Abstract
The shortest synthetic routes to nine cis-pheromones containing a variety of functionality, including an unconjugated (E,Z) diene, are reported. These lepidopteran pheromones are used extensively for pest control, and were easily prepared using ruthenium-based Z-selective cross metathesis, highlighting the advantages of this method over less efficient ways to form Z-olefins. Important insight into the mechanism of Z-selective metathesis was uncovered during experimentation and subsequently explored.
Keywords: Z-olefin, insect pheromone, olefin metathesis, seed oil derivatives, unconjugated diene
The use of insect sex pheromones to limit specifically targeted pest populations has gained increasing popularity as a viable, safe, and environmentally-friendly alternative to insecticides. While broad spectrum insecticides are toxic compounds that have been shown to adversely affect human health,[1] extensive studies have revealed that insect pheromones are nontoxic and safe for human consumption at the levels used in pest control practices.[2] Female sex pheromones are mainly employed in pest control in a process termed mating disruption. This involves dispersing pheromones over a large area, overloading the sensory organs of male insects and preventing them from locating and mating with females who are releasing a much smaller amount of the same pheromone blends; this strategy has proven to reduce specific insect populations dramatically.[3] To date, the United States Environmental Protection Agency (EPA) has approved approximately twenty lepidopteran female sex pheromones as active ingredients for pest control.[2]
The lepidopteran order of insects includes extensive families of butterflies and moths whose larvae can devastate critical and valuable crops; it is estimated that insects destroy approximately 13% of food crops in the United States each year.[1] The majority of known lepidopteran sex pheromones are straight-chained hydrocarbon acetates, alcohols, and aldehydes containing one to three double bonds with various olefin geometries. The facile formation of trans-olefin containing pheromones using olefin metathesis has been reported, however the efficient synthesis of cis-containing pheromones has remained a challenge.[4] Current routes to such species involve wasteful processes including the Lindlar hydrogenation of alkynes and Wittig reactions, among others.[5] Due to difficulty in completely removing the palladium hydrogenation catalyst and the high toxicity of the lead reagent necessary to prevent over-reduction, the Lindlar hydrogenation of alkynes is not an optimal route to Z-olefins. Similarly, the Wittig reaction is not ideal due to the use of stoichiometric reagents and difficulty removing phosphine oxide byproducts.
A family of functional-group tolerant, ruthenium-based Z-selective metathesis catalysts was recently disclosed and a monounsaturated cis-olefin containing pheromone was synthesized using the optimized nitrato-type catalyst 1 (Figure 1). Catalyst 1 has been shown to be an improvement over previously reported carboxylate-substituted catalysts in terms of activity, stability, and selectivity.[6] We desired to further demonstrate the efficacy of 1 by synthesizing a set of insect pheromones with diverse functionality while simultaneously investigating the reactivity of catalyst 1 in more complicated cross metathesis reactions.
Figure 1.
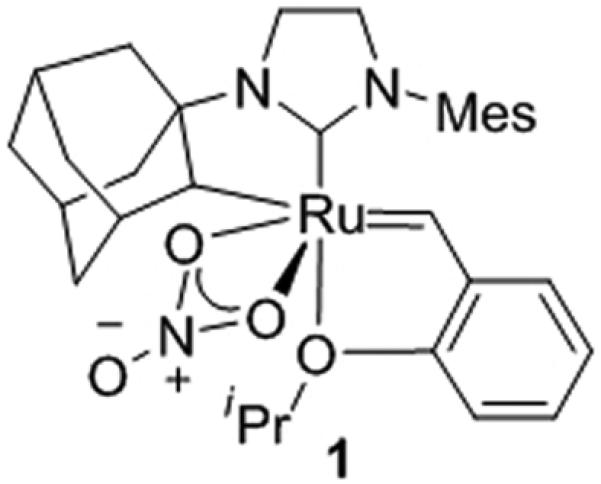
Previously reported chelated catalyst for Z-selective olefin metathesis (1).
