Abstract
Background
Aerobic physical capacity plays an important role in reducing morbidity and mortality rates in subjects with cardiovascular diseases. This action is often related to an improvement in the autonomic modulation of heart rate variability (HRV). However, controversies remain regarding the effects of physical training on cardiac autonomic control in healthy subjects. Therefore, our objective was to investigate whether aerobic capacity interferes with the autonomic modulation of HRV and whether gender differences exist.
Methods
Healthy men and women (N=96) were divided into groups according to aerobic capacity: low (VO2: 22-38 ml/kg-1 min-1), moderate (VO2: 38-48 ml/kg-1 min-1) and high (VO2 >48 ml/kg-1 min-1.) We evaluated the hemodynamic parameters and body composition. The autonomic modulation of HRV was investigated using spectral analysis. This procedure decomposes the heart rate oscillatory signal into frequency bands: low frequency (LF=0.04-0.15Hz) is mainly related to sympathetic modulation, and high frequency (HF=0.15-0.5Hz) corresponds to vagal modulation.
Results
Aerobic capacity, regardless of gender, determined lower values of body fat percentage, blood pressure and heart rate. In turn, the spectral analysis of HRV showed that this parameter did not differ when aerobic capacity was considered. However, when the genders were compared, women had lower LF values and higher HF values than the respective groups of men.
Conclusion
The results suggest that aerobic physical capacity does not interfere with HRV modulation; however, the cardiac modulatory balance differs between genders and is characterized by a greater influence of the autonomic vagal component in women and by the sympathetic component in men.
Introduction
Low cardiorespiratory capacity is directly associated with an increase in the morbidity and mortality rates in both male and female subjects with cardiovascular diseases, regardless of the presence of other risk factors [1-3].
In this sense, the increase in aerobic capacity through the regular practice of physical exercises has been suggested to be a non-pharmacological means to prevent or treat a number of diseases, mainly those characterized as chronic degenerative, such as hypertension, diabetes mellitus and obesity [4,5].
In fact, there is evidence that moderate-to-high levels of cardiorespiratory capacity reduce the risks of mortality resulting from cardiovascular diseases or other causes in both genders, and this cardioprotective effect is independent of age, race, adiposity or other health conditions [3,6-9].
Despite this evidence, the causes of reduced morbidity via practicing physical exercises, which increases an individual’s aerobic capacity, have not been completely elucidated yet. It is common to report the improvement in cardiac autonomic control as one of the adaptations responsible for this reduction [10,11]. However, many clinical and experimental studies assessing autonomic control under different perspectives have shown conflicting results in terms of the adaptations induced by exercise on the autonomic modulation of heart rate variability (HRV) [12-15]. In fact, these conflicting results seem to be more related to healthy subjects because most studies demonstrate that the regular practice of physical exercises improves HRV in subjects with chronic degenerative diseases, such as hypertension, obesity and diabetes [10,11,13,16].
Moreover, some studies have reported that the higher level of aerobic capacity improves cardiac autonomic function in healthy subjects, suggesting the hypothesis that high-performance athletes have a better autonomic HRV modulation than sedentary subjects or those who practice physical activities for leisure and/or health reasons [17-19]. Another debate is whether the possible physical exercise-induced autonomic adaptations on HRV modulation would be the same for both genders [10,13,14,16,18] because very few studies have evaluated the differences in the cardiac autonomic modulation between healthy men and women with different levels of aerobic capacity. Our hypothesis is that sedentary men and women have different modulation patterns and that aerobic physical capacity can interfere with such patterns in a gender-specific way.
Therefore, the objective of the present study was to investigate whether different levels of aerobic capacity can influence cardiac autonomic modulation in healthy subjects and whether there are differences between men and women.
Methods
Participants
A total of 96 healthy subjects (53 men and 43 women), aged between 18 and 40 years, participated in the study. All subjects were normotensive and had normal cardiopulmonary function. The exclusion criteria were as follows: a high body mass (index > 25 kg/m2); the use of drugs or other addictive substances (tobacco and alcohol); a diagnosis of asthma, sleep apnea, or hypertension (blood pressure > 140/90 mmHg); metabolic disorders, including diabetes, dyslipidemia, pre-diabetes, and diabetes types 1 and 2; the chronic use of medications or substances that might interfere with cardiac autonomic function or the cardiovascular system; and the presence of musculoskeletal disorders that would prevent the subjects from performing the evaluations.
Prior to the experimental procedures, all volunteers were selected by anamnesis, which provided data regarding each participant’s physical fitness and health status. Regarding their athletic profile, the volunteers were questioned about their daily and weekly physical activities, including the type, frequency, and workload. All volunteers who were included in the “low aerobic capacity” group (sedentary) reported that they were not following any type of physical activity program and had only a small caloric expenditure in daily activities, such as walking and climbing stairs. All volunteers included in the “moderate aerobic capacity” (trained) group reported that they were following a physical activity program at the gym or outdoors once per day for 5 days per week, with an average time between 1 hour and 30 minutes and 2 hours. These volunteers were participating in amateur competitions, such as marathons, for recreational purposes and to maintain their health and quality of life. The volunteers included in the “high aerobic capacity” group (athletes) had daily training sessions (4-5 hours) divided into two periods (morning and night) 7 days per week to improve athletic performance. All volunteers in this group were elite athletes who had participated in national and international sports competitions in their respective modalities. The modalities practiced by both the “moderate” and the “high” groups were considered as aerobic in nature (long-distance races, such as marathons and triathlons). Finally, all the information regarding the athletic profile was compared with the results obtained from cardiopulmonary stress testing (ergospirometry).
After the screening, the selected volunteers were divided into three subgroups according to aerobic physical capacity, which was determined using a maximum ergospirometric test on a treadmill as follows: low aerobic capacity (VO2max: 22-38 ml kg-1 min-1), moderate aerobic capacity (VO2max: 38-48 ml kg-1 min-1), and high aerobic capacity (VO2max: > 48 ml kg-1 min-1).
