.31 ± 0.47 compared to 0.45 ± 0.39 A405, respectively (P = 0.0002) (Fig. 2E). Using a 95% confidence interval, the range of lysate-specific IgG levels and total ZGP for survivors falls between 0.47 to 0.65 and 1.16 to 1.46 A405, respectively, whereas the range for nonsurvivors is between 0.04 to 0.52 and 0.15 to 0.75 A405, respectively. Overall, IgG levels corresponding to survival could be measured more precisely with ZGP-specific IgG than total ZEBOV-specific IgG levels.
Humoral immune responses in NHPs before and after challenge
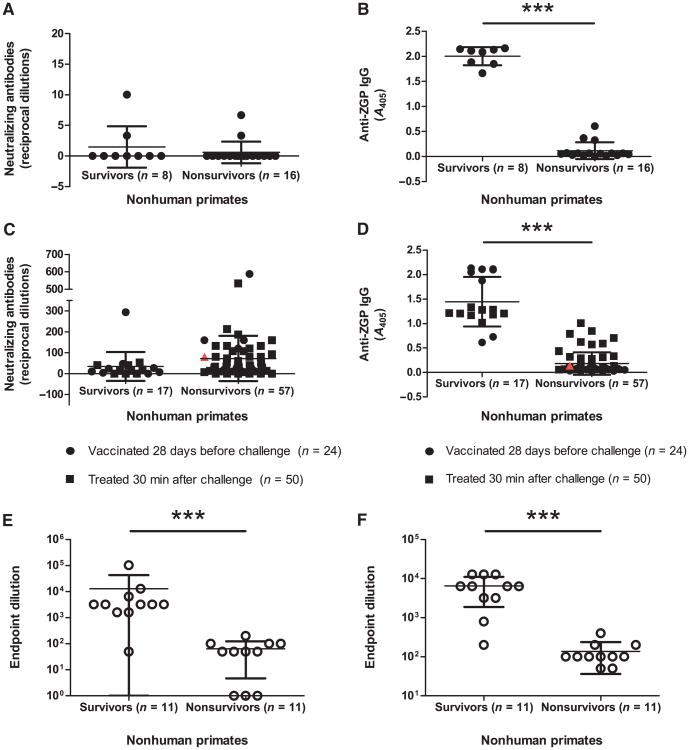
Because ZEBOV disease progression in humans is most closely mimicked in the NHP animal model, cellular and humoral immune responses were characterized in surviving versus nonsurviving NHPs before or after a lethal challenge with 1000 plaque-forming units (PFU) of ZEBOV. NHPs were either vaccinated 28 days before or treated 30 min after challenge. Blood samples were analyzed either at 28 days after vaccination to characterize the immune response immediately before challenge or at 7 days after challenge, which represents antibody levels generated as a result of treatment and ZEBOV exposure. Circulating NAb could not be detected before challenge (Fig. 3A). Because the ZGP-specific immune response correlated with survival to a higher degree than overall IgG immune responses in guinea pigs, only ZGP-specific IgG immune responses were evaluated before and after challenge in NHPs. Anti-ZGP IgG levels before challenge were 2.00 ± 0.18 and 0.12 ± 0.17 A405 for surviving and nonsurviving NHPs, respectively (P = 0.0001) (Fig. 3B). Using a 95% confidence interval, the range of total ZGP-specific IgG levels before challenge was between 1.85 to 2.16 and 0.03 to 0.21 A405 for survivors and nonsurvivors, respectively.
Fig. 3.
Humoral immune response in cynomolgus macaques. (A) Serum NAb levels 28 days after vaccination. Antibody levels are reported as reciprocal dilutions. (B) Total serum IgG levels 28 days after vaccination. Antibody levels are reported as A405. (C) Serum NAb levels 7 days after challenge. Antibody levels are reported as reciprocal dilutions. (D) Total serum IgG levels 7 days after challenge. Antibody levels are reported as A405. (E and F) Total serum IgG levels immediately before (E) or 6 days after (F) challenge for VSV-vaccinated NHPs. Antibody levels are reported as endpoint dilutions. An infected, untreated NHP is included (red triangle) as a control for immune responses indicative of disease progression. Error bars represent means ± SD.
On day 7 after challenge, total anti-ZGP IgG levels from survivor and nonsurvivor NHPs were 1.45 ± 0.51 and 0.18 ± 0.23 A405, respectively (P < 0.0001) (Fig. 3C), whereas NAb levels for survivor and nonsurvivor NHPs were 35 ± 69 and 70 ± 110, respectively (P > 0.05) (Fig. 3D). Using a 95% confidence interval, the range of total ZGP-specific IgG levels after challenge was between 1.19 to 1.71 and 0.12 to 0.25 A405 for survivors and nonsurvivors, respectively. NHP survivors treated 30 min after challenge typically exhibited lower ZGP-specific IgG titers than those vaccinated 28 days before challenge.