Nine lepidopteran female sex pheromones currently approved by the EPA as insecticide alternatives were chosen as synthetic targets, and it was proposed that the chosen species could be formed at ambient temperatures from renewable and commercially-available chemicals with a minimal amount of steps and low catalyst loadings of 1.[ 7 ] Alcohol-substituted species were chosen as substrates because simple manipulations, such as acetylation or oxidation, could be subsequently carried out to produce the desired pheromones. It is envisioned that the methods developed could be elaborated to quickly synthesize other well-studied insect pheromones containing cis-olefins not yet approved by the EPA for pest control.
We proposed that a number of the pheromone targets could be synthesized by the Z-selective cross metathesis of various terminal olefins with the seed oil derivatives oleyl alcohol (2) and 11-eicosenol (4). These long chain primary alcohols contain one 1,2-disubstituted cis-double bond and are obtained by the transesterification and reduction of a number of seed oils, including canola and jojoba oil. The metathesis of seed oils offers new synthetic routes to high value products from renewable resources with high chemoselectivity.[8] Currently, plant oils are a particularly important renewable raw material for the chemical industry, and products derived from them are used heavily as surfactants, cosmetic products, and lubricants.[9] We envisioned that using these alcohol-substituted starting materials would provide simple, cheap, and renewable routes to high value insect pheromones.
It was found that 1-hexene could be reacted with the aforementioned seed oil derivatives in the presence of catalyst 1 (1 mol %) to yield the desired cross products in good yields with high cis-selectivity (Scheme 1). The reaction of 1-hexene with oleyl alcohol directly produced 3 in 77% yield and with 86% of the Z-olefin. The corresponding product (5) derived from 11-eicosenol was obtained in 75% yield and with 86% Z-olefin, and was subsequently oxidized under Swern conditions to yield pheromone 6. The syntheses reported here are the shortest routes to form pheromones 3 and 6.[5]
Scheme 1.
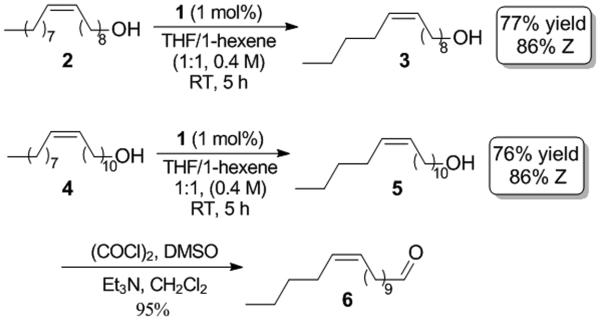
Syntheses of pheromones 3 and 6.
In order to investigate the mechanism of formation of pheromone cross products, the reaction of 1-hexene and oleyl alcohol (2) to produce 3 was monitored over time using quantitative 13C NMR (Figure 2).[ 10 ] As shown, 2 quickly converts to the terminal olefin, 9-decenol, through an ethenolysis reaction. Formation of a ruthenium methylidene is thus required and we proposed that this species was generated from homodimerization of 1-hexene. The resulting 9-decenol then slowly reacts with 1-hexene to produce the desired pheromone cross product 3; the cis:trans ratio of 3 was also monitored and did not change to any appreciable extent over the course of the reaction. It is proposed that internal olefins cannot react directly to produce the desired cross products, but must first be converted to the corresponding terminal olefins via ethenolysis, and only then can they undergo a cross metathesis reaction with another terminal olefin. In order to test this assertion, the internal olefins cis-5-decene and 4 were reacted in the presence of catalyst 1 to produce compound 5. If the proposition was true, since a methylidene cannot be formed due to the absence of ethylene and terminal olefins, there should be no conversion to the desired cross product because the internal olefins cannot be degraded. Indeed, reacting the two internal olefins for up to 19 hours led to no formation of 5, as monitored by gas chromatography. We believe that this is a general feature of cross metathesis reactions catalyzed by 1 and is possibly due to the high steric demands associated with forming trisubstituted ruthenacyclobutane intermediates.