Volunteers were screened at the Exercise Physiology Laboratory, Department of Biomechanics, Medicine and Rehabilitation, School of Medicine of Ribeirão Preto, University of São Paulo, Brazil. The study protocol was conducted in accordance with the ethical standards established by the Helsinki Declaration of 1975 and was approved by the Ethics Committee on Human Research, School of Medicine of Ribeirão Preto, University of São Paulo, Brazil (Protocol #4820/2011). All subjects were informed about the procedures and non-invasive experiments that would be performed in this study. After agreeing to participate in the study, all subjects signed an informed consent form.
Study Design
Each volunteer was evaluated in the morning (08:00 to 10:00 am) during two laboratory visits. They were previously instructed not to perform intense physical activity and to avoid the consumption of alcoholic and caffeinated beverages for 48 hours prior to testing, and to sleep at least 8 hours and eat soft food 2 hours before testing.
Hemodynamic parameters
Systolic arterial pressure (SAP), diastolic arterial pressure and mean arterial pressure (MAP) were obtained using a mercury column sphygmomanometer with the auscultatory method. Heart rate (HR) was obtained using an electrocardiographic digital recorder (ML866 PowerLab, ADInstruments, Bella Vista, Australia). Evaluations were performed before experimental procedures and after a 15-minute rest period.
Spectral Analysis of HRV
HR recordings for HRV spectral analysis via electrocardiograms (ML866 PowerLab, ADInstruments, Bella Vista, Australia) were performed between 9:00 and 10:00 a.m. for 40 minutes while volunteers were in a supine position. Time series were obtained from adjacent R-R intervals (iRR) and then divided into segments of 200 beats, which were superimposed over the segments of 100 beats obtained from the previous series. After calculating the mean and variance of each segment, a spectral analysis was performed using the autoregressive model [20]. The oscillatory components present in the stationary segments from beat to beat of the iRR were calculated based on the resources of Levinson-Durbin, according to Akaike’s criteria [21]. This procedure allows the automatic quantification of the central frequency and influence of each relevant oscillatory component that is present in the interval series. The oscillatory components were classified as low frequency (LF) and high frequency (HF), and they showed fluctuations in the frequency ranges of 0.04-0.15 Hz and 0.15-0.5 Hz, respectively [20,21]. The power of the LF and HF components in the variability of the iRR was also expressed in normalized units and was obtained by calculating the percentage of variability in the LF and HF and considering the total power after subtracting the very low frequency (VLF) component (frequencies < 0.04 Hz). This normalization procedure minimizes the effects of total power changes on the variability of the absolute values of the LF and HF components [20,21]. Additionally, the LF/HF ratio was calculated to establish an index for the assessment of the cardiac autonomic modulation.
Ergospirometric test
The maximum oxygen uptake (VO2max) was assessed by a maximal exercise test on a treadmill (Super ATL Millenium®, Inbramed/Inbrasport, Brazil) according to a previously published protocol [22]. The analysis of exhaled gases (O2 and CO2) was performed using a metabolic device (Ultima™ CardiO2, Medical Graphics Corp., St. Paul, MN, USA).
Anthropometric parameters
Body mass index values were obtained using the formula W/H2, where W is the weight in kilograms and H is the height of the subject in meters. Body composition was evaluated using the bioelectrical impedance method (Quantum BIA 101; Q-RJL Systems, Clinton Township, MI, USA).
Statistical Analysis
The results are shown as the mean ± S.E.M. (standard error of the mean). The effects of gender and aerobic physical capacity were assessed by two-way analysis of variance (ANOVA). When appropriate, post hoc comparisons were performed using the Student-Newman–Keuls test. Differences were considered significant when p<0.05. All statistical tests were performed with SigmaStat 3.5 software (Systat Software Inc., San Jose, CA, USA). The sample size calculation was performed using the “GraphPad StatMate 2.0”, confidence level of 95%, Power 80%, using the LF and HF variables in normalized units. It was been suggested the number of 12 volunteers in each group.
Results
The characteristics and hemodynamic parameters of the subjects are presented in Table 1. The men had higher BMI values than women, regardless of the level of aerobic capacity (gender factor, F(1,90): 27.9, p<0.001). In turn, the assessment of body fat percentage showed an inverse relationship with the level of aerobic capacity in both men and women. However, men had the lowest values at all levels of aerobic capacity. With regard to the hemodynamic parameters (Table 1), both men and women from the moderate and high aerobic capacity groups had lower HR values than the subjects from the low aerobic capacity group (aerobic physical capacity factor, F(2,90): 71, p<0.001). However, there was no statistically significant difference between men and women when the groups were compared. An inverse relationship was observed between aerobic capacity and AP in both men and women (SAP, aerobic physical capacity factor, F(2,90): 11.4, p<0.001; DAP, aerobic physical capacity factor, F(2,90): 7.78, p<0.001; MAP, aerobic physical capacity factor, F(2,90): 10.8, p<0.001). Additionally, women always demonstrated lower values than men (SAP, gender factor, F(2,90): 18.9, p<0.001; DAP, gender factor, F(2,90): 13.3, p<0.001; MAP, gender factor, F(2,90): 18.2, p<0.001).
