Abstract
Drug interactions with plasma proteins influence their pharmacokinetics and pharmacodynamics. We aimed to test whether a strong quantitative relationship exists between plasma free fraction (fP) and lipophilicity for low molecular weight nonacidic drug-like compounds. We measured the n-octanol-buffer distribution coefficients at pH 7.4 (mlogD) of 18 diverse radiotracers (<470 Da) used for brain imaging with positron emission tomography in vivo. Lipophilicities were also computed as clogD with two software packages. The fP values for monkeys and humans were determined by ultrafiltration and transformed into mlogDpr/pl values representing the log10 of the within phase partition of the radiotracers between plasma proteins and remaining plasma. mlogDpr/pl correlated strongly with mlogD for human (mlogDpr/pl = 0.733mlogD−0.780, r2 = 0.74) and monkey (mlogDpr/pl = 0.780mlogD−1.15, r2 = 0.83), but less strongly with clogD. These relationships were significantly different between species (P = 0.006). Removal of eight fluorinated compounds from the datasets raised r2 to 0.81 and 0.91 for humans and monkeys, respectively. For the tested compounds, we conclude that n-octanol-buffer (pH 7.4) distribution strongly models that between plasma proteins and remaining plasma and moreover that mlogD accounts for over 74% of compound mlogDpr/pl and is a strong determinant of fP.
Keywords: Protein binding, drug-like properties, physicochemical properties, logP, structure-property relationship, lipophilicity, drug, radiotracer, logD, plasma free fraction
INTRODUCTION
The interaction of administered drugs with plasma proteins has long been of interest, since essentially only unbound drug is capable of traversing biological membranes to exert pharmacological action.1,2 Furthermore, the extent to which a drug binds to plasma proteins will influence its distribution, rate of metabolism, and excretion.1,2 In our laboratory, we are primarily interested in developing and applying radiotracers (radiopharmaceuticals) for imaging protein targets within brain with the technique of positron emission tomography (PET). Commonly, such radiotracers are structurally related to central nervous system drugs. The penetration of such radiotracers across the blood–brain barrier after intravenous administration depends on many factors,3–5 including the plasma free fraction (fP), which may be defined as the fraction of drug in plasma that is not bound to plasma proteins.6 For detailed pharmacokinetic analysis of the behavior of PET radiotracers aimed at deriving important imaging output parameters, accurate unbiased measurement of fP may be required in subjects under study, whether animal or human.6 In view of the aforementioned considerations, the interaction of drug-like compounds with plasma proteins7,8 and the prediction of these interactions9,10 have merited considerable study. Lipophilicity has long been recognized as an important property influencing many aspects of drug–protein interactions, and consequently drug pharmacokinetic and pharmacodynamic behavior.1 The most widely used index of drug lipophilicity is the n-octanol-water partition coefficient P, usually expressed as log10 of this value (logP).11,12 As many drugs also exist in ionized forms at physiological pH (pH 7.4), the log10 of the partition of all forms of the drug between n-octanol and buffer at pH 7.4 (logD) is usually considered more relevant to physiological phenomena. Nowadays, estimates of lipophilicity may be computed from simple depictions of drug structure with various commercially available software packages to give the corresponding parameters clogP and clogD.* Because of the great ease of these computations, clogP and clogD values have become quite prevalent in the literature, especially for large datasets where alternative experimental determinations would be laborious.
The extent to which logD can represent the interactions of drugs with plasma proteins is a fundamental and well-discussed question.10 Many experimental studies have observed or have derived different relationships between logD and fP for various types of dataset. For example, Lázníček et al.13 studied the plasma protein binding of organic acids across three animal species and humans and established relationships of the type fp = 1/(1 + aDb), where a depends on species and b is approximately equal to 1. van de Waterbeemd et al.14 found sigmoidal relationships between plasma protein binding (i.e., 1−fp) and logD that were appreciably dissimilar among acidic, basic, and neutral compounds. Laruelle et al.4 reported a strong linear relationship between fp and logP for a small set (n = 4) of congeneric imaging radiotracers. However, Saiakhov et al.15 found a poor linear relationship (r2 = 0.279) between 1−fp and logP (logKow) for a large dataset (n = 154) of diverse drugs. Yamazaki and Kanaoka16 found strong nonlinear relationships between clogD and plasma protein binding among quite large datasets of diverse basic, neutral, and acidic pharmaceuticals. By contrast, Kratochwil et al.17 found poor correlations among a large set of both acidic and basic drugs between logD and binding affinity to human serum albumin, the most prevalent protein in plasma.
We are interested in testing the extent to which lipophilicity accounts for fP among nonacidic PET radiotracers produced for brain imaging in our laboratory, because an understanding of the quantitative relationship between lipophilicity and its possible species dependence could be useful in further PET radiotracer development. Moreover, reliable quantification of the role of lipophilicity in drug–plasma protein interactions may be generally useful for drug design, and also facilitate the development of in silico predictive methods.10,15,18,19
In this study, we examined the relationship between fP and lipophilicity for a moderately sized set of well-diversified low molecular weight nonacidic compounds. These compounds serve in our laboratory as radiotracers for brain molecular imaging with PET and their availability in radioactive form and in high radiochemical purity facilitated fP and lipophilicity measurements, and provided opportunity to study the relationships between these two parameters. We found that the log10 of compound partition between plasma proteins and the remainder of plasma correlates strongly with measured logD value in both the human and the monkey. Accordingly, logD is the major determinant of fP in both species, and may be considered a useful parameter for the prima facie estimation of fP for candidate nonacidic low molecular weight drugs or indeed brain imaging radiotracers.
MATERIALS AND METHODS
Statistics
Linear regression analysis between one dependent and one independent variable was performed by the least squares method with GraphPad Prism for Windows software (GraphPad Software; San Diego, California). Statistical significance for the uppertail F-test was set at the 5.0% level (α = 0.05) for the appropriately calculated degrees of freedom. Regression lines and associated 95% confidence intervals were plotted with GraphPad Prism for Windows software. Analysis of covariance was used to test the hypothesis of whether two regression lines were equivalent. Significance of the test was performed at the 5% level (α = 0.05). Student’s t-test was used to test for differences in means of two samples.