To determine whether this observation also applied to other vaccine platforms, we also analyzed serum samples from an independent NHP survival study with a VSV-based vaccine with respect to ZGP-specific IgG levels immediately before or day 7 after challenge. End-point dilution titers of 12,805 ± 29,905 and 64 ± 59 were obtained for survivors and nonsurvivors, respectively, immediately before challenge (P = 0.0003) (Fig. 3E), whereas endpoint dilution titers of 6491 ± 4616 and 136 ± 100 were observed for survivors and non-survivors, respectively, 7 days after challenge (P = 0.0001) (Fig. 3F).
Cellular immune responses in NHPs
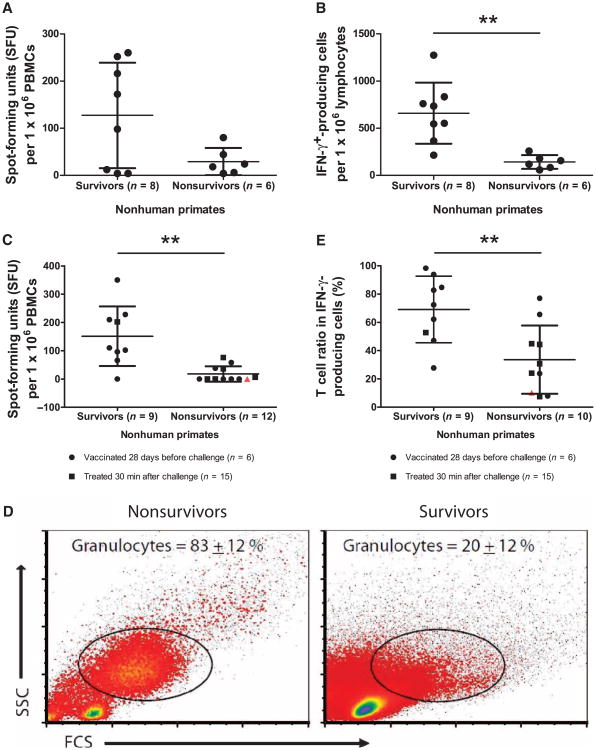
In an effort to define the cellular immune response in surviving and nonsurviving NHPs, the numbers of ZGP-specific IFN-γ–secreting cells were measured in parallel with the expression levels of CD4 and CD8 T cell markers 14 days after vaccination or 7 days after ZEBOV challenge. Before challenge, the occurrence of IFN-γ–secreting cells was not statistically different between survivors and nonsurvivors. Upon ex vivo stimulation by ZGP peptides, the occurrence of IFN-γ–secreting cells in nonsurvivors compared to survivors was 29 ± 29 and 127 ± 112 spot-forming units (SFU) per million peripheral blood mononuclear cells (PBMCs), respectively (P > 0.05), as measured by ELISPOT (Fig. 4A). However, analysis of flow cytometry [fluorescence-activated cell sorting (FACS)] data on lymphocytes revealed a difference between survivors and nonsurvivors at 660 ± 324 and 142 ± 72 IFN-γ–secreting cells per million lymphocytes, respectively (P = 0.0013). At early time points after infection, the occurrence of IFN-γ–secreting cells upon in vitro stimulation by ZGP peptides was lower in nonsurvivors compared to survivors with 18 ± 27 and 152 ± 105 SFU per million PBMCs, respectively (P = 0.0013), as measured by ELISPOT (Fig. 4C). However, analysis of FACS data revealed a much higher proportion of granulocytes in samples from the nonsurvivors compared to survivors (Fig. 4D), which may be responsible for the lower rate of IFN-γ–secreting cells in nonsurvivors. Therefore, FACS analysis on the T cell ratio of total IFN-γ–producing cells likely offers a better estimate of the impact of cell-mediated immunity after exposure. After ZEBOV challenge, IFN-γ was mainly produced by T cells at 69 ± 24% and is higher in survivor animals compared to nonsurvivors (P = 0.0057), in which T cells only represent 34 ± 24% of IFN-γ–positive cells (Fig. 4E).
Fig. 4.