Table 1. Effects of gender and aerobic physical capacity on characteristics and hemodynamic values.
|
Low (N = 35)
|
Moderate (N = 36)
|
High (N = 25)
|
Gender Factor
|
Aerobic Physical Capacity Factor
|
Interaction
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Men (n=18)
|
Women (n=17)
|
Men (n=22)
|
Women (n=14)
|
Men (n=13)
|
Women (n=12)
|
F(DF) | P | F(DF) | P | F(DF) | P | ||||||||||||
| Characteristics | |||||||||||||||||||||||
| Age, years | 27 ± | 1.2 | 27 ± | 1.3 | 29 ± | 1.4 | 27 ± | 1.7 | 32 ± | 2.1 | 31 ± | 1.6 | F(1,90):0.28 | >0.05 | F(2,90):4.68 | 0.012 | F(2,90):0.04 | >0.05 | |||||
| Height, cm | 177 ± | 1.6 | 166 ± | 1.3a | 176 ± | 1.4 | 165 ± | 1.4b | 175 ± | 1.8 | 160 ± | 2.5c | F(1,90):84.9 | <0.001 | F(2,90):2.78 | >0.05 | F(2,90):0.71 | >0.05 | |||||
| Weight, Kg | 73 ± | 1.7 | 61 ± | 1.5a | 74 ± | 1.7 | 61 ± | 1.4b | 72 ± | 2.1 | 54 ± | 1.9 c e | F(1,90):101 | <0.001 | F(2,90):3.9 | 0.022 | F(2,90):1.08 | >0.05 | |||||
| BMI, Kg/m2 | 23.3 ± | 0.3 | 21.9 ± | 0.5a | 23.7 ± | 1.6 | 22.4 ± | 0.4b | 23.3 ± | 0.5 | 20.8 ± | 0.4c | F(1,90):27.9 | <0.001 | F(2,90):2.9 | >0.05 | F(2,90):1.03 | >0.05 | |||||
| % Body Fat | 17.0 ± | 0.3 | 21.7 ± | 0.6a | 13.0 ± | 0.3a | 13.9 ± | 0.3d | 10.1 ± | 0.2 a b | 12.2 ± | 0.4 c d e | F(1,90):70.8 | <0.001 | F(2,90):258 | <0.001 | F(2,90): 15.1 | <0.001 | |||||
| VO2peak, ml Kg-1 min-1 | 31.7 ± | 0.7 | 30.4 ± | 0.6 | 43.6 ± | 0.5a | 41.8 ± | 0.8d | 62.1 ± | 1.0 a b | 54.7 ± | 1.5c d e | F(1,90):0.08 | >0.05 | F(2,90):268 | <0.001 | F(2,90):2.66 | >0.05 | |||||
| Hemodynamic Values | |||||||||||||||||||||||
| Heart Rate, bpm | 81 ± | 2.7 | 82 ± | 2.8 | 65 ± | 1.7a | 68 ± | 1.6d | 52 ± | 1.8 a b | 55 ± | 2.0 d e | F(1,90):1.1 | >0.05 | F(2,90):71 | <0.001 | F(2,90):0.28 | >0.05 | |||||
| SAP, mmHg | 120 ± | 2.7 | 111 ± | 2.2a | 115 ± | 1.6a | 107 ± | 1.6b | 108 ± | 2.9a | 100 ± | 2.5c d e | F(1,90):18.9 | <0.001 | F(2,90):11.4 | <0.001 | F(2,90):0.06 | >0.05 | |||||
| DAP, mmHg | 76 ± | 2.6 | 67 ± | 1.9a | 69 ± | 1.5a | 65 ± | 2.5 | 65 ± | 2.9a | 58 ± | 2.1c d e | F(1,90):13.3 | <0.001 | F(2,90):7.78 | <0.001 | F(2,90): 0.69 | >0.05 | |||||
| MAP, mmHg | 93 ± | 2.5 | 84 ± | 1.9a | 87 ± | 1.3a | 82 ± | 2.0b | 83 ± | 2.8a | 75 ± | 1.9c d e | F(1,90):18.2 | <0.001 | F(2,90):10.8 | <0.001 | F(2,90):0.4 | >0.05 | |||||
Values are the means ± S.E.M. BMI, body mass index; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure. aP<0.05 vs. Men Low Aerobic Capacity Group; bP<0.05 vs. Men Moderate Aerobic Capacity Group; cP<0.05 vs. Men High Aerobic Capacity Group; dP<0.05 vs. Women Low Aerobic Capacity Group; eP<0.05 vs. Women Moderate Aerobic Capacity Group.