Compound Selection and Preparation
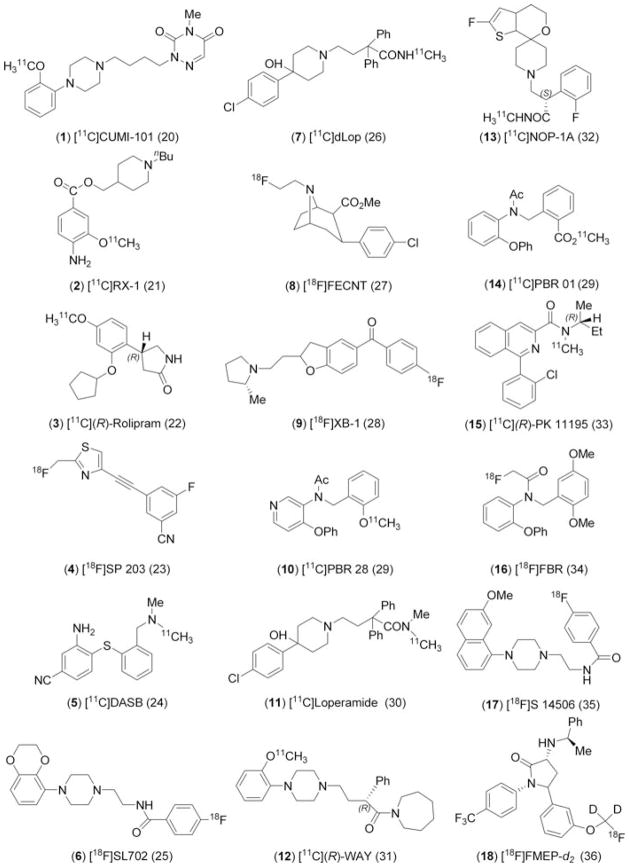
Eighteen compounds for study were selected from among the radiotracers being produced in our laboratory for PET brain imaging experiments in monkey and human subjects. These compounds were labeled with either short-lived carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 109.7 min) and obtained in high radiochemical purity (>99%), as described previously.20–36 All were radiochemically stable in monkey and human plasma, except [18F]SP203, which is unstable in monkey plasma.23 The radiotracers are all low molecular weight (260–470 Da) nonacidic compounds with low total polar surface area (tPSA; range: 30–65 Å) (Table 1). They span a wide range of lipophilicity (Table 1), and represent 12 distinct core structural classes (Chart 1). Eight of the compounds contain one or more fluorine atoms. Most of the compounds are quite basic (Table 1) and exist as interconverting neutral (non-zwitterionic) and positively charged species at physiological pH (pH 7.4).
Table 1.
Measured and Computed Properties of Radiotracers 1 – 18, Listed in Ascending Order of mlogD
| Entry | Radiotracer | M. wt.a | tPSA b (Å) | pKac |
clogP
|
clogD
|
mlogDd |
fP
|
mlogDpr/pl
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pallas | ACD | Pallas | ACD | Monkeye | Humane | Monkeye | Humane | ||||||
| 1 | [11C]CUMI-101 | 372.5 | 56 | 9.25 9.13 |
1.49 | 0.00 | −0.47 | −0.19 | 1.13 ± 0.02 | 0.594 ± 0.016 | 0.442 ± 0.003* | −0.165 ± 0.029 | 0.101 ± 0.006* |
| 2 | [11C]RX-1 | 320.5 | 65 | 8.47 | 2.16 | 2.62 | 1.06 | 2.39 | 1.77 ± 0.01 | 0.450 ± 0.0289 | ND | 0.087 ± 0.051 | ND |
| 3 | [11C](R)-Rolipram | 275.3 | 48 | 7.61 | 2.35 | 1.41 | 1.93 | 1.41 | 2.16 ± 0.01 | 0.238 ± 0.0545 | 0.0580 ± 0.0327* | 0.512 ± 0.132 | 1.26 ± 0.26* |
| 4 | [18F]SP203 | 260.2 | 36 | 0.39 | 3.66 | 2.97 | 3.66 | 2.97 | 2.18 ± 0.22 | ND | 0.0550 ± 0.0165 | ND | 1.25 ± 0.13 |
| 5 | [11C]DASB | 283.4 | 34 | 7.28 | 2.37 | 2.87 | 2.12 | 1.49 | 2.47 ± 0.02 | 0.120 ± 0.015 | 0.109 ± 0.029 | 0.868 ± 0.063 | 0.921 ± 0.124 |
| 6 | [18F]SL702 | 385.4 | 54 | 8.39 | 2.70 | 2.69 | 1.67 | 2.68 | 2.50 ± 0.01 | 0.238 ± 0.052 | ND | 0.511 ± 0.122 | N.D. |
| 7 | [11C]dLop | 463.0 | 53 | 8.05 | 4.51 | 3.71 | 3.77 | 3.04 | 2.60 ± 0.04 | 0.154 ± 0.018 | 0.123 ± 0.015 | 0.742 ± 0.063 | 0.853 ± 0.060 |
| 8 | [18F]FECNT | 325.8 | 30 | 6.99 | 3.47 | 3.31 | 3.33 | 2.28 | 2.64 ± 0.02 | 0.0873 ± 0.0255 | 0.0577 ± 0.0045 | 1.03 ± 0.14 | 1.21 ± 0.04 |
| 9 | [18F]XB-1 | 351.4 | 30 | 9.29 | 4.79 | 4.84 | 2.90 | 2.28 | 2.95 ± 0.01 | 0.0193 ± 0.0044 | 0.0175 ± 0.0014f | 1.71 ± 0.11 | 1.75 ± 0.03f |
| 10 | [11C]PBR28 | 348.4 | 51 | 6.58 | 2.56 | 3.30 | 2.50 | 3.29 | 3.01 ± 0.00 | 0.0560 ± 0.0125 | 0.0367 ± 0.0060 | 1.24 ± 0.11 | 1.42 ± 0.07 |
| 11 | [11C]Loperamide | 477.0 | 44 | 8.01 | 4.55 | 4.26 | 3.85 | 3.53 | 3.04 ± 0.00 | 0.0512 ± 0.0036 | ND | 1.27 ± 0.11 | ND |
| 12 | [11C](R)-WAY | 435.6 | 36 | 8.66 | 4.29 | 5.00 | 3.01 | 4.83 | 3.16 ± 0.35 | 0.0273 ± 0.0097 | 0.0063 ± 0.0015* | 1.57 ± 0.15 | 2.20 ± 0.10* |
| 13 | [11C]NOP-1A | 420.5 | 42 | 7.47 | 3.61 | 5.21 | 3.27 | 4.36 | 3.41 ± 0.07 | 0.114 ± 0.005 | 0.0977 ± 0.0152 | 0.892 ± 0.02 | 0.969 ± 0.074 |
| 14 | [11C]PBR01 | 375.4 | 56 | 4.40 | 3.29 | 4.06 | 3.29 | 4.06 | 3.90 ± 0.02 | 0.0098 ± 0.0075 | 0.0072 ± 0.0086 | 2.15 ± 0.49 | 2.41 ± 0.620 |
| 15 | [11C](R)-PK11195 | 352.9 | 33 | 3.53 | 4.89 | 4.58 | 4.89 | 4.58 | 3.97 ± 0.18 | 0.0207 ± 0.0085 | 0.0047 ± 0.0006* | 1.71 ± 0.21 | 2.33 ± 0.06* |
| 16 | [18F]FBR | 395.4 | 48 | 4.99 | 3.96 | 4.09 | 3.95 | 4.09 | 4.05 ± 0.02 | 0.0217 ± 0.0078 | 0.0040 ± 0.0030* | 1.68 ± 0.18 | 2.52 ± 0.44* |
| 17 | [18F]S14506 | 392.5 | 33 | 8.39 | 4.14 | 4.08 | 3.11 | 3.93 | 4.14 ± 0.06 | 0.0059 ± 0.0023 | 0.0037 ± 0.0009f | 2.25 ± 0.17 | 2.44 ± 0.11f |
| 18 | [18F]FMPEP- d2 | 474.5 | 42 | 6.86 | 4.74 | 5.36 | 4.63 | 5.32 | 4.24 ± 0.08 | 0.0041 ± 0.0001 | 0.0050 ± 0.0017 | 2.39 ± 0.01 | 2.31 ± 0.14 |
Significantly lower than monkey value (P < 0.05).