Cell-mediated immune response in cynomolgus macaques. (A) Number of IFN-γ–secreting cells per million PBMCs 14 days after vaccination. (B) Number of IFN-γ–secreting cells per million lymphocytes 14 days after vaccination. (C) Number of IFN-γ–secreting cells per million PBMCs 7 days after challenge. (D) Flow cytometry analysis of cell populations present in sample from survivors and nonsurvivors 7 days after challenge. Granulocytes represent the major proportion of cells in nonsurvivor animals, whereas cells from survivors are mainly lymphocytes. The percentages of granulocytes are indicated on the dot plot. (E) Percentage of T cells in IFN-γ–producing cells 7 days after challenge. An infected, untreated NHP is included (red triangle) as a control for immune responses indicative of disease progression. Error bars represent means ± SD.
Innate immune response in NHPs
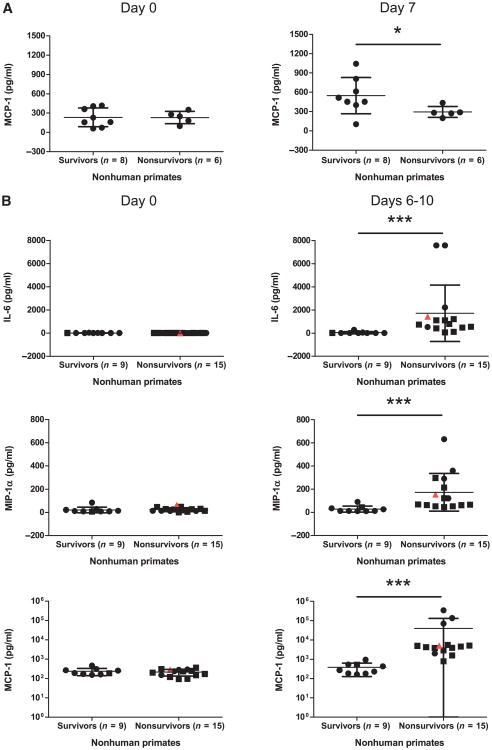
In the search for alternative host markers of positive versus negative outcomes before and after ZEBOV challenge, the expression profile of several cytokines and chemokines was evaluated at 7 days after vaccination and 6 to 10 days after challenge from Ad-CAGoptZGP–vaccinated NHP serum samples. Cytokine levels between the different groups did not differ significantly immediately before vaccination or challenge. However, an increase in monocyte chemoattractant protein-1 (MCP-1) levels was observed for survivors over nonsurvivors to measured levels of 549 ± 282 and 295 ± 85 pg/ml (P = 0.0451) on day 7 after vaccination (Fig. 5A).
Fig. 5.
Cytokine and chemokine levels in cynomolgus macaques. (A and B) Serum cytokine and chemokine levels for surviving and nonsurviving NHPs evaluated (A) immediately before and 7 days after vaccination or (B) immediately before and 6 to 10 days after challenge. Circles represent NHPs vaccinated 28 days before challenge, whereas squares represent NHPs treated 30 min after challenge. An infected, untreated NHP is included (red triangle) as a control for immune responses indicative of disease progression. Error bars represent means ± SD.
Several cytokines showed increases early after ZEBOV exposure. Measured levels of interleukin-6 (IL-6) were 1720 ± 2437 and 40 ± 92 (P = 0.0001) for nonsurvivors and survivors, respectively; macrophage inflammatory protein-1α (MIP-1α) levels were 174 ± 164 and 28 ± 26 (P = 0.0003) for nonsurvivors and survivors, respectively; and measured levels of MCP-1 were 39,575 ±91,741 and 383 ± 255 pg/ml (P < 0.0001) for nonsurvivors and survivors, respectively, on days 6 to 10 after challenge (Fig. 5B).
Discussion
Whether and which immune responses correlate with protection against ZEBOV infection has been a matter of intense debate and is in general a controversial area in the study of infectious diseases. An immune correlate of protection is defined as “a specific immune response to a vaccine that is closely related to protection against infection, disease, or other defined end point” (21). A correlate of protection must be consistent and reliable as evidenced by statistical analysis; otherwise, it is not a reasonable measure of immunity to infection. Furthermore, a correlate of protection induced by vaccination is not necessarily the same as the mechanism that eliminates infection (22); thus, it may contribute to but is not necessarily solely responsible for protection.