Figure 2.
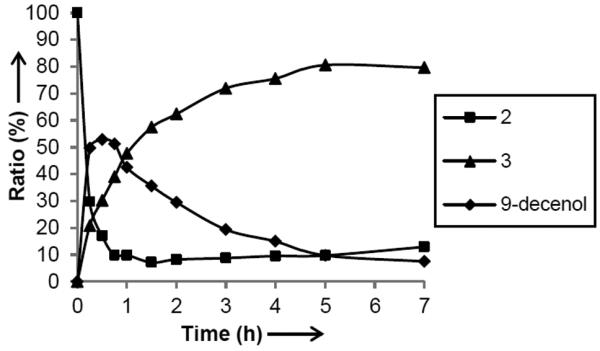
Time course experiment monitoring relative amounts of 2, 9-decenol, and the desired pheromone 3.
Cross metathesis of the same seed oil derivatives with 1-butene proved to be more difficult than with 1-hexene (Scheme 2). As with all reactions presented in this study, ethylene generated must be allowed to escape in order to obtain reasonable conversions, so a fixed amount of the gaseous substrate could not be used. The optimal conditions involved a slow bubble of 1-butene in the reaction solution and slightly higher catalyst loading of 1 (2 mol %). Reaction with oleyl alcohol and 11-eicosenol followed by acetylation of the cross products led to formation of pheromones 8 and 10 in modest yields (40-47%) and slightly reduced cis-selectivity (76-77%).[11] A significant amount of the corresponding terminal olefins derived from ethenolysis of 2 and 4 was detected, suggesting that the lower yields are due to the inability of 1-butene to efficiently react with the terminal olefins under the conditions tested.[12]
Scheme 2.
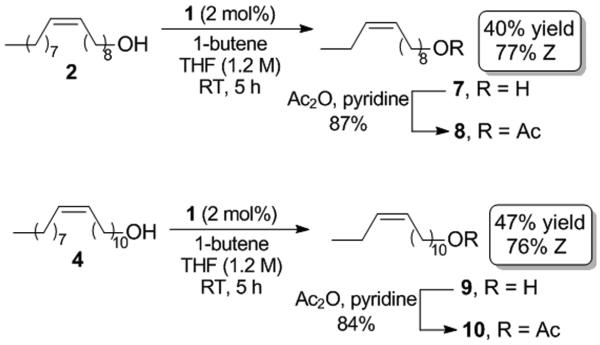
Syntheses of pheromones 8 and 10.
Cross metathesis of the terminal olefins 8-nonenol and 1-pentene led to formation of pheromone 12 in good yield (73%) with high cis-selectivity (86%) (Scheme 3). Subsequent acetylation of 12 provided pheromone 13 in 90% yield. Compound 13 was alternatively synthesized by the direct reaction of 8-nonenyl acetate and 1-pentene which proceeded in analogous overall yields (~65%) and Z-selectivities (~85%) when compared to the above two step sequence. The reaction of 1-pentene with 8-nonenol resulted in slightly higher yields compared to the reaction with 8-nonenyl acetate, which is likely a result of the higher selectivity alcohol-containing substrates exhibit for formation of the desired cross products.[13] Regardless, it has been demonstrated that Z-olefins with acetate functionality can be easily prepared using catalyst 1. The successful use of functionalized terminal olefins in this methodology is attractive due to the wide variety of commercially available α-olefins containing alcohol and acetate functional groups.
Scheme 3.
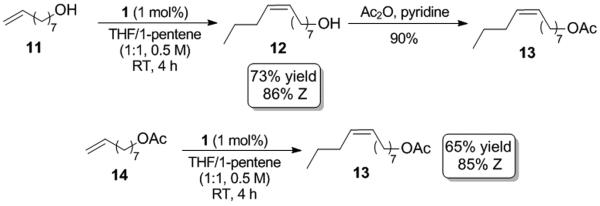
Syntheses of pheromones 12 and 13.