ND, not determined.
For nonradioactive compound.
Total polar surface area from Chem-Draw Ultra 11.0.
Computed with Pallas 3.70; compound 1 has 2 values.
Mean ± SD for n = 6.
Mean ± SD for n = 3.
Measured on pooled human plasma that had been stored at −70°C.
Chart 1.
Structures of radiotracers (1 – 18) used in this study. References to the radiotracers are given in parentheses.
Estimation of Compound pKa
Estimates of compound pKa were obtained with Pal-las 3.70 software (Compudrug, S. San Francisco, California).
Computation and Measurement of Compound Lipophilicity
Pallas 3.70 software and also ACD software (version 9.04; Advanced Chemistry Development, Inc.; Toronto, Canada) were used to compute two compound lipophilicity parameters, clogD (the log10 of compound partition between n-octanol and buffer at pH 7.4), and clogP (the partition of the neutral compound microspecies between n-octanol and water). Each software uses the depicted two-dimensional structure of the compound as input for computation.
Compound lipophilicity was also measured using the radiotracer as the log10 of its partition between n-octanol and sodium phosphate buffer (pH 7.4, 0.15 M) (mlogD) at room temperature, according to a previously published method.29 The mean and standard deviation of six lipophilicity measurements are reported for each compound (Table 1). Computed (clogD) and measured (mlogD) values were each correlated to mlogDpr/pl by regression analysis.
Measurement of Compound Plasma Free Fraction
All animal studies were performed in accordance with the Guide for the Use of Laboratory Animals37 and the National Institutes of Health Animal Care and Use Committee. Thirteen rhesus monkeys (Macaca mulatta) were used. All experiments involving humans were approved by the Institutional Research Board of the NIH. The fp of each compound was measured with a radiotracer of greater than 99% radiochemical purity at room temperature in either arterial monkey or human plasma, or both. Monkey plasma was prepared from blood taken from fasted male animals that had been immobilized with ketamine and that were maintained in anesthesia with about 1.5% isoflurane in oxygen in readiness for PET imaging experiments. Human plasma was prepared from blood taken from consenting conscious adults preceding radiotracer injection for PET imaging experiments, or, in a few exceptional cases, from pooled human blood that had been stored at −70°C, as indicated in Table 1. Each determination of fp entailed addition of radiochemically pure radiotracer (20–70 μCi), as a solution in saline or saline–ethanol (2–10 μL), to a prepared non-radioactive plasma sample (≈650 μL) followed by mixing and measure ments of fp on three aliquots (200 μL each) by ultrafiltration (Centrifree; Millipore, Billerica, Massachusetts), as detailed previously.38 The mean value constituted one measurement. This procedure was performed thrice for each radiotracer and the results expressed as mean ± SD for the three measurements (Table 1). Except where pooled blood was used, no animal or human contributed to more than one of the three measurements. Because of the frequency of PET experiments, fp data were sometimes available for some radiotracers in more than three subjects. In such cases, in order to achieve equal weighting of data between radiotracers, three values were selected randomly, that is, by lottery with replacement.
Relationship of Within-Phase Partition of Compound Between Plasma Proteins and Remaining Plasma to Lipophilicity
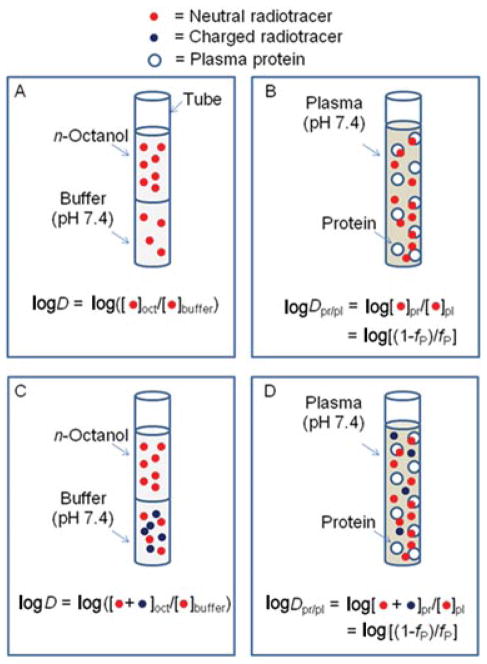
Compound fp values for monkey and human were transformed into within-phase partition coefficients between plasma proteins and remaining plasma (Dpr/pl), where Dpr/pl = (1−fp)/fp and expressed as log10 of this value (mlogDpr/pl) (Fig. 1). For each species, these values were then correlated by regression analysis with clogP, clogD, or mlogD as independent variables. Compound datasets were also divided into those for fluorine-containing (n = 8) and nonfluorine-containing radiotracers (n = 10), and the data reanalyzed.
Figure 1.
Diagram illustrating the analogy between logD (partition of radiotracer between n-octanol and pH 7.4 buffer) and logDpr/pl (partition of radiotracer between dissolved plasma proteins and remaining plasma) for neutral radiotracer (panels A and B) and for a basic radiotracer having protonated species at pH 7.4 (panels C and D). The models assume that charged species are excluded from n-octanol and from binding to plasma proteins.