We evaluated the importance of humoral immunity independent to the cell-mediated immune response in relation to protection from ZEBOV infection in the three animal species that have been extensively used to model EBOV infection and pathogenicity. Survival and immunological studies were first performed in knockout mice with deletions of different regulatory and effector arms of the adaptive immune system. Although the B cell knockout mice produced IFN-γ in response to ZGP peptide stimulation in vitro, this response was insufficient for survival. Thus, failure to generate ZGP-specific B cell–mediated immunity is an indicator of mortality from ZEBOV challenge in mice. CD8+ T cell knockout mice were unable to generate a ZGP-specific CD8+ T cell response but still demonstrated a substantial number of IFN-γ–secreting cells in response to ZGP peptide stimulation, suggesting that other cellular subsets, such as CD4+ T cells and natural killer (NK) cells, could be responsible for the observed IFN-γ response. This observation is consistent with past reports indicating the importance of NK and CD4+ T cells for successful immunization in mice against ZEBOV (23, 24).
Although serum anti-ZEBOV NAb levels in guinea pigs and NHPs correlated weakly with or did not correlate with survival, respectively, total ZGP- and lysate-specific IgG levels varied significantly between survivors and nonsurvivors before and after ZEBOV challenge in NHPs. Indeed, the highlighted guinea pig that had a significant ZGP-specific IgG response succumbed to disease much later than the other treated animals, suggesting that serum antibodies may have delayed its death. Indeed, in more than 96 outbred NHPs, ZGP-specific IgG levels correlated with the highest degree of statistical significance to host survival, with >99.9% confidence immediately before ZEBOV challenge and also 7 days after exposure. On the basis of this observation, the induction of a specific antibody response early after challenge may be important and at a minimum may correlate with survival from ZEBOV infection, which is in accordance with previously published data (25). The viral GP is essential for the viral life cycle and is the main determinant of ZEBOV pathogenicity, where GP1,2 initiates attachment and fusion of the viral and host membranes, leading to endothelial cell disruption and cytotoxicity (26, 27). Furthermore, secreted GP inhibits neutrophil activation and thereby host immune responses (28). This suggests that ZGP-specific antibodies could possibly disrupt several viral functions, leading to the control of ZEBOV infection and disease.
The importance of cell-mediated immunity independent to the humoral immune response was also evaluated. Although activation markers of the cellular response did not correlate with survival in mice and NHP after vaccination, the NHP ELISPOT assays showed a significant difference in numbers of IFN-γ –producing cells between surviving and nonsurviving animals after exposure. However, these data should be interpreted with caution as the concomitant FACS data showed vastly different blood profiles between surviving and nonsurviving NHPs, including an unusually high proportion of granulocytes in nonsurvivors, which was presumably induced by ZEBOV disease progression. Because the ELISPOT assays did not distinguish between cell types, this could explain the observed lower rate of IFN-γ–producing cells, because lymphocytes are known as the main producers of IFN-γ. FACS analysis of lymphocytes at 14 days after vaccination revealed that an elevated IFN-γ response contributes positively to survival. Furthermore, an early IFN-γ response mediated by a significantly higher proportion of T cells was observed in surviving NHPs 7 days after exposure, which is consistent with several previous studies linking the cell-mediated response with a reduction in viral load and hence a better outcome against EBOV infection (29–31).
NHPs showing detectable clinical signs of disease that survived the lethal challenge exhibited a decreased humoral but not cell-mediated immune response. These observations were mainly associated with NHPs treated 30 min after ZEBOV challenge and could be because NHPs vaccinated 28 days before challenge had more time to establish a complete, specific IgG response and thus raise their chances of survival, whereas NHPs treated after exposure had little time and thus had to rely on other additional components of the immune response to limit viral spread and disease progression early on during infection until the humoral response was sufficiently activated. These data are also consistent with results from knockout mice, which suggest that the B cell but not CD8+ T cell response is sufficient for survival. Indeed, recent studies demonstrated that passive transfer of ZGP-specific serum antibodies conferred 100% protection from ZEBOV in mice and guinea pigs (32, 33), and treatment of NHPs with immune sera or ZGP-specific monoclonal antibodies resulted in complete survival of lethally infected animals (19, 20). Thus, ZEBOV-specific antibodies alone may be sufficient to control ZEBOV replication, leading to survival and consequently may be able to be used to predict protection.
The innate immune responses in survivor versus nonsurvivor NHPs were characterized early after vaccination or after infection with ZEBOV to find early markers correlating to potential vaccine efficacy and because dysregulated activation of the immune response has been previously postulated as a possible cause of fatal outcome in EBOV patients (34). Up-regulation of MCP-1 after vaccination was associated with survival; however, increased MCP-1 after lethal challenge was observed largely from nonsurvivors, suggesting that MCP-1 can have a beneficial role after vaccination but is associated with a negative outcome when up-regulated after challenge with ZEBOV. Other proinflammatory markers, including IL-6 and MIP-1α, were also significantly up-regulated in nonsurvivors after challenge and may be indicative of disease progression. Although these cytokine profiles are part of the immune fingerprint correlating to survival or nonsurvival against ZEBOV, testing of antibody levels is more cost-effective and statistically more accurate than measuring cytokine profiles. However, monitoring cytokine and chemokine profiles as well as the T cell response will help complement the antibody data to illustrate a complete picture of the resulting immune responses after vaccination and after exposure and should be performed whenever feasible to situate the patient in relation to evolution of the disease. Notably, cytokine fingerprints could be good candidates as markers of disease severity and progression or favorable versus unfavorable outcome, helping decision-making for patient management.