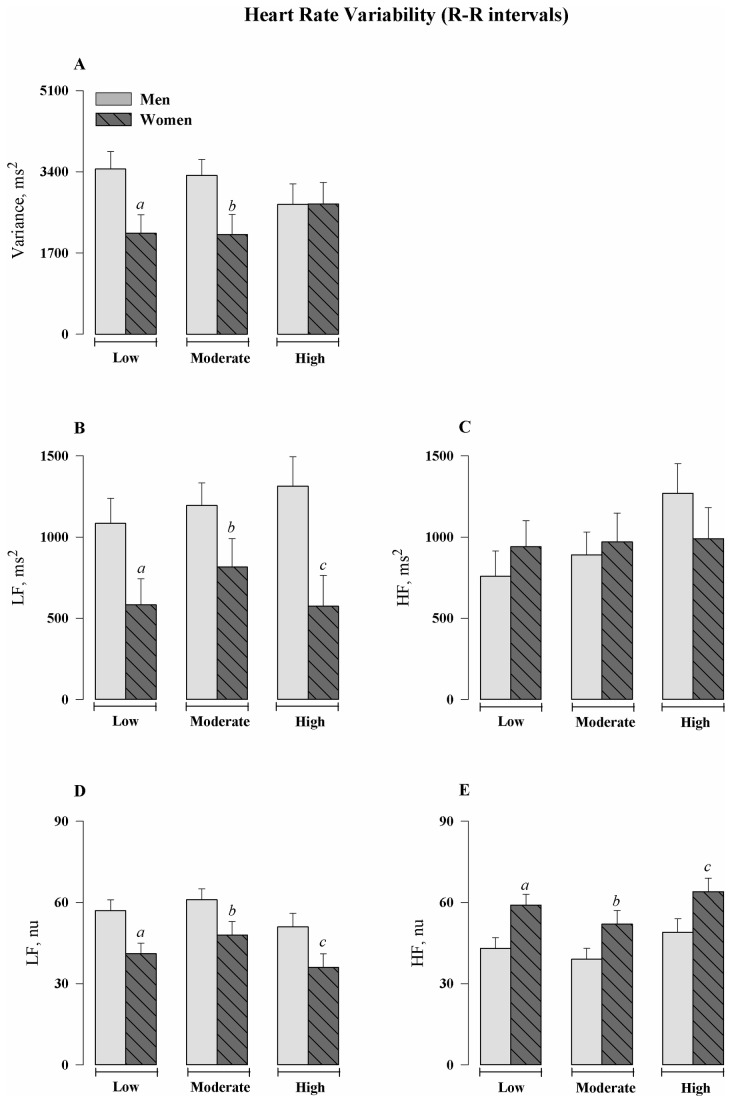
Table 2 and figure 1 show the results of spectral analysis. Women, regardless of aerobic physical capacity, had a lower total variance (gender factor, F(1,90): 6.89, p=0.010), smaller oscillations in the LF bands in the absolute (gender factor, F(1,90): 15.6, p<0.001) and normalized (gender factor, F(1,90): 15.8, p<0.001) units, and greater oscillations in the HF band in normalized units (gender factor, F(1,90): 15.8, p<0.001) than men. In addition, the groups constituted of women had lower values of LF/HF ratio than men (gender factor, F(1,90): 10.9, p=0.001). In turn, the level of aerobic capacity did not influence any of the spectral parameters evaluated in both men and women.
Table 2. Effects of gender and aerobic physical capacity on heart rate variability.
|
Low (N = 35)
|
Moderate (N = 36)
|
High (N = 25)
|
Gender Factor
|
Aerobic Physical Capacity Factor
|
Interaction
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Men (n=18)
|
Women (n=17)
|
Men (n=22)
|
Women (n=14)
|
Men (n=13)
|
Women (n=12)
|
F(DF) | P | F(DF) | P | F(DF) | P | ||||||||||||
| Spectral Parameters | |||||||||||||||||||||||
| RRi, ms | 758 ± | 29 | 755 ± | 30 | 938 ± | 26a | 885 ± | 33d | 1164 ± | 34 a b | 1119 ± | 36 d e | F(1,90):1.7 | >0.05 | F(2,90):70.7 | <0.001 | F(2,90):0.4 | >0.05 | |||||
| Variance, ms2 | 3456 ± | 368 | 2115 ± | 379a | 3323± | 333 | 2086 ± | 418b | 2716 ± | 433 | 2726 ± | 451 | F(1,90):6.89 | 0.010 | F(2,90):0.02 | >0.05 | F(2,90):1.59 | >0.05 | |||||
| LF, ms2 | 1084 ± | 154 | 583 ± | 159a | 1194 ± | 139 | 816 ± | 175b | 1314 ± | 181 | 574 ± | 189c | F(1,90):15.6 | <0.001 | F(2,90):0.60 | >0.05 | F(2,90):0.56 | >0.05 | |||||
| HF, ms2 | 758 ± | 156 | 940 ± | 160 | 890 ± | 141 | 970 ± | 177 | 1270 ± | 183 | 990 ± | 191 | F(1,90):0.195 | >0.05 | F(2,90):1.34 | >0.05 | F(2,90):0.93 | >0.05 | |||||
| LF, nu | 57 ± | 4 | 41 ± | 4a | 61 ± | 4 | 48 ± | 5b | 51 ± | 5 | 36 ± | 5c | F(1,90):15.8 | <0.001 | F(2,90):3.03 | >0.05 | F(2,90):0.07 | >0.05 | |||||
| HF, nu | 43 ± | 4 | 59 ± | 4a | 39 ± | 4 | 52 ± | 5b | 49 ± | 5 | 64 ± | 5c | F(1,90):15.8 | <0.001 | F(2,90):3.03 | >0.05 | F(2,90):0.07 | >0.05 | |||||
| LF/HF | 1.81 ± | 0.31 | 0.91 ± | 0.30a | 2.09 ± | 0.28 | 1.14 ± | 0.35b | 1.51 ± | 0.36 | 0.69 ± | 0.37c | F(1,90):10.9 | 0.001 | F(2,90):1.17 | >0.05 | F(2,90):0.02 | >0.05 | |||||
Values are the means ± S.E.M. LF, low frequency; HF, high frequency; nu, normalized units. aP<0.05 vs. Men Low Aerobic Capacity Group; bP<0.05 vs. Men Moderate Aerobic Capacity Group; cP<0.05 vs. Men High Aerobic Capacity Group; dP<0.05 vs. Women Low Aerobic Capacity Group; eP<0.05 vs. Women Moderate Aerobic Capacity Group.
Figure 1. Heart Rate Variability (R-R intervals).
(A) Total variance of heart rate obtained by means of series of R–R interval (ms2). (B and C) Spectral power density of heart rate in low (LF) and high frequencies (HF) in absolute values. (D and E) Spectral power density of heart rate in the LF and HF bands in normalized units (nu), respectively. Values are means ± S.E.M. aP<0.05 vs. Men Low Aerobic Capacity Group; bP<0.05 vs. Men Moderate Aerobic Capacity Group; cP<0.05 vs. Men High Aerobic Capacity Group.
Discussion
This study investigated in detail the HRV and its relationship with aerobic capacity, addressing the differences between genders. The results of the present study show that higher levels of aerobic capacity improve the hemodynamic response, which was demonstrated by lower basal HR, SAP, DAP, and MAP values, independent of gender. However, women presented the lowest pressure values compared to men at each level of aerobic capacity investigated. Regarding the spectral parameters of HRV, the level of aerobic capacity did not induce any difference when same-gender subjects were compared to each other, although sinus bradycardia was observed in the high aerobic capacity group. However, when the comparison was performed between genders, there were differences at each level of physical conditioning.