RESULTS AND DISCUSSION
In this study, our main findings were strong quantitative but species-distinct linear relationships between measured compound lipophilicity (mlogD) and the parameter mlogDpr/pl derived from our measurements of fp in both human and nonhuman primate plasma. mlogDpr/pl represents the log10 of the equilibrium partition of compound between plasma proteins and remaining plasma and is a parameter that may be considered analogous to the log10 of the equilibrium partition of compound between n-octanol and pH 7.4 buffer (logD) (Fig. 1).
At the outset of this study, we were interested in how well commercial software might predict logD as a measure of compound lipophilicity, because computations are easily performed from simple two-dimensional structural representations. Use of the radiotracers generally allowed compound lipophilicities to be measured with high precision across a wide range of values as evident from the associated relatively small standard deviations, except for compounds 4 and 12, which gave 10% and 11% coefficient of variance, respectively (Table 1). Compound logD values, when computed with either Pallas or ACD software and compared with measured values, showed regression lines with regression coefficients (slopes) greater than unity, but only moderately strong correlations (Fig. 2; Table 2, entries 1 & 2). Lipophilicity (logD) computed from ACD software correlated somewhat more strongly with measured lipophilicity (mlogD) (r2 = 0.740) than did lipophilicity computed from Pallas software (r2 = 0.619). (It should be noted that the results from each software package are not expected to correspond because they apply different methods to calculate lipophilicity from two-dimensional structure). Although these correlations may be considered to be strong, these computational methods clearly fail to predict very accurate values. In fact, for ACD and Pallas software, the root mean squared errors were 0.76 and 0.80 log10 units, respectively. Our observations are similar to those of Tetko and Poda39 who, with the use of versions of Pallas, ACD and other software packages, found high root mean squared errors of 1.0 to 1.5 log10 units in the estimation of logD among very large datasets (17,861 and 640) of diverse organic compounds.
Figure 2.
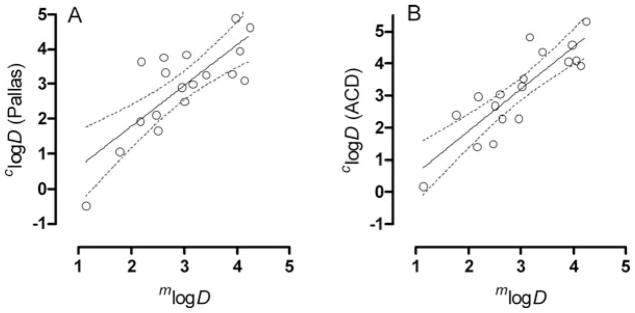
Plots of clogD from Pallas software (panel A) and ACD software (panel B) versus mlogD for the radiotracers 1 – 18 showing the regression lines and bounds of 95% confidence. The regression lines are defined in Table 2.
Table 2.
Data from Regression Analyses of Dependent Variables Y and Independent Variables X, Where the Line is Defined as Y = mX + c, with m Representing Slope and c the Value of Y at X = 0
| Entry | X | Y | na | mb | cb | r2 b | Pb |
|---|---|---|---|---|---|---|---|
| 1 | mlogD | clogD (Pallas) | 18 | 1.17 ± 0.23 | −0.544 ± 0.706 | 0.619 | 0.0001 |
| 2 | mlogD | clogD (ACD) | 18 | 1.31 ± 0.19 | −0.736 ± 0.600 | 0.740 | <0.0001 |
| 3 | clogD (ACD) | mlogDpr/pl (human) | 15 | 0.384 ± 0.053 | 0.352 ± 0.184 | 0.553 | <0.0001 |
| 4 | clogD (ACD) | mlogDpr/pl (monkey) | 17 | 0.394 ± 0.045 | −0.0365 ± 0.0498 | 0.561 | <0.0001 |
| 5 | clogD (Pallas) | mlogDpr/pl (human) | 15 | 0.393 ± 0.070 | 0.394 ± 0.229 | 0.426 | <0.0001 |
| 6 | clogD (Pallas) | mlogDpr/pl (monkey) | 17 | 0.428 ± 0.053 | −0.0283 ± 0.168 | 0.569 | <0.0001 |
| 7 | clogP (ACD) | mlogDpr/pl (human) | 15 | 0.351 ± 0.062 | −0.292 ± 0.241 | 0.430 | <0.0001 |
| 8 | clogP (ACD) | mlogDpr/pl (monkey) | 17 | 0.407 ± 0.050 | −0.268 ± 0.193 | 0.574 | <0.0001 |
| 9 | clogP (Pallas) | mlogDpr/pl (human) | 15 | 0.461 ± 0.089 | −0.0674 ± 0.333 | 0.385 | <0.0001 |
| 10 | clogP (Pallas) | mlogDpr/pl (monkey) | 17 | 0.504 ± 0.070 | −0.571 ± 0.256 | 0.519 | <0.0001 |
| 11 | mlogD | mlogDpr/pl (human) | 15 | 0.733 ± 0.067 | −0.651 ± 0.212 | 0.738 | <0.0001 |
| 12 | mlogD | mlogDpr/pl (monkey) | 17 | 0.780 ± 0.050 | −1.15 ± 0.16 | 0.832 | <0.0001 |
| 13 | mlogD | mlogDpr/pl (human; fluoro) | 7 | 0.635 ± 0.120 | −0.364 ± 0.416 | 0.595 | <0.0001 |
| 14 | mlogD | mlogDpr/pl (monkey; fluoro) | 7 | 0.784 ± 0.130 | −1.19 ± 0.45 | 0.659 | <0.0001 |
| 15 | mlogD | mlogDpr/pl (human; nonfluoro) | 8 | 0.824 ± 0.084 | −0.870 ± 0.246 | 0.814 | <0.0001 |
| 16 | mlogD | mlogDpr/pl (monkey; nonfluoro) | 10 | 0.798 ± 0.049 | −1.17 ± 0.14 | 0.906 | <0.0001 |
n = number of data points used in regression analysis, with one data point per compound; means were used for measured values.
Values computed with GraphPad Prism for Windows software.