The immune response represents a very complex network of highly regulated interactions and optimal crosstalk between T and B cells that is essential for antibody production (35). Immune responses from different protocols were analyzed with respect to survival outcome to favor statistical analysis from large groups of animals, and the findings suggest that the humoral immune response, specifically ZGP-specific IgG, plays a role in providing protection against ZEBOV challenge. This finding was independent of vaccine platform choice and timing of ZEBOV exposure. This strongly reinforces the idea that humoral immunity plays the major role in protection against EBOV infection, whereas cell-mediated immunity plays a supporting role in protection, becoming more prominent when vaccine-induced antibody levels are suboptimal. Therefore, vaccines that elicit a robust ZGP-specific IgG response are more likely to generate a protective immune status. For instance, a higher antigen dose (36), administration of vaccine via intraperitoneal as opposed to the intratracheal, intranasal, or oral routes (37), or the use of a strong T helper 2 (TH2) adjuvant, such as MF59 oil-in-water emulsion or alum (38, 39), can all promote increased humoral responses, and it would be interesting to combine these strategies with current experimental EBOV strategies to optimize vaccine efficacy.
Although the exact mechanism of antibody-mediated protection remains elusive, the antibodies could be working in several ways—through antibody-dependent cell-mediated cytotoxicity, by inhibiting virus budding, or by blocking virus entry. Elucidating the mechanisms of antibody protection involving different points of action will require in-depth analysis and likely several years while many promising Ebola vaccines are already awaiting clinical trials. Overall, the present study indicates that ZGP-specific IgG levels after vaccination correlate with protection against ZEBOV infection and therefore should be the method of choice for routine testing after vaccination as well as monitoring post-exposure patients in outbreak situations.
Materials and Methods
Construction and production of adenoviral vectors
Molecular clones of E1/E3-deleted AdHu5 vectors expressing ZGP were generated, rescued, and termed Ad-CAGoptZGP (40). Large-scale infections (5 × 108 cells) were initiated from positive transfectants and purified by cesium chloride (41). Particle number and infectivity of vectors were determined by standard optical density and immunodetection of the AdHu5 hexon protein, respectively, with the Adeno-X rapid titer kit (Clontech) according to the manufacturer's instructions. Several Ad-CAGoptZGP preparations were generated and quantified for both infectious particle and total particle number. Preparations with a ratio of at least 1:200 infectious to total particle were used in this study.
Animal models, vaccination, and challenge
C57BL/6J wild-type and various strains of female mice, including Rag-1, IFN-γ, B cell, CD4+, or CD8+ T cell knockouts (Jackson Laboratory), were used for this study. Mice were immunized by intramuscular injection of 1 × 1010 total particles of Ad-CAGoptZGP diluted in phosphate-buffered saline (PBS) and then challenged 28 days later by intraperitoneal injection with 1000 × LD50 (median lethal dose) of the MA-ZEBOV strain Mayinga (42).
Female Hartley strain of guinea pigs (Charles River) were administered intramuscularly or intranasally with 1 × 1010 total particles of Ad-CAGoptZGP diluted in PBS, either in the presence or in the absence of preexisting immunity induced intramuscularly or intranasally with 1 × 1011 total particles of Ad-CAGlacZ, 28 days before vaccination. Guinea pigs were challenged 28 days after vaccination by intraperitoneal injection with 1000 × LD50 of GA-ZEBOV strain Mayinga (43).
Healthy male and female cynomolgus macaques (Macaca fascicularis) (2.5 to 11 kg) (Primus Bio-Resources) were administered intramuscularly, intranasally, or intranasally/intratracheally with 1 × 1010 to 4 × 1010 infectious units (IFU) of Ad-CAGoptZGP either in the presence or in the absence of preexisting immunity induced as described previously, supplemented with or without 3 × 109 to 2 × 109 PFU/kg per NHP of AdHu5 expressing IFN-α (DEF201) (44) either 28 days before challenge or 30 min after challenge. Recombinant universal type I IFN-α (0.44 μg/kg) (PBL Interferon Source) was administered intramuscularly to NHPs on days 5 to 21 after challenge. In the VSV study, animals were vaccinated intramuscularly, intranasally, or orally with 2 × 107 PFU VSVΔG-ZGP (8, 45). NHPs were challenged with 1000 PFU ZEBOV, strain Kikwit (46), per animal and sampled on days 0, 3, 7, 14, 21, and 28 after challenge. In addition, terminal samples were harvested from moribund NHPs before euthanasia.