Recent studies have shown that moderate-to-high cardiorespiratory capacity is related to adaptations of several cardiovascular performance parameters, thus decreasing the risk of cardiac events and mortality [3,23]. In agreement with these studies, the present study shows that aerobic physical training promotes important adaptations, which is addressed below.
Heart Rate and Arterial Pressure
Aerobic physical training promoted important hemodynamic changes. In the present study, an accentuated sinus bradycardia was observed in high-performance athletes regardless of gender. In fact, sinus bradycardia is a phenomenon observed in athletes as a possible result of autonomic adaptations and/or intrinsic cardiac alterations, mainly involving the sinus node [17,24]. This reduction of heart rate, when associated with athletes, denotes improved performance.
Another favorable effect on cardiovascular health resulting from an increase in aerobic capacity is a decrease in AP. Consistent with the findings reported in the literature, our study shows that the subjects with better aerobic capacity had AP values lower than those with poor aerobic capacity. The mechanisms involved in the physical training effect on AP are not completely elucidated. Some studies suggest the involvement of several mechanisms, such as changes in the activity of the autonomic nervous system, which is mainly characterized by a reduction in the sympathetic influence. An alternative mechanism involves a decrease of serum vasoconstrictor factor levels and an increase in the endothelium-derived dilator factor levels, resulting in a reduction in the peripheral vascular resistance, as evidenced by low levels of norepinephrine and plasmatic renin activity, resulting in an increase in endothelium-induced vasodilatation [25-28].
Regarding the difference in AP response between genders, women showed lower SAP, DAP and MAP values than men, with women in the high aerobic capacity group having AP values smaller than those in other groups. The cause of such a finding is unknown; however, there are studies showing that women exhibit differences in several aspects of hemodynamic regulation compared to men. Among these aspects, one can cite low cardiac responses to baroreceptor activation, low plasmatic renin activity, increased vascular 1-adrenergic response and lower levels of circulating catecholamines compared to men [29-31]. Moreover, several studies indicate that gender differences in the hemodynamic regulation of arterial pressure might be related to sexual hormones [32-35]. In fact, data from the literature suggest that estrogens have a cardioprotective effect in premenopausal women [35-38] because there is a significant increase in the MAP values after menopause, which may be associated with a higher incidence of cardiovascular diseases [34,39,40]. Despite the cardioprotective effect of estrogens, this is not the only factor involved in the AP increase observed after menopause. Another hormone that plays a role in AP regulation is testosterone. Studies report that testosterone, among other factors, induces an increase in MAP by activating the angiotensin-renin system [32-34]. Therefore, testosterone seems to have a harmful effect on MAP, participating more actively in the process of inducing sexual dimorphism.
Heart Rate Variability
The present study demonstrates that the level of aerobic capacity does not promote changes in the spectral parameters of HRV when same-gender subjects were compared to each other. However, when men were compared to women, differences in each level of aerobic capacity were observed. Women presented the lowest values of LF oscillations and highest values of HF oscillations compared to their male counterparts, regardless of the level of aerobic capacity.
Some studies have demonstrated that analyzing the HRV to quantify the modulating influence of both autonomic components (i.e., sympathetic and parasympathetic) on the heart can also be an important tool in aiding the prognosis of cardiovascular stress and conditioning [41-43]. In fact, this process allows for the non-invasive evaluation of the cardiac autonomic function in different pathophysiological situations, including overtraining syndrome [44,45].
In this sense, the basal indices of cardiac autonomic modulation can be influenced by different conditions, including gender. When genders were compared, regardless of the level of physical conditioning, men presented an autonomic balance in favor of sympathetic modulation characterized by greater LF oscillations, whereas women presented an autonomic balance in favor of HF oscillations. These discrepant results were more evident in well-conditioned subjects (i.e., the moderate and high aerobic capacity groups), showing that gender can indeed influence cardiac autonomic modulation [31,46-48]. In accordance with these observations, the literature has shown that middle-aged women have the greatest variations in the spectral indices of HRV and lowest values of AP at rest conditions compared to men of the same age group. This finding indicates that the female population would present an increased vagal cardiac modulation, which would be evidenced by higher HF values and lower LF values [30,46-48]. However, it was also shown that such a discrepancy in the spectral parameters of HRV decreases between men and women when their age is above 50 years old [46]. It has been suggested that female hormones, especially β-estradiol [49], facilitate vagal cardiac function activation [50]. Therefore, the reduction in the vagal modulation predominantly in women older than 50 years might be attributed to the hypothesis that such a facilitating effect is promoted by female sexual hormones [50]. Conversely, it is speculated that the higher indices of sympathetic autonomic modulation in men might be mainly attributed to their physical constitution, comprised of a greater muscle sympathetic nerve activity [51] as well as a higher number of sympathetic ganglionic neurons compared to women, which would result in a more favorable sympathetic modulation balance [47,48,52].
Regardless of the differences between the genders observed in the present study, several studies have reported that practicing physical exercises regularly improves cardiac autonomic modulation, thus suggesting the hypothesis that athletes or well-conditioned subjects have a better pattern of cardiac autonomic modulation [17,19]. This improvement would be mostly associated with an increase in HRV, which is mainly characterized by a greater influence of the parasympathetic autonomic component on the heart [53-55]. In this way, some studies have shown that athletes possess an HRV greater than that of low-performance subjects [17] and that subjects practicing moderate-to-intense physical activities have an autonomic modulation of HR better than that of sedentary subjects, thus providing evidence of the predominance of vagal modulation (HF) on the sinus node [19]. This finding was also observed when only women were studied [48-55].