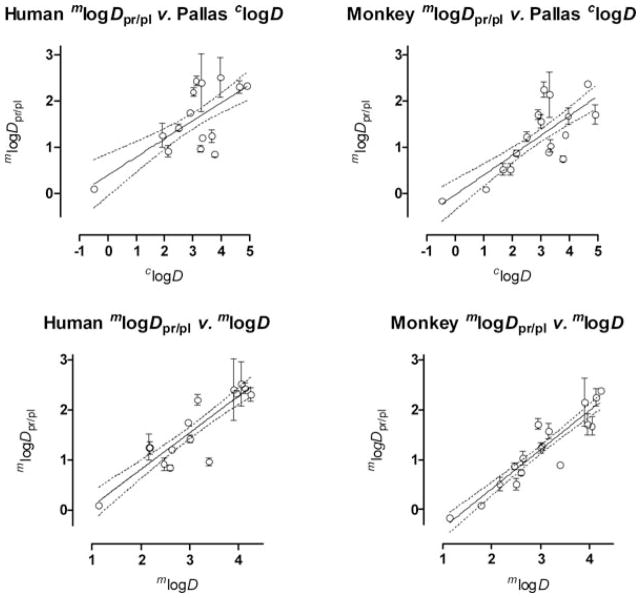
Nevertheless, we proceeded to test whether clogD from either software program might correlate with mlogDpr/pl in monkey and human plasma. The use of the radiotracers allowed for quite precise determinations of compound fP and the derived parameter mlogDpr/pl across a wide range of values as evident from the associated relatively small standard deviations (Table 1). From either program, significant correlations were found between clogD and mlogDpr/pl for monkey and human (Fig. 3, Table 2, entries 3–6). Slopes of the regression lines were much lower than unity and the correlation coefficients were not especially high (Table 2). The ACD software gave a somewhat stronger correlation (r2 = 0.553; Table 2, entry 3) for the human data than the Pallas software (r2 = 0.426; Table 2, entry 5).
Figure 3.
mlogDpr/pl versus clogD and mlogD showing regression lines along with the 95% confidence intervals for human and monkey plasma samples. Where error bars are not visible they are within the symbol size. The regression lines are defined in Table 2.
Because the partition coefficient of uncharged compound between n-octanol and water (logP) is also readily computed from a two-dimensional representation of compound structure, and without the need for an additional uncertain internal estimation of compound pKa, we also tested for correlation between clogP and mlogDpr/pl in each species. Significant correlations were found. However, correlation coefficients were found to be weaker than for clogD (Table 2, c.f. entries 7 & 8), except in the case of monkey mlogDpr/pl versus clogP from ACD software, which gave an almost negligible improvement (r2 = 0.574 versus 0.569; Table 2, c.f. entries 8 and 6). Only in the single case of lipophilicity calculated with Pallas software and for the monkey data did we find that clogP was appreciably more sensitive than clogD to changes in mlogDpr/pl (Table 2, c.f. entries for m in 10 and 6). Overall, we concluded that clogD gave stronger correlations than clogP with mlogDpr/pl for our datasets. Our findings may be compared with those of Lobell and Sivarajah18 for human and uncharged (i.e., neutral) and positively charged sets of compounds with molecular weights <825 Da. They found linear relationships between mlogDpr/pl and AlogP98, where the latter was a logP value computed with QSAR+ module of Cerius2 (version 4.6, Acclerys Inc. San Diego, California). Regression lines had similar regression coefficients (m ≈ 0.45) to those found in this study for neutral and basic compounds (Table 3). Their large datasets gave somewhat higher correlation coefficients (r2 ≤ 0.67) (Table 3).
Table 3.
Comparison of m and c Values for Relationships mlogDpr/pl = mclogP + c in Human and Monkey for Different Sources of clogP and Organic Compound Classes
| Entry | Species | Source of clogP | Compound class | n | r2 | m | c |
|---|---|---|---|---|---|---|---|
| 1a | Human | Cerius2 (version 4.6) | Uncharged | 78 | 0.63 | 0.449 | −0.416 |
| 2a | Human | Cerius2 (version 4.6) | Positively charged | 63 | 0.67 | 0.463 | −1.097 |
| 3b | Human | ACD | Neutral/basic | 15 | 0.430 | 0.351 | −0.834 |
| 4b | Monkey | ACD | Neutral/basic | 17 | 0.574 | 0.407 | −0.268 |
| 5b | Human | Pallas 3.0 | Neutral/basic | 15 | 0.385 | 0.461 | −0.067 |
| 6b | Monkey | Pallas 3.0 | Neutral/basic | 17 | 0.519 | 0.504 | −0.571 |
From Ref. 18.
From this study.
We proceeded to test whether mlogDpr/pl might correlate more strongly with measured lipophilicity (mlogD). For both monkey and human, strong and highly significant linear correlations were found between mlogDpr/pl and mlogD (Fig. 3; Table 2, entries 11 and 12). For human, the quantitative relationship was found to be
| (1) |
and for monkey,
| (2) |
Therefore, the use of mlogD in place of computed values resulted in much improved sensitivity (higher slope or correlation coefficient) and also in much stronger correlation (higher r2). Equations 1 and 2 may be algebraically transformed to give the following relations between fP and D for human and monkey, respectively.
| (3) |
| (4) |
Species differences are important considerations in drug development and also in our research area of PET radiotracer development, where results in animals, usually rodents or monkeys, inform decisions on whether to advance radiotracer evaluations into humans. Only a few previous studies have compared fP for drug-like compounds among species.13,19,40,41 Comparison of the monkey and human fP data for 14 of the 18 compounds in this study showed statistically significant species differences (P < 0.05) for five compounds (Table 1). In all five cases, fP was lower in human than in monkey. The small set of other published studies also consistently reported lower fP in human than in other species.13,19,40,41 We found that the regression lines from the mlogD and mlogDpr/pl values for human and monkey were significantly different by covariance analysis (P < 0.006). The monkey data show the higher coefficients of regression and correlation and this may have a strong biological basis. Thus, the managed monkey population, from which the data in this study were obtained, is more homogeneous with regard to factors that include genetics, gender, diet, fasting state, absence of disease, and exposure to medications than the sampled humans. Such factors are well known to affect the binding of drugs to plasma proteins.7,8 Moreover, there are documented intrinsic species differences in the nature of drug binding sites for serum albumin,42–44 the most abundant drug-binding protein in blood,7,8 and also for serum α1-acid glycoprotein.45 Table 4 compares the linear relationships between mlogDpr/pl and mlogD established in this study for neutral and basic compounds in monkey and human (Eqs 1 and 2) with those established by Lázníček et al.13 for low molecular weight carboxylic acids in several species (mouse, rat, rabbit, and human). The regression coefficients (slopes m) for the relationships established by Lázníček et al.13 (Table 4, entries 1–4) are approximately 1 and significantly higher than for the relationships that we obtained for neutral and basic compounds in monkey and human (Table 4, entries 5 & 6). Acidic compounds are generally considered to bind preferentially to serum albumin whereas basic compounds are considered to bind preferentially to the much less abundant serum α1-acid glycoprotein.2,7,8 The differences in slopes between the equations obtained by Lázníček et al.13 for acid compounds and those for the regression lines expressed in Eqs. 1 and 2 may reflect these general differences in acid/base binding preference as well as species differences in protein binding sites.