All animal procedures and scoring sheets were approved by the Institutional Animal Care Committee at the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) according to the guidelines of the Canadian Council on Animal Care. All infectious work was performed in the “Biosafety Level 4” facility at NML, PHAC.
Statistical analysis
Results from survival groups were compared with the two-tailed Mann-Whitney nonparametric t test, which does not assume that biological sample values conform to a Gaussian distribution. Significance values of P < 0.05 were considered significant (*), P < 0.01 were considered highly significant (**), and P < 0.001 were considered extremely significant (***). All statistical analyses were performed on GraphPad Prism v.5.01 software. All error bars indicate 1 SD from the mean.
NAb assay
Sera harvested from animals immediately before vaccination or challenge 28 days after vaccination or 6 to 10 days after challenge were inactivated at 56°C for 45 min. Twofold serial dilutions of each sample were mixed with 100 transducing units of ZEBOV encoding the enhanced green fluorescent protein (eGFP) reporter gene and incubated at 37°C for 60 min. The mixture was transferred onto subconfluent VeroE6 cells and incubated for 90 min at 37°C in 5% CO2. Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum (FBS) was added, and plates were incubated at 37°C in 5% CO2 for 48 hours. The highest serum dilution with cells scoring for greater than 50% reduction in quantity of eGFP expression under a fluorescence microscope was considered positive for NAb, and neutralization titers were reported as the reciprocal of this dilution.
ZGP-specific IgG ELISA
Immulon 2 HB Flat Bottom MicroTiter ELISA plates (Thermo Scientific) were coated with 50 ng of His-ZEBOV-GP capture antigen diluted in PBS (47). Plates were washed with PBS/0.1% Tween 20 and then blocked for 90 min with 5% skim milk powder/PBS/0.2% Tween 20 at 37°C (47). For Ad-CAGoptZGP studies, samples were diluted at 1:1600 for serum harvested before challenge or at 1:50 for serum harvested after challenge in 5% skim milk powder/PBS/0.2% Tween 20. For VSVΔG-ZGP studies, samples were diluted until endpoint titer was reached. Diluted serum was incubated with capture antigen for 60 min at 37°C A secondary antibody for mouse [horseradish peroxidase (HRP)–conjugated rat anti-mouse antibody to mouse IgG] (Jackson Laboratory), for guinea pig (HRP-conjugated goat anti–guinea pig antibody to guinea pig IgG) (KPL), or for NHP (HRP-conjugated goat anti-human antibody to IgG) (KPL) was added and incubated for 60 min at 37°C HRP substrate was added and incubated at room temperature for 30 min. Plates were read with the VMax Kinetic ELISA Microplate Reader (Molecular Devices), the data were analyzed with CellMaxPro software, and results were reported as the optical density measured by A405.
Indirect ZEBOV ELISA
γ-Irradiated sucrose gradient-purified ZEBOV was used as the capture antigen. Heat-inactivated guinea pig or NHP sera were diluted at 1:50 with 5% skim milk powder/PBS/0.2% Tween 20. All other steps were performed as described previously.
Cellular immune responses
The frequency of splenocytes or PBMCs secreting IFN-γ was measured with the mouse or human IFN-γ ELISPOT detection kit (BD Bio-sciences), respectively, according to the manufacturer's instructions. Briefly, 96-well ELISPOT plates were coated overnight at 4°C with purified anti-mouse IFN-γ or anti-human IFN-γ antibody. Free unspecific binding sites were blocked with RPMI 1640 (Invitrogen) supplemented with 10% FBS for at least 2 hours at room temperature. Splenocytes harvested from mice 10 days after vaccination were ground against a fine mesh filter in L-15 medium (Gibco), and mononuclear cells were isolated and resuspended in L-15. Whole blood collected in EDTA-containing vacutainer tubes was diluted with an equal volume of PBS and layered on a Ficoll density gradient (GE Healthcare). Fifteen–amino acid oligomer peptide pools (167 peptides in total) with 10–amino acid overlaps were obtained (GenScript) for the entire ZGP. Each peptide was resuspended in dimethyl sulfoxide, diluted in RPMI 1640 medium (supplemented with 10% FBS, 1% penicillin, 1% streptomycin, 1% l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 1% Hepes buffer, and 5 × 10−3 M 2-mecaptoethanol), and added to 5 × 105 splenocytes or PBMCs to give a final concentration of 2.5 or 5 μg/ml, respectively. ELISPOT plates were incubated for 18 hours at 37°C and 5% CO2. Samples were washed and incubated with biotinylated anti-mouse IFN-γ or anti-human IFN-γ for 2 hours at room temperature, followed by incubation with HRP-conjugated streptavidin (BD Biosciences) for 1 hour at room temperature. Spots were visualized with 3-amino-9-ethylcarbazole as substrate (BD Biosciences). IFN-γ–positive cells were visualized as spots on the ELISPOT membrane and counted with an ELISPOT plate reader (Cell Technology).