We next attempted to synthesize a pheromone containing an unconjugated diene and were pleased to find that the Z-selective cross metathesis of oleyl alcohol and trans-1,4-hexadiene using catalyst 1 led to selective formation of compound 15, which was subsequently acetylated to yield pheromone 16 (Scheme 4). Previous reported syntheses of 16 require at least six steps from commercially available starting materials.[14] We were able to form 16 starting from renewable and commercially available reagents in two steps with good overall yield (60%) and high cis-selectivity (88%). It should be noted that only trace amounts of products derived from metathesis of the internal double bond of the trans-1,4-hexadiene starting material, such as compound 17, were detected. Despite significant research efforts, the selective synthesis of unconjugated and conjugated dienes using previous generations of metathesis catalysts has remained difficult.[ 15 ] This is the first example of the cross metathesis of an unconjugated diene that exhibits chemoselectivity based on olefin geometry.[ 16 ] The observed chemoselectivity suggests that this could be a powerful and general tool to construct (E,Z) dienes. The selective formation of 15 over 17 seems to be a consequence of the inability of 1 to react with 1,2-disubsituted trans-olefins. Since it has been proposed that all internal olefins must first undergo an ethenolysis reaction before they can react further, the observation that trans-olefins are unreactive suggests that catalyst 1 could be a capable Z-selective ethenolysis catalyst.[17]
Scheme 4.
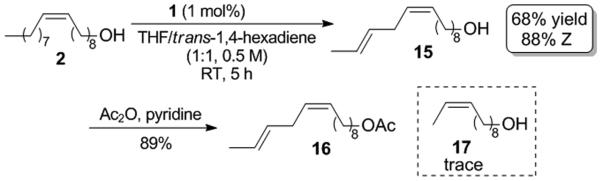
Synthesis of pheromone 16.
Two of the synthetic targets do not follow the general definition of lepidopteran insect pheromones; the major component pheromone of the Douglas fir tussock moth (20) contains ketone functionality, while the gypsy moth pheromone disparlure (24) contains an epoxide. We proposed that both pheromones would require more involved syntheses compared to the above compounds, but could be readily accessible using Z-selective metathesis methodology.
A number of syntheses of 20 have been reported using as few as four steps, however construction of the cis-olefin was predominantly achieved by the semi-reduction of alkynes with a Lindlar catalyst or the Wittig reaction.[18] Our synthesis of 20 was carried out as shown in Scheme 5. Secondary alcohol 18, formed by the addition of 4-pentenyl lithium to 10-undecenal, was subsequently reacted with 1-heptene in a Z-selective cross metathesis reaction with 0.5 mol % catalyst loading of 1 to yield 19 in good yield (70%) with high Z-selectivity (88%). This was then oxidized to the desired ketone-containing pheromone 20 using a Swern reaction in 47% overall yield and 88% of the Z-olefin, in three steps from commercially available starting materials.
Scheme 5.
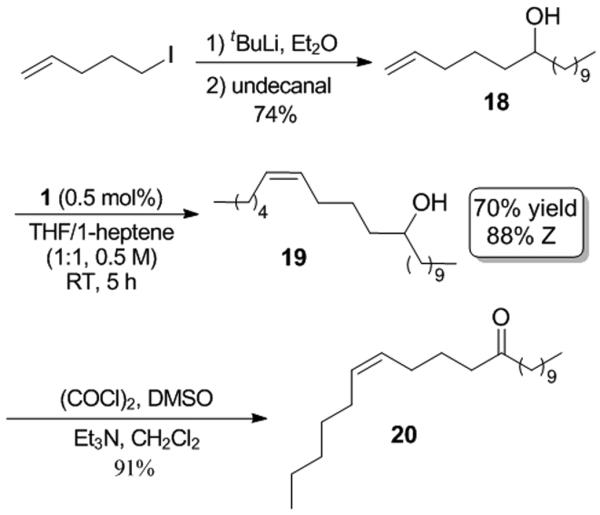
Synthesis of pheromone 20.