However, no alteration in cardiac autonomic modulation was observed in our study when the groups of subjects with different aerobic capacities were compared to each other, regardless of the evaluated gender. Thus, our results suggest that the aerobic capacity level, which is characterized by a greater VO2peak response and low basal HR, did not induce changes in the spectral parameters of HRV, which has also been corroborated by other authors [12,24,56,57]. When this gender comparison was performed, we found no further differences between men and women.
These discrepant results suggest that other components of aerobic physical conditioning may contribute to the difference in cardiac autonomic modulation in healthy subjects. For example, resistance strength is a determinant of aerobic capacity that is independent of the VO2max [58,59]. In addition, although aerobic physical capacity may influence HRV, it does not affect HRV in a dose-dependent manner with increasing levels of physical activity [60].
Meanwhile, other mechanisms may have contributed to these results. Because of the presence of respiratory sinus bradycardia, as evidenced by low values of basal HR in the high aerobic capacity group and the absence of changes in the autonomic modulation of HRV, physical exercise may have played a direct role in the sinus node modulation in these subjects, although this parameter was not investigated in the present study.
Conclusions
Our results suggest that cardiovascular and autonomic responses, regardless of the level of aerobic capacity, are different between men and women, as the former present a profile of cardiac autonomic modulation that favors the sympathetic component, whereas the latter present a profile that favors the vagal component. In addition, the level of aerobic physical capacity did not interfere with the autonomic modulation of HRV in the healthy subjects, despite the presence of sinus bradycardia in the groups with high aerobic capacity.
Acknowledgments
The authors express their sincere gratitude to all volunteers who participated in this study.
Funding Statement
This study was supported by the FAPESP (2011/07878-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chase NL, Sui X, Lee DC, Blair SN (2009) The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens 22: 417–424. doi: 10.1038/ajh.2009.6. PubMed: 19197248. [DOI] [PubMed] [Google Scholar]
- 2. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y et al. (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035. doi: 10.1001/jama.2009.681. PubMed: 19454641. [DOI] [PubMed] [Google Scholar]
- 3. Lee D-C, Sui X, Ortega FB, Kim Y-S, Church TS et al. (2011) Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med 45: 504–510. doi: 10.1136/bjsm.2009.066209. PubMed: 20418526. [DOI] [PubMed] [Google Scholar]
- 4. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO et al. (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. PubMed: 17671236. [DOI] [PubMed] [Google Scholar]
- 5. Rosamond W, Flegal K, Furie K, Go A, Greenlund K et al. (2008) Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. PubMed: 18086926. [DOI] [PubMed] [Google Scholar]
- 6. Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH et al. (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401. doi: 10.1001/jama.1989.03430170057028. PubMed: 2795824. [DOI] [PubMed] [Google Scholar]
- 7. Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R et al. (1993) Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 328: 533–537. doi: 10.1056/NEJM199302253280803. PubMed: 8426620. [DOI] [PubMed] [Google Scholar]
- 8. Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA et al. (2003) Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA 290: 1600–1607. doi: 10.1001/jama.290.12.1600. PubMed: 14506119. [DOI] [PubMed] [Google Scholar]
- 9. Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P et al. (2008) Exercise capacity and mortality in black and white men. Circulation 117: 614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. PubMed: 18212278. [DOI] [PubMed] [Google Scholar]
- 10. Goulopoulou S, Baynard T, Franklin RM, Fernhall B, Carhart R Jr et al. (2010) Exercise training improves cardiovascular autonomic modulation in response to glucose ingestion in obese adults with and without type 2 diabetes mellitus. Metab Clin Exp 59: 901–910. doi: 10.1016/j.metabol.2009.10.011. PubMed: 20015524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cozza IC, Di Sacco THR, Mazon JH, Salgado MCO, Dutra SGV et al. (2012) Physical exercise improves cardiac autonomic modulation in hypertensive patients independently of angiotensin-converting enzyme inhibitor treatment. Hypertens Res 35: 82–87. doi: 10.1038/hr.2011.162. PubMed: 21956728. [DOI] [PubMed] [Google Scholar]
- 12. Bosquet L, Gamelin F-X, Berthoin S (2007) Is aerobic endurance a determinant of cardiac autonomic regulation? Eur J Appl Physiol 100: 363–369. doi: 10.1007/s00421-007-0438-3. PubMed: 17440748. [DOI] [PubMed] [Google Scholar]
- 13. Piotrowicz E, Baranowski R, Piotrowska M, Zieliński T, Piotrowicz R (2009) Variable effects of physical training of heart rate variability, heart rate recovery, and heart rate turbulence in chronic heart failure. Pacing Clin Electrophysiol 32 Suppl 1: S113–S115. doi: 10.1111/j.1540-8159.2008.02266.x. PubMed: 19250071. [DOI] [PubMed] [Google Scholar]