Table 4.
Comparison of m and c Values for Relationships mlogDpr/pl = mmlogD + c in Various Species and for Different Organic Compound Classes
| Entry | Species | Compound class | n | r2 | m | c |
|---|---|---|---|---|---|---|
| 1a | Mouse | Acidic | 11 | 0.884 | 1.04 | −2.15 |
| 2a | Rat | Acidic | 11 | 0.931 | 1.01 | −1.75 |
| 3a | Rabbit | Acidic | 11 | 0.945 | 1.02 | −1.28 |
| 4a | Human | Acidic | 11 | 0.970 | 0.994 | −1.10 |
| 5b | Monkey | Neutral/basicc | 17 | 0.832 | 0.780 | −1.15 |
| 6b | Human | Neutral/basicc | 15 | 0.738 | 0.733 | −0.651 |
| 7b | Monkey | F-noncontaining neutral/basic | 10 | 0.906 | 0.798 | −1.17 |
| 8b | Monkey | F-containing neutral/basic | 7 | 0.659 | 0.784 | −1.10 |
| 9b | Human | F-noncontaining neutral/basic | 8 | 0.814 | 0.824 | −0.870 |
| 10b | Human | F-containing neutral/basic | 7 | 0.595 | 0.635 | −0.364 |
From Ref. 13.
From this study.
Includes fluorine-containing and nonfluorine-containing compounds.
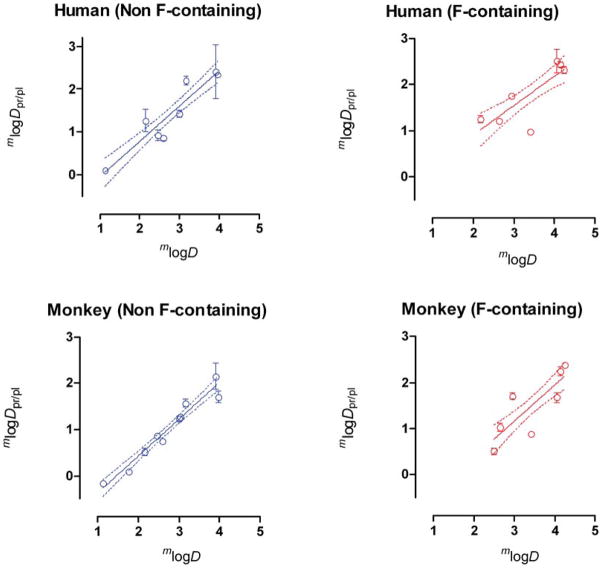
A high proportion of our set of neutral/basic compounds, eight from 18, contains one or more fluorine atoms. Fluorine atoms are known to confer unusual and even unexpected partition properties in small organic molecules.46 Highly fluorinated molecules tend to be neither oil nor water soluble, and are often therefore described as fluorophilic. Because the presence of fluorine atoms in a molecule may cause lipophilicity and hydrophobicity to diverge, logP may not truly reflect lipophilicity.47 Even incorporation of a low number of fluorine atoms into a drug-like molecule may have major consequences for disposition or protein binding. In view of these considerations, we examined the impact of removing the eight fluorine-containing compounds from our datasets. For both human and monkey, this removal resulted in enhanced correlations of mlogDpr/pl with mlogD (Fig. 4; Table 2, entries 11 & 12), with the monkey data again showing the stronger correlation. The correlations for the dataset of fluorine-containing compounds were much weaker (Fig. 4; Table 2, entries 13 & 14). The data for the fluorine compounds happen to appear mainly on the high side of the range of mlogDpr/pl and mlogD values and show more dispersion about the regression line than for the nonfluorine-containing compounds. This may explain the observed differences in the strength of correlations of fluorine-containing and nonfluorine-containing compounds. Covariance analysis showed no statistical difference between the regression lines for fluorine-containing and non-fluorine-containing compound datasets (P = 0.563 for monkey and P = 0.482 for human).
Figure 4.
Regression lines for human and monkey mlogDpr/pl versus mlogD for fluorine-containing radiotracers and nonfluorine-containing radiotracers. Where error bars are not visible they are within the symbol size. The regression lines are defined in Table 2.
The correlation coefficient for the relationship between monkey mlogDpr/pl and mlogD for the structurally noncongeneric dataset of nonfluorinated compounds is remarkably high (r2 = 0.906; n = 10) (Table 2). This implies that the two-phase n-octanol-pH 7.4 buffer system is an exceptionally robust model for the single-phase partition of compounds between plasma proteins and the remainder of plasma, and accounts for about 90% of this partition. It is useful to reflect on the meaning of the parameter logDpr/pl. In the hypothetical case of a compound binding to a single binding site in only one protein in plasma, Dpr/pl would relate by the law of mass action to the equilibrium binding constant KA, according to the expression:
| (5) |
where [protein] is the protein concentration and therefore with fP by:
| (6) |
In pharmacology, ligand affinity to a protein is usually expressed as the equilibrium dissociation constant KD, the reciprocal of KA, with a low value representing high affinity binding. From Eqs. 5 and 6, it follows that KD relates to Dpr/pl by the expression:
| (7) |
and therefore with fP by:
| (8) |
For a low fP value of 0.01 for a compound binding to a protein present at 0.6 mM, such as albumin in blood,7,8 the KD would be 6 μM16 and for binding to the much less prevalent serum α1-acid glycoprotein, with a typical blood concentration of ~20 μM,8 the KD would be 0.2 μM. These equilibrium dissociation constants are consistent with being due to relatively weak nonspecific interactions compared with the strong specific interactions of ligands that are needed for binding to proteins with KD values in the nanomolar or even subnanomolar range. For example, in an extreme case, SP203 (4) binds specifically to metabotropic glutamate subtype five receptors with 37 pM affinity.23 Of the ligands listed in Table 1, all fP values exceed 0.0037, and they therefore bind to blood proteins with relatively low affinity.
As mentioned in the Introduction, different studies have reported different mathematical relationships between lipophilicity and fp. Some are linear4,15 and some are sigmoidal,14 plus some are of the logarithmic type reported here (Eqs. 1 and 2).13 The sigmoidal relationships between fP (or 1−fP) and logD are in fact congruent with the logarithmic relationships because a plot of log10[(1−fp)/fp] versus fp is sigmoidal.10 The linear relationship reported by Laruelle et al.4 is for only four structurally congeneric and similarly basic compounds ([11C]DASB (5) and congeners) covering a narrow logD range of 2.44–3.31. Re-expression of the data as mlogDpr/pl versus logD actually improves the correlation coefficient (from r2 = 0.93 to 0.98) and the statistical significance of the correlation from P = 0.035 to 0.011. In fact, there is no physical basis for expecting a linear relationship between fP and lipophilicity (logD or logP). Therefore, the poor linear relationship reported by Saiakhov et al.15 (r2 = 0.29, n = 154) is, in fact, as would be expected.