Many routes to enantiopure 24 have been reported, and have led to the determination that (+)-24 is significantly more active than (−)-24 as a chemical attractant. Despite this, the racemic form (±)-24 has been shown to disrupt mating as effectively as (+)-24 and is extensively employed in pest control practices. [19] Multiple reported syntheses of (±)-24 exist and require no fewer than four steps.[5h,20] Our synthesis was carried out as shown in Scheme 6. First, cross metathesis of 4-pentenol and 1-dodecene afforded 21 in moderate yield (62%) and high Z-selectivity (84%).[ 21 ] Alcohol 21 was tosylated and reacted with isobutylmagnesium bromide to produce the desired aliphatic intermediate 23.[22] Subsequent epoxidation of 23 with mCPBA yielded (±)-24 in four steps from commercially available starting materials.
Scheme 6.
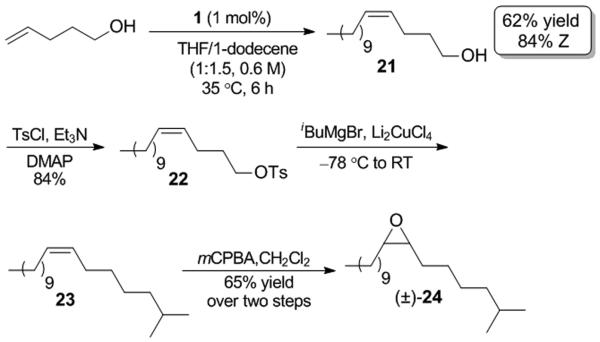
Synthesis of pheromone (±)-24.
Current industrial syntheses of (±)-24 similarly proceed through epoxidation of alkyl intermediate 23, which can be formed either by Wittig or acetylenic routes.[5h] Use of the Wittig reaction on an industrial scale is generally difficult because of the large amount of phosphine oxide byproducts that must be removed, and problems cooling the reaction mixture enough to promote adequate cis-selectivity. Still, (±)-24 can be formed in five steps with 60% overall yield and 88% Z-olefin. The industrial scale synthesis of (±)-24 using the poisoned hydrogenation of a disubstitited acetylene can be completed in four overall steps with 98% Z-olefin. However, on an industrial scale, forming disubsituted acetylenes can be expensive and requires the use of large amounts of liquid ammonia, temperature control can be a problem and lead to incomplete reduction, and double bond migration and isomerization can occur leading to impurities. The use of methathesis as the key step to form (±)-24 is attractive because cheap α-olefins can be used, unreacted starting material can be recycled, and all reactions can be run at mild temperatures. It is envisioned that all of the synthetic routes outlined above can be adapted for large scale syntheses.
In summary, the facile synthesis of nine lepidopteran female sex pheromones has been achieved using ruthenium-based Z-selective olefin metathesis. These pheromones are approved by the EPA as pest control agents, however it is conceived that other analogous pheromones can be synthesized in a similar manner, further promoting their use as insecticide alternatives. The synthesis of these compounds provided valuable insight into the reactivity and selectivity of catalyst 1, which is markedly distinct from previous generations of metathesis catalysts. The development of new Z-selective catalysts and operating on a larger scale should make this methodology even more selective and efficient. Compounds containing a variety of functional groups, including alcohols, acetates, aldehydes, ketones, and epoxides were easily prepared in a minimal number of steps from commercial sources, including several seed oil derivatives; the syntheses of all pheromones described above are the most concise to date. It has been demonstrated that ruthenium-based Z-selective metathesis provides an attractive route to form complex internal olefins in good yields with high cis-selectivity, and could emerge as a viable alternative to other popular methods used to form cis-olefins such as the partial hydrogenation of alkynes and the Wittig reaction.