- 14. Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN et al. (2009) The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health 99: 921–928. doi: 10.2105/AJPH.2007.133165. PubMed: 19299682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Souza HCD, De Araújo JE, Martins-Pinge MC, Cozza IC, Martins-Dias DP (2009) Nitric oxide synthesis blockade reduced the baroreflex sensitivity in trained rats. Auton Neurosci 150: 38–44. doi: 10.1016/j.autneu.2009.04.007. PubMed: 19443278. [DOI] [PubMed] [Google Scholar]
- 16. Figueroa A, Baynard T, Fernhall B, Carhart R, Kanaley JA (2007) Endurance training improves post-exercise cardiac autonomic modulation in obese women with and without type 2 diabetes. Eur J Appl Physiol 100: 437–444. doi: 10.1007/s00421-007-0446-3. PubMed: 17406886. [DOI] [PubMed] [Google Scholar]
- 17. Dixon EM, Kamath MV, McCartney N, Fallen EL (1992) Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res 26: 713–719. doi: 10.1093/cvr/26.7.713. PubMed: 1423437. [DOI] [PubMed] [Google Scholar]
- 18. Tulppo MP, Mäkikallio TH, Seppänen T, Laukkanen RT, Huikuri HV (1998) Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol 274: H424–H429. PubMed: 9486244. [DOI] [PubMed] [Google Scholar]
- 19. Hautala AJ, Mäkikallio TH, Kiviniemi A, Laukkanen RT, Nissilä S et al. (2003) Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 285: H1747–H1752. doi: 10.1152/ajpheart.00202.2003. PubMed: 12816748. [DOI] [PubMed] [Google Scholar]
- 20. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065. doi: 10.1161/01.CIR.93.5.1043. PubMed: 8598068. [DOI] [PubMed] [Google Scholar]
- 21. Malliani A, Pagani M, Lombardi F, Cerutti S (1991) Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492. doi: 10.1161/01.CIR.84.2.482. PubMed: 1860193. [DOI] [PubMed] [Google Scholar]
- 22. Kokkinos P, Sheriff H, Kheirbek R (2011) Physical inactivity and mortality risk. Cardiol. Res Pract: 2011: 924945 doi: 10.4061/2011/924945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinelli FS, Chacon-Mikahil MPT, Martins LEB, Lima-Filho EC, Golfetti R et al. (2005) Heart rate variability in athletes and nonathletes at rest and during head-up tilt. Braz J Med Biol Res 38: 639–647. doi: 10.1590/S0100-879X2005000400019. PubMed: 15962191 /S0100-879X2005000400019 [DOI] [PubMed] [Google Scholar]
- 24. Fagard RH, Cornelissen VA (2007) Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil 14: 12–17. doi: 10.1097/HJR.0b013e3280128bbb. PubMed: 17301622. [DOI] [PubMed] [Google Scholar]
- 25. James GD, Sealey JE, Müller F, Alderman M, Madhavan S et al. (1986) Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl 4: S387–S389. doi: 10.1097/00004872-198608000-00001. PubMed: 3553481. [DOI] [PubMed] [Google Scholar]
- 26. Poveda JJ, Riestra A, Salas E, Cagigas ML, López-Somoza C et al. (1997) Contribution of nitric oxide to exercise-induced changes in healthy volunteers: effects of acute exercise and long-term physical training. Eur J Clin Invest 27: 967–971. doi: 10.1046/j.1365-2362.1997.2220763.x. PubMed: 9395795. [DOI] [PubMed] [Google Scholar]
- 27. Boegli Y, Gremion G, Golay S, Kubli S, Liaudet L et al. (2003) Endurance training enhances vasodilation induced by nitric oxide in human skin. J Invest Dermatol 121: 1197–1204. doi: 10.1046/j.1523-1747.2003.12518.x. PubMed: 14708626. [DOI] [PubMed] [Google Scholar]
- 28. Cornelissen VA, Fagard RH (2005) Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 46: 667–675. doi: 10.1161/01.HYP.0000184225.05629.51. PubMed: 16157788. [DOI] [PubMed] [Google Scholar]
- 29. Reckelhoff JF, Zhang H, Granger JP (1998) Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439. doi: 10.1161/01.HYP.31.1.435. PubMed: 9453341. [DOI] [PubMed] [Google Scholar]
- 30. Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS et al. (2001) Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol 91: 2611–2618. PubMed: 11717226. [DOI] [PubMed] [Google Scholar]
- 31. Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D et al. (2005) Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. PubMed: 15687139. [DOI] [PubMed] [Google Scholar]
- 32. Reckelhoff JF, Zhang H, Srivastava K (2000) Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483. doi: 10.1161/01.HYP.35.1.480. PubMed: 10642345. [DOI] [PubMed] [Google Scholar]
- 33. Reckelhoff JF (2001) Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208. doi: 10.1161/01.HYP.37.5.1199. PubMed: 11358929. [DOI] [PubMed] [Google Scholar]
- 34. Rogers J, Sheriff DD (2004) Role of estrogen in nitric oxide- and prostaglandin-dependent modulation of vascular conductance during treadmill locomotion in rats. J Appl Physiol 97: 756–763. doi: 10.1152/japplphysiol.00115.2004. PubMed: 15247204. [DOI] [PubMed] [Google Scholar]
- 35. Kienitz T, Quinkler M (2008) Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79. doi: 10.1159/000119417. PubMed: 18319594. [DOI] [PubMed] [Google Scholar]
- 36. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA et al. (1995) Prevalence of hypertension in the US adult population. Results Third Natl Health Nutr Examination Surv: 1988-1991. Hypertension 25: 305–313 [DOI] [PubMed] [Google Scholar]
- 37. van Ittersum FJ, van Baal WM, Kenemans P, Mijatovic V, Donker AJ et al. (1998) Ambulatory--not office--blood pressures decline during hormone replacement therapy in healthy postmenopausal women. Am J Hypertens 11: 1147–1152. doi: 10.1016/S0895-7061(98)00165-4. PubMed: 9799030. [DOI] [PubMed] [Google Scholar]