Flow cytometry analysis
The frequency of CD4+ or CD8+ cells producing IFN-γ and IL-2 was assessed by flow cytometry. PBMCs were isolated as aforementioned and seeded at 106 cells per well in ELISPOT medium. Cells were stimulated overnight with different peptide pools (5 μg/ml) in the presence of GolgiPlug (1 μl/ml) (BD Biosciences). Peripheral blood lymphocytes were then stained with peridinin chlorophyll protein (PerCp)– Cy5.5–conjugated mouse anti-human CD4 and allophycocyanin (APC)–conjugated mouse anti-human CD8 antibodies (BD Biosciences), followed by 20-min incubation in Cytofix/Cytoperm (BD Biosciences). Intracellular cytokines were detected after staining with fluorescein isothiocyanate (FITC)–conjugated mouse anti-human IFN-γ and phycoerythrin (PE)–conjugated anti-human IL-2 (BD Biosciences) diluted in Perm/Wash buffer (BD Biosciences). At least 300,000 events were analyzed with four-color flow cytometer (BD Biosciences) in the bio-containment level 4 laboratory of the NML, PHAC.
Cytokine and chemokine single and multiplex analysis
NHP serum was analyzed for 28 cytokines and chemokines with the Monkey Cytokine Magnetic 28-Plex Panel (Invitrogen) according to the manufacturer's instructions. Serial dilutions of cytokine standards were prepared and added to a Millipore MultiScreen-BV filter plate. Magnetic beads were added in parallel to each well and washed twice. Incubation buffer was first added to each well, where serum samples were diluted 1:2 with assay diluent. Samples were incubated on a plate shaker at 500 rpm in the dark for 2 hours at room temperature. The plate was then applied to a magnetic separator (Invitrogen) and washed twice. Biotinylated anti-human multicytokine reporter was added to each well and incubated on a plate shaker at 500 rpm in the dark for 1 hour at room temperature. The plate was applied to the magnetic separator and washed twice, and then streptavidin-PE was added directly after a 1:10 dilution in streptavidin diluent. The plate was incubated on a plate shaker at 500 rpm in the dark for 30 min at room temperature, applied to the magnetic separator, washed twice, resuspended in wash buffer, and shaken for 1 min. The plate was read with the Qiagen LiquiChip 200 Workstation. Cytokine concentrations were calculated with the Luminex xPONENT 3.1 software.
Acknowledgments
We thank A. Marzi, A. Bello, A. Grolla, G. Schumer, J. Gren, M. Gray, S. Jones, J. Strong, and K. Tran for either their technical assistance or their donation of crucial reagents. We also thank N. Beausoleil, C. De Graff, K. Azaransky, and M. French from Veterinary Technical Services for all animal husbandry support, in addition to M. VanderLoop for the veterinary assistance. Funding: This research was supported by the PHAC and funded by a grant from the Chemical, Biological, Radiological and Nuclear Research and Technology Initiative to G.P.K. and the Japan Initiative for Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology to A.T. G.W. is the recipient of a Doctoral Research Award from the Canadian Institute for Health Research.