Footnotes
Dr. David VanderVelde is thanked for his assistance with NMR characterization and experiments. Mr. Zachary K. Wickens and Dr. Daryl P. Allen are thanked for helpful discussion related to pheromone synthesis. This work was financially supported by the NIH (NIH 5R01GM031332-27), the NSF (CHE-1048404), and NSERC (fellowship to V. M. M.). Materia Inc. is acknowledged for the generous donation of metathesis catalyst 1.
Supporting information for this article is available on the WWW under http://www.angewandte.org.
Contributor Information
Myles B. Herbert, Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 (USA)
Dr. Vanessa M. Marx, Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 (USA)
Richard L. Pederson, Materia Inc., 60 N. San Gabriel Blvd., Pasadena, CA, 91107 (USA)
Robert H. Grubbs, Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 (USA).
References
- [1].Pimintel D. Chem. Br. 1991:646. [Google Scholar]
- [2]. [accessed 5/10/2012];Lepidopteran Pheromones Fact Sheet. http://www.epa.gov/oppbppd1/biopesticides/ingredients/factsheets/factsheet_lep_pheromones.htms/ingredients/factsheets/factsheet_lep_pheromones.htm.
- [3].a) Welter SC, Pickel C, Millar J, Cave F, Van Steenwyk RA, Dunley J. J. Califor. Agric. 2005;59:17. [Google Scholar]; b) Howse PE, Stevens IDR, Jones OT. Insect Pheromones and their Use in Pest Management. Chapman & Hall; New York: 1998. pp. 314–338. [Google Scholar]
- [4].Pederson RL, Fellows IM, Ung TA, Ishihara H, Hajela SP. Adv. Synth. Catal. 2002;344:728. [Google Scholar]
- [5].The synthesis of alkenyl alcohols, acetates, and aldehydes containing a Z-olefin using classical approaches have been widely reported: Petrushkina EA, Kalinin VN. Russ. J. Gen. Chem. 2008;78:1897. Li J-M, Yong J-P, Aisa HA. Chem. Nat. Compd. 2008;44:224. Chobanyan ZA. Russ. J. Appl. Chem. 2004;77:2036. Subbaraman AS, Mithran S, Mamdapur VR. Molecules. 1998;3:35. Maruoka K, Oishi M, Yamamoto H. J. Am. Chem. Soc. 1996;118:2289. Schaub B, Blasér G, Schlosser M. Tetrahedron Lett. 1985;26:307. Horiike M, Tanouchi M, Hirano C. Agric. Biol. Chem. 1978;42:1963. Henrick CA. Tetrahedron. 1977;33:1845.
- [6].a) Endo K, Grubbs RH. J. Am. Chem. Soc. 2011;133:8525. doi: 10.1021/ja202818v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Keitz BK, Endo K, Herbert MB, Grubbs RH. J. Am. Chem. Soc. 2011;133:9686. doi: 10.1021/ja203488e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Keitz BK, Endo K, Patel PR, Herbert MB, Grubbs RH. J. Am. Chem. Soc. 2012;134:693. doi: 10.1021/ja210225e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Keitz BK, Fedorov A, Grubbs RH. J. Am. Chem. Soc. 2012;134:2040. doi: 10.1021/ja211676y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Separation of E-isomers from the desired Z-isomers was beyond the scope of this paper, however, the undesired E-pheromone is generally inert for pest control applications. If isolation of the pure Z-isomer is necessary, chromatography with silver nitrate has been shown to be effective at this type of separation: Williams CM, Mander LN. Tetrahedron. 2001;57:425.
- [8].a) Biermann U, Bornscheuer E, Meier MAR, Metzger JO, Shäfer HJ. Angew. Chem. Int. Ed. 2011;50:3854. doi: 10.1002/anie.201002767. [DOI] [PubMed] [Google Scholar]; b) Marvey BB. Int. J. Mol. Sci. 2008;9:1393. doi: 10.3390/ijms9081393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meier MAR, Metger JO, Shubert US. Chem. Soc. Rev. 2007;36:1788. doi: 10.1039/b703294c. [DOI] [PubMed] [Google Scholar]
- [10].See supporting information for NMR conditions.