- 38. Seely EW, Walsh BW, Gerhard MD, Williams GH (1999) Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension 33: 1190–1194. doi: 10.1161/01.HYP.33.5.1190. PubMed: 10334810. [DOI] [PubMed] [Google Scholar]
- 39. Calhoun DA, Oparil S (1998) The Sexual Dimorphism of High Blood Pressure. Cardiol Rev 6: 356–363. doi: 10.1097/00045415-199811000-00012. PubMed: 10348960. [DOI] [PubMed] [Google Scholar]
- 40. August P, Oparil S (1999) Hypertension in women. J Clin Endocrinol Metab 84: 1862–1866. doi: 10.1210/jc.84.6.1862. PubMed: 10372676. [DOI] [PubMed] [Google Scholar]
- 41. Baumert M, Brechtel L, Lock J, Hermsdorf M, Wolff R et al. (2006) Heart rate variability, blood pressure variability, and baroreflex sensitivity in overtrained athletes. Clin J Sport Med 16: 412–417. doi: 10.1097/01.jsm.0000244610.34594.07. PubMed: 17016118. [DOI] [PubMed] [Google Scholar]
- 42. Kiviniemi AM, Hautala AJ, Mäkikallio TH, Seppänen T, Huikuri HV et al. (2006) Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R-R interval. Eur J Appl Physiol 96: 686–692. doi: 10.1007/s00421-005-0130-4. PubMed: 16416318. [DOI] [PubMed] [Google Scholar]
- 43. Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP (2007) Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 101: 743–751. doi: 10.1007/s00421-007-0552-2. PubMed: 17849143. [DOI] [PubMed] [Google Scholar]
- 44. Mazon J, Gastaldi A, Di Sacco T, Cozza I, Dutra S et al. (2013) Effects of training periodization on cardiac autonomic modulation and endogenous stress markers in volleyball players. Scand J Med Sci Sports 23: 114–120. doi: 10.1111/j.1600-0838.2011.01357.x. PubMed: 21812826. [DOI] [PubMed] [Google Scholar]
- 45. Perseguini NM, Takahashi ACM, Rebelatto JR, Silva E, Borghi-Silva A et al. (2011) Spectral and symbolic analysis of the effect of gender and postural change on cardiac autonomic modulation in healthy elderly subjects. Braz J Med Biol Res 44: 29–37. doi: 10.1590/S0100-879X2010007500137. PubMed: 21140100. [DOI] [PubMed] [Google Scholar]
- 46. Huikuri HV, Pikkujämsä SM, Airaksinen KE, Ikäheimo MJ, Rantala AO et al. (1996) Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 94: 122–125. doi: 10.1161/01.CIR.94.2.122. PubMed: 8674168. [DOI] [PubMed] [Google Scholar]
- 47. Kuo TB, Lin T, Yang CC, Li CL, Chen CF et al. (1999) Effect of aging on gender differences in neural control of heart rate. Am J Physiol 277: H2233–H2239. PubMed: 10600841. [DOI] [PubMed] [Google Scholar]
- 48. Tezini GCSV, Silveira LCR, Villa-Clé PG Jr, Jacinto CP, Di Sacco THR et al. (2009) The effect of aerobic physical training on cardiac autonomic control of rats submitted to ovariectomy. Menopause 16: 110–116. doi: 10.1097/GME.0b013e318182d352. PubMed: 18978639. [DOI] [PubMed] [Google Scholar]
- 49. Du XJ, Dart AM, Riemersma RA (1994) Sex differences in the parasympathetic nerve control of rat heart. Clin Exp Pharmacol Physiol 21: 485–493. doi: 10.1111/j.1440-1681.1994.tb02545.x. PubMed: 7982279. [DOI] [PubMed] [Google Scholar]
- 50. Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G et al. (1996) Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251. PubMed: 8847310. [DOI] [PubMed] [Google Scholar]
- 51. Beaston-Wimmer P, Smolen AJ (1991) Gender differences in neurotransmitter expression in the rat superior cervical ganglion. Brain Res. Dev Brain Res 58: 123–128. doi: 10.1016/0165-3806(91)90244-D. [DOI] [PubMed] [Google Scholar]
- 52. Migliaro ER, Contreras P, Bech S, Etxagibel A, Castro M et al. (2001) Relative influence of age, resting heart rate and sedentary life style in short-term analysis of heart rate variability. Braz J Med Biol Res 34: 493–500. doi: 10.1590/S0100-879X2001000400009. PubMed: 11285461. [DOI] [PubMed] [Google Scholar]
- 53. Middleton N, De Vito G (2005) Cardiovascular autonomic control in endurance-trained and sedentary young women. Clin Physiol Funct Imaging 25: 83–89. doi: 10.1111/j.1475-097X.2004.00594.x. PubMed: 15725306. [DOI] [PubMed] [Google Scholar]
- 54. Sztajzel J, Jung M, Sievert K, Bayes De Luna A (2008) Cardiac autonomic profile in different sports disciplines during all-day activity. J Sports Med Phys Fit 48: 495–501. PubMed: 18997654. [PubMed] [Google Scholar]
- 55. Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM et al. (2009) Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiol (Oxf) 195: 339–348. doi: 10.1111/j.1748-1716.2008.01897.x. PubMed: 18774947. [DOI] [PubMed] [Google Scholar]
- 56. Perini R, Veicsteinas A (2003) Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 90: 317–325. doi: 10.1007/s00421-003-0953-9. PubMed: 13680241. [DOI] [PubMed] [Google Scholar]
- 57. Brunetto AF, Roseguini BT, Silva BM, Hirai DM, Guedes DP (2005) Effects of gender and aerobic fitness on cardiac autonomic responses to head-up tilt in healthy adolescents. Pediatr Cardiol 26: 418–424. doi: 10.1007/s00246-004-0808-0. PubMed: 16374693. [DOI] [PubMed] [Google Scholar]
- 58. Péronnet F, Thibault G (1989) Mathematical analysis of running performance and world running records. J Appl Physiol 67: 453–465. PubMed: 2759974. [DOI] [PubMed] [Google Scholar]
- 59. Melanson EL (2000) Resting heart rate variability in men varying in habitual physical activity. Med Sci Sports Exerc 32: 1894–1901. doi: 10.1097/00005768-200011000-00012. PubMed: 11079519. [DOI] [PubMed] [Google Scholar]
- 60. Ellestad MH (1986) Stress Testing: Principles and Practices, 3rd Edition. Philadelphia: F A Davis. [Google Scholar]