Footnotes
Author contributions: G.W. designed and conducted the experiments and wrote the paper. J.S.R. conducted the experiments and wrote the paper. S.P., A.P., X.Q., and J.A. conducted the experiments. J.H. and Y.Z. provided scientific input. A.T. and H.F. provided scientific support. G.P.K. designed and conducted the experiments and wrote the paper.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Wilson JA, Bosio CM, Hart MK. Ebola virus: The search for vaccines and treatments. Cell Mol Life Sci. 2001;58:1826–1841. doi: 10.1007/PL00000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart MK. Vaccine research efforts for filoviruses. Int J Parasitol. 2003;33:583–595. doi: 10.1016/s0020-7519(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81:6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, Murphy BR, Collins PL, Sanchez A. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J Virol. 2006;80:2267–2279. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Sanchez A, Ward JM, Murphy BR, Collins PL, Bukreyev A. A paramyxovirus-vectored intranasal vaccine against Ebola virus is immunogenic in vector-immune animals. Virology. 2008;377:255–264. doi: 10.1016/j.virol.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 9.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of Ebola virus. J Virol. 2009;83:7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Greenough K, Wilson JM. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 13.Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, Nichol ST, Nabel GJ. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]
- 14.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, Jahrling PB, Smith JF. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19:142–153. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JA, Hart MK. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol. 2001;75:2660–2664. doi: 10.1128/JVI.75.6.2660-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE. Passive transfer of antibodies protects immunocompetent and imunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J Virol. 2001;75:4649–4654. doi: 10.1128/JVI.75.10.4649-4654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl. 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, Reimann KA, Bao S, Rao S, Roederer M, Jahrling PB, Koup RA, Nabel GJ. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 19.Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, Ortiz RA, Prugar LI, Pratt WD. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci USA. 2012;109:5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. Successful treatment of Ebola virus–infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4:138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 21.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA. Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 23.Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: Protection in mice requires CD4+ T cells. J Virol. 2002;76:9176–9185. doi: 10.1128/JVI.76.18.9176-9185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warfield KL, Perkins JG, Swenson DL, Deal EM, Bosio CM, Aman MJ, Yokoyama WM, Young HA, Bavari S. Role of natural killer cells in innate protection against lethal Ebola virus infection. J Exp Med. 2004;200:169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: Implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 29.Bradfute SB, Warfield KL, Bavari S. Functional CD8+ T cell responses in lethal Ebola virus infection. J Immunol. 2008;180:4058–4066. doi: 10.4049/jimmunol.180.6.4058. [DOI] [PubMed] [Google Scholar]
- 30.Olinger GG, Bailey MA, Dye JM, Bakken R, Kuehne A, Kondig J, Wilson J, Hogan RJ, Hart MK. Protective cytotoxic T-cell responses induced by Venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol. 2005;79:14189–14196. doi: 10.1128/JVI.79.22.14189-14196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debré P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 32.Qiu X, Alimonti JB, Melito PL, Fernando L, Ströher U, Jones SM. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin Immunol. 2011;141:218–227. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Qiu X, Fernando L, Melito PL, Audet J, Feldmann H, Kobinger G, Alimonti JB, Jones SM. Ebola GP-specific monoclonal antibodies protect mice and guinea pigs from lethal Ebola virus infection. PLoS Negl Trop Dis. 2012;6:e1575. doi: 10.1371/journal.pntd.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4:e837. doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 36.Kaur S, Kaur T, Garg N, Mukherjee S, Raina P, Athokpam V. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis. Parasitol Res. 2008;103:1413–1419. doi: 10.1007/s00436-008-1150-x. [DOI] [PubMed] [Google Scholar]
- 37.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, McGhee JR, Kiyono H. Nasal immunization induces Haemophilus influenzae–specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180:122–132. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 38.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O'Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–6454. [PubMed] [Google Scholar]
- 40.Gao G, Zhou X, Alvira MR, Tran P, Marsh J, Lynd K, Xiao W, Wilson JM. High throughput creation of recombinant adenovirus vectors by direct cloning, green-white selection and I-Sce I-mediated rescue of circular adenovirus plasmids in 293 cells. Gene Ther. 2003;10:1926–1930. doi: 10.1038/sj.gt.3302088. [DOI] [PubMed] [Google Scholar]
- 41.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, Jones S, Wilson JM. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006;346:394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 42.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1998;178:651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 43.Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, Jahrling PB. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999;179(Suppl. 1):S203–S217. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 44.Wu JQ, Barabé ND, Huang YM, Rayner GA, Christopher ME, Schmaltz FL. Pre- and post-exposure protection against Western equine encephalitis virus after single inoculation with adenovirus vector expressing interferon alpha. Virology. 2007;369:206–213. doi: 10.1016/j.virol.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Ströher U, Feldmann H, Jones SM. Mucosal immunization of cynomolgus macaques with the VSVΔG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, Huggins JW, LeDuc JW, Peters CJ. Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl. 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama E, Yokoyama A, Miyamoto H, Igarashi M, Kishida N, Matsuno K, Marzi A, Feldmann H, Ito K, Saijo M, Takada A. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin Vaccine Immunol. 2010;17:1723–1728. doi: 10.1128/CVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]