- [11].Alternative methods, including pressurization of the reaction vessel with 1-butene, and a slow stream of 1-butene into the headspace of the reaction vessel, resulted in highly reduced yields and Z-selectivity. The cause of this Z-degradation is not known but will be investigated in a subsequent report.
- [12].A small amount of terminal olefin products derived from ethenolysis of the seed oil derivatives was generated during the corresponding reaction with 1-hexene as well. The reaction of 9-decenol with 1-butene to produce 7 produced analogous yields and Z-selectivities when compared to the reaction of oleyl alcohol and 1-butene.
- [13].A much larger amount of the undesired homodimer was detected for reaction of 8-nonenyl acetate compared to 8-nonenol.
- [14].a) Hornyánszky G, Rohály J, Novák L. Synth. Commun. 2008;38:1533. [Google Scholar]; b) Ortiz A, Quesada A, Sanchez A. J. Chem. Ecol. 2004;30:991. doi: 10.1023/b:joec.0000028463.43564.40. [DOI] [PubMed] [Google Scholar]; c) Matveeva ED, Erin AS, Leshcheva IF, Kurts AL. Russ. J. Org. Chem. 2000;36:765. [Google Scholar]
- [15].Funk TW, Efskind J, Grubbs RH. Org. Lett. 2005;7:187. doi: 10.1021/ol047929z. [DOI] [PubMed] [Google Scholar]
- [16].The Z-selective homodimerization of conjugated 1,3-dienes was recently reported for molybdenum and tungsten olefin metathesis catalysts: Townsend EM, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2012;134:11334. doi: 10.1021/ja303220j.
- [17].This phenomenon was first reported for a Z-selective molybdenum metathesis catalyst: Marinescu SC, Levine DS, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2011;133:11512. doi: 10.1021/ja205002v.
- [18].There are many reported syntheses of compound 20. Selected examples: Jindal R, Devi A, Kad GL, Singh J. Indian J. Chem. 2010;49:495. Sect B. Jones DM, Kamijo S, Dudley GB. Synlett. 2006;6:936. Hayes JF, Shipman M, Twin H. J. Org. Chem. 2002;67:935. doi: 10.1021/jo016164v.
- [19].a) Zhigang W, Jianfeng Z, Peiqiang H. Chin. J. Chem. 2012;30:23. [Google Scholar]; b) Kim S-G. Synthesis. 2009;14:2418. [Google Scholar]; c) Prasad KR, Anbarasan P. J. Org. Chem. 2007;72:3155. doi: 10.1021/jo070060o. [DOI] [PubMed] [Google Scholar]; d) Kounmbis AE, Chronopoulos DD. Tetrahedron Lett. 2005;46:4353. [Google Scholar]; e) Iwaki S, Marumo S, Saito T, Yamada M, Katagiri K. J. Am. Chem. Soc. 1974;96:7842. [Google Scholar]
- [20].a) Bykov VI, Goletiani AR, Butenko TA, Finkel’shtein E. Sh. Dokl. Akad. Nauk. 2002;384:496. [Google Scholar]; b) Bykov VI, Butenko TA, Petrova EB, Finkelshtein E. Sh. Tetrahedron. 1999;55:8249. [Google Scholar]; c) Tolstikov GA, Odinokov VN, Galeeva RI, Bakeeva RS. Tetrahedron Lett. 1978;21:1857. [Google Scholar]
- [21].The moderate yield here is not surprising given that the homodimerization of 4-pentenol has proven to be a difficult reaction. See Reference 6 (c).
- [22].It should be noted that species 14 resembles a number of completely hydrocarbon pheromones, and this synthetic route should enable facile access to such species. For an example, see: Carlson DA, Mayer MS, Sihacek DZ. Science. 1971;76:174. doi: 10.1126/science.174.4004.76.