Do the quantitative relationships between compound logD and fP, derived from our model, have practical value? The relationships have some practical limitations. First, at least 10% of the variance in fp is not accounted for by lipophilicity (logD). Second, small errors in the estimation or measurement of logD can lead to sizable errors in the estimation of fp. Because of the sigmoidal relationship between fP and logD, the relative magnitude of the error in fp depends on the true value of logD. Our set of PET brain-imaging radiotracers has fp values in human that range widely from 0.44 for radiotracer 1 to 0.0037 for radiotracer 18 (Table 1). By Eq. 3, a compound with a logD value of 0.816 would be expected to give an fp value in human of 0.5. A +0.1 log10 unit error in the logD value would predict an fp value of 0.4999, that is, a negligible error in fp. However, for very low values of fp, errors become much more appreciable for the same error in logD. Thus, a compound with a logD value of 3.869 would be expected to give an fp value of 0.005. A +0.1 log10 unit error in the logD value would predict an fp value of 0.0042, that is, a −16% error in fp. In most cases, we measured logD with errors of <0.1 log10 unit (Table 1). Hence, mlogD values might be used to estimate fp with reasonable accuracy, especially for moderate values. Although, from a practical standpoint, it may be just as easy to measure fp as to measure logD, even with a nonradioactive compound, measurement of logD may be more convenient because fP measurement requires regulatory approvals for animal or human blood sampling. As experienced here, present computational methods for estimating logD give sizeable root mean squared errors (≈0.8 log10 units). Major advances are therefore still needed in the accuracy of computational estimations of logD for fp in any species of interest to be predictable with good reliability.
CONCLUSIONS
For the set of structurally diverse nonacidic drug-like compounds studied here, mlogD accounts for over 73% of mlogDpr/pl in humans and over 83% in monkey, despite the great structural dissimilarity between n-octanol and plasma proteins. The derived linear relationships (Eqs. 1 and 2) are statistically different between species. With the exception of one value among 13, monkey fP values were consistently higher than human values. The much weaker correlation of mlogDpr/pl with clogD reflects appreciable errors intrinsic to computation of clogD and points to the present limited value of computed lipophilicity for the development of in-silico methods for the prediction of fP.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIMH). The authors are grateful to Mr. J. Hong, Ms. C.L. Morse, Dr. Y. Zhang, and Dr. E. Luong for radiotracer syntheses, and to the NIH Clinical PET Center (Chief Dr. P. Herscovitch) for radioisotope production.
Footnotes
In this paper we use the prefix c to indicate computed value and the prefix m to indicate measured value.
References
- 1.Smith DA, van de Waterbeemd H, Walker DK. Pharmacokinetics and metabolism in drug design. Weinheim, Germany: Wiley VCH; 2001. [Google Scholar]
- 2.Schmidt S, Gonzalez D, Derendof H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–1122. doi: 10.1002/jps.21916. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Laruelle M, Slifstein M, Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imaging Biol. 2003;5:363–375. doi: 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Pike VW. PET radiotracers: Crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson GR. Plasma and tissue binding considerations in drug disposition. Drug Metab Rev. 1983;14:427–465. doi: 10.3109/03602538308991396. [DOI] [PubMed] [Google Scholar]
- 8.Israeli ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 9.van de Waterbeeemd H, Gifford E. ADMET in silico modeling: Towards prediction paradise. Nat Rev Drug Discov. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- 10.Hall LM, Hall LH, Kier LB. Methods for predicting the affinity of drugs and drug-like compounds for human plasma proteins: A review. Curr Comput Aided Drug Des. 2009;5:90–105. [Google Scholar]
- 11.Leo A, Hansch C, Elkins D. Partition coefficients and their uses. Chem Rev. 1971;71:525–616. [Google Scholar]
- 12.Smith RN, Hansch C, Ames MM. Selection of a reference partitioning system for drug design work. J Pharm Sci. 1975;64:599–606. doi: 10.1002/jps.2600640405. [DOI] [PubMed] [Google Scholar]
- 13.Lázníček M, Květina J, Mazák J, Krch V. Plasma protein binding-lipophilicity relationships: Interspecies comparison of some organic acids. J Pharm Pharmacol. 1987;3:79–83. doi: 10.1111/j.2042-7158.1987.tb06949.x. [DOI] [PubMed] [Google Scholar]
- 14.van de Waterbeemd H, Smith DA, Jones BC. Lipophilicity in PK design: Methyl, ethyl, futile. J Comput Aided Mol Des. 2001;15:273–286. doi: 10.1023/a:1008192010023. [DOI] [PubMed] [Google Scholar]
- 15.Saiakhov RD, Stefan LR, Klopman G. Multiple computer-automated structure evaluation model of the plasma protein binding affinity of diverse drugs. Perspectives Drug Discovery Des. 2000;19:133–155. [Google Scholar]
- 16.Yamazaki K, Kanaoka M. Computational prediction of the plasma protein-binding percent of diverse pharmaceutical compounds. J Pharm Pharmacol. 2004;93:1480–1494. doi: 10.1002/jps.20059. [DOI] [PubMed] [Google Scholar]
- 17.Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs: A new approach. Biochem Pharmacol. 2002;64:1355–1374. doi: 10.1016/s0006-2952(02)01074-2. [DOI] [PubMed] [Google Scholar]
- 18.Lobell M, Sivarajah V. In silico prediction of aqueous solubility, human plasma protein binding and volume of distribution of compounds from calculated pKa and AlogP98 values. Mol Diversity. 2003;7:69–87. doi: 10.1023/b:modi.0000006562.93049.36. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson MP. Plasma protein binding affinity and its relationship to molecular structure: An in-silico analysis. J Med Chem. 2007;50:101–112. doi: 10.1021/jm060981b. [DOI] [PubMed] [Google Scholar]
- 20.Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung S-C, Tamir S, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging. 2007;34:1050–1060. doi: 10.1007/s00259-006-0324-y. [DOI] [PubMed] [Google Scholar]
- 21.Xu R, Hong J, Morse CL, Pike VW. Synthesis, structure-affinity relationships, and radiolabeling of selective high-affinity 5-HT4 receptor ligands as prospective imaging probes for positron emission tomography. J Med Chem. 2010;53:7035–7047. doi: 10.1021/jm100668r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita M, Zoghbi SS, Crescenzo MS, Hong J, Musachio JL, Lu J-Q, Lio J-S, Seneca N, Tipre DN, Cropley VL, Imaizumo M, Gee AD, Seidel J, Green MV, Pike VW, Innis RB. Quantification of brain phosphodiesterase 4 in rat with (R)[11C]rolipram-PET. NeuroImage. 2005;26:1201–1210. doi: 10.1016/j.neuroimage.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Siméon FG, Brown AK, Zoghbi SS, Patterson VM, Innis RB, Pike VW. Synthesis and simple18F-labeling of 3-fluoro-5-(2-(2-(fluoromethyl)thiazol-4-yl)ethynyl)benzonitrile as a high affinity radioligand for imaging monkey brain metabotropic glutamate subtype-5 receptors with positron emission tomography. J Med Chem. 2007;50:3256–3266. doi: 10.1021/jm0701268. [DOI] [PubMed] [Google Scholar]
- 24.Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S, Liow JS, Zoghbi SS, Gladding RL, Innis RB, Pike VW. Evaluation of [18F]SL702 as a prospective agonist PET radioligand for brain 5-HT1Areceptors in mice and monkey. NeuroImage. 2008;41:T157. [Google Scholar]
- 26.Lazarova N, Zoghbi SS, Hong J, Seneca N, Tuan E, Gladding RL, Liow J-S, Taku A, Innis RB, Pike VW. Synthesis and evaluation of [N-methyl-11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem. 2008;51:6034–6043. doi: 10.1021/jm800510m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, Shah J, Musachio JL, Pike VW, Innis RB. PET imaging of the dopamine transporter with18F-FECNT: A polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–527. [PubMed] [Google Scholar]
- 28.Bao X, Lu S, Liow J-S, Zoghbi SS, Jenko KJ, Clark DT, Morse CL, Gladding RL, Innis RB, Pike VW. Radiolabeling and evaluation of [18F]XB-1 in monkey as a prospective histamine subtype 3 receptor PET radioligand. J Label Compd Radiopharm (in press) 2011;54(Suppl 1):S83. [Google Scholar]
- 29.Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Lu S, Fujita M, Innis RB, Pike VW. Synthesis and evaluation in monkey of two sensitive11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- 30.Zoghbi SS, Liow JS, Yasuno F, Hong J, Tuan E, Lazarova N, Gladding RL, Pike VW, Innis RB. 11C-Loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J Nucl Med. 2008;49:649–656. doi: 10.2967/jnumed.107.047308. [DOI] [PubMed] [Google Scholar]
- 31.McCarron JA, Zoghbi SS, Shetty HU, Vermeulen ES, Wikström HV, Ichise M, Yasuno F, Halldin C, Innis RB, Pike VW. Synthesis and initial evaluation of [11C](R)-RWAY in monkey - a new, simply labeled antagonist radioligand for imaging brain 5-HT1Areceptors with PET. Eur J Nucl Med Mol Imaging. 2007;34:1670–1682. doi: 10.1007/s00259-007-0460-z. [DOI] [PubMed] [Google Scholar]
- 32.Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, Hong J, Zoghbi SS, Fujita M, Toledo MA, Diaz N, Gackenheimer SL, Tauscher JT, Barth VN, Innis RB. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem. 2011;54:2687–2700. doi: 10.1021/jm101487v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage. 2010;49:2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briard E, Zoghbi SS, Siméon FG, Imaizumi M, Gourley JP, Shetty HU, Lu S, Fujita M, Innis RB, Pike VW. Single-step high-yield radiosynthesis and evaluation of a sensitive18F-labeled ligand for imaging brain peripheral benzodiazepine receptors with PET. J Med Chem. 2009;52:688–699. doi: 10.1021/jm8011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu S, Liow JS, Zoghbi SS, Hong J, Innis RB, Pike VW. Evaluation of [11C]S14506 and [18F]S14506 in rat and monkey as agonist PET radioligands for brain 5-HT1Areceptors. Curr Radiopharm. 2010;3:9–18. doi: 10.2174/1874471011003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, Felder CC, Halldin C, Schaus JM. Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J Med Chem. 2009;51:5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark J, Baldwin R, Bayne K, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Bobb JW, Smith OA, Steggerda JA-D, Van de Ber JL. Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources. Washington, DC: National Research Council; 1996. [Google Scholar]
- 38.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: β-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 39.Tetko IV, Poda GI. Application of ALOGPS 2.1 to predict logD distribution coefficient for Pfizer proprietary compounds. J Med Chem. 2004;47:5601–5604. doi: 10.1021/jm049509l. [DOI] [PubMed] [Google Scholar]
- 40.Acharya MR, Sparreboom A, Sausville EA, Conley BA, Doroshow JH, Venitz J, Figg WD. Interspecies differences in plasma protein binding of MS-275, a novel histone deacetylase inhibitor. Cancer Chemother Pharmacol. 2006;57:275–281. doi: 10.1007/s00280-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 41.Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs–revisited. Curr Opin Drug Discov Devel. 2004;7:507–511. [PubMed] [Google Scholar]
- 42.Kosa T, Maruyama T, Otagiri M. Species differences of serum albumins: I. Drug binding sites. Pharm Res. 1997;14:1607–1612. doi: 10.1023/a:1012138604016. [DOI] [PubMed] [Google Scholar]
- 43.Kosa T, Maruyama T, Otagiri M. Species differences of serum albumins: II. Chemical and thermal stability. Pharm Res. 1998;15:449–454. doi: 10.1023/a:1011932516717. [DOI] [PubMed] [Google Scholar]
- 44.Kosa T, Maruyama T, Sakai N, Yonemura N, Yahara S, Otagiri M. Species differences of serum albumins: III. Analysis of structural characteristics and ligand binding properties during N-B transitions. Pharm Res. 1998;15:592–598. doi: 10.1023/a:1011986028529. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto K, Sukimoto K, Nishi K, Maruyama T, Suenaga A, Otagiri M. Characterization of ligand binding sites on the α1-acid glycoprotein in human, bovines and dogs. Drug Metab Pharmacokinet. 2002;17:300–306. doi: 10.2133/dmpk.17.300. [DOI] [PubMed] [Google Scholar]
- 46.Goss KU, Bronner G. What is so special about the sorption behavior of highly fluorinated compounds? J Phys Chem A. 2006;110:9518–9522. doi: 10.1021/jp062684o. [DOI] [PubMed] [Google Scholar]
- 47.Bégué J-P, Bonnet-Delpon D. Bioorganic and medicinal chemistry of fluorine. New Jersey: John Wiley & Sons, Inc; 2008. [Google Scholar]