Abstract
Objective
The aim of the study reported here was to identify whether a stem cell biomarker, Lin28, may predict the pathologic tumor response to neoadjuvant chemotherapy for patients with locally advanced gastric cancer.
Methods
The study enrolled 47 patients with gastric cancer who underwent neoadjuvant chemotherapy followed by surgery between July 2004 and March 2012. Cancer tissue was biopsied by gastroscopy and Lin28 expression in the tissue was measured by immunohistochemistry. Statistical analyses were performed to identify the relationship between Lin28 expression and tumor regression grade.
Results
Of the 47 cases, pathologic nonresponse was observed in 29 (61.7%) and pathologic response in 18 (38.3%). Receiver-operating characteristic curve analysis showed that the histoscore of Lin28 expression with 0.325 as a cutoff value could differentiate between pathologic response and nonresponse. Multivariable analysis showed that Lin28 expression was an independent predictive factor for pathologic response to neoadjuvant chemotherapy (P = 0.006).
Conclusion
Lin28 expression was associated with pathologic tumor response in locally advanced gastric cancer patients undergoing neoadjuvant chemotherapy. This may suggest that Lin28 can serve as a predictive biomarker for neoadjuvant chemotherapy in patients with gastric cancer.
Keywords: neoadjuvant chemotherapy, pathologic tumor response, stem cell biomarker, immunohistochemistry
Introduction
Gastric cancer is now the fourth most common cancer worldwide, and the third most common cause of death from cancer.1 Preoperative chemotherapy is currently wildly used in local advanced gastric cancer because it may improve patient survival. However, there may be risk in delaying surgery in unresponsive cases. Several studies2,3 have found biomarkers in patients that can predict the chemotherapy response before chemotherapy, but there are still limitations of these for clinical use. Lin28 is a highly conserved RNA-binding protein4 that has been shown to participate in inducing pluripotent stem cells and to the maintenance of stem cell-like cells in cancer.5 Recent studies suggest that Lin28 functions as an oncogene, which may promote malignant transformation and tumor progression.6 Moreover, several recent studies have demonstrated that Lin28 expression correlates with the survival of patients with malignant diseases.7 Our previous study showed that Lin28 expression is associated with poor survival in gastric cancer patients8 and other research has demonstrated that the expression of Lin28 is correlated with radioresistance in human cancer cells.9 However, as far as we are aware, there is no evidence to support the correlation of Lin28 expression and chemotherapy response in gastric cancer.
The study reported here aimed to find the relationship between Lin28 expression and chemotherapy response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer, with the intention of determining whether Lin28 could be a marker that may predict the efficacy of neoadjuvant chemotherapy in gastric cancer patients.
Patients and methods
Tissue was collected from 47 patients with locally advanced gastric cancer by endoscopic biopsy from July 2004 to March 2012 at the Department of Surgical Oncology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China. Inclusion criteria and exclusion criteria, pre-treatment staging, and surgical procedure were consistent with our previously published study.10 All patients received standard neoadjuvant chemotherapy or radio-chemotherapy followed by curative resection (R0 resection) and adjuvant chemotherapy.
Assessment of pathologic response
After surgery, all tumor specimens were examined by pathologists. Pathologic response was evaluated according to Becker’s criteria, in which residual tumor cells were microscopically evaluated.11 Briefly, tumor regression was classified into three grades: Grade 1, complete or subtotal regression (<10% residual tumor per tumor bed); Grade 2, partial tumor regression (10%–50% residual tumor per tumor bed); and Grade 3, minimal or no tumor regression (>50% residual tumor per tumor bed). All patients with Grade 1 or 2 regression were defined as having experienced “tumor regression,” while the definition of Grade 3 present “pathologic nonresponse” was taken from by Liu et al.12
Immunohistochemical staining for detection of Lin28 expression
Immunohistochemical analysis to determine Lin28 expression was performed on formalin-fixed, paraffin-embedded specimens. The slides were de-paraffinized in xylene and rehydrated in gradient ethanol solutions. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 10 minutes. The slides were immersed in 10 mM citric buffer (pH 6.0) with heating for 15 minutes for antigen retrieval. Nonspecific binding was blocked by preincubation with 10% fetal calf serum in phosphate-buffered saline with 0.01% sodium azide, and the slides were incubated in a humid chamber for 1 hour with an antibody against Lin28 (rabbit polyclonal antibody, cat no 11724-1-AP, PTGLAB (Proteintech Group, Inc, Chicago, USA); 1:75). Following this, the slides were incubated with undiluted EnVision™ HRP complex (Dako Denmark A/S, Glostrup, Denmark) for 60 minutes, then visualized with diaminobenzidine (Dako Denmark A/S) and counterstained with hematoxylin.
For substitute negative controls, the primary antibody was replaced with phosphate-buffered saline. Positive control was normal testis tissue known to exhibit high expression of Lin28. Evaluation of immunoreactivity for Lin28 was conducted according to the intensity and distribution of cell cytoplasm staining by diaminobenzidine chromogen. Cell cytoplasm staining intensity was scored using a weighted-histoscore method.13 The intensity of staining was graded as 3+ (dark brown; strongly positive), 2+ (brown; moderately positive), 1+ (light brown; weekly positive), and negative. The final histoscores were calculated as follows:
Scoring was undertaken by two independent observers who did not know the clinical outcomes. Discrepant scoring was reevaluated by the two observers and agreement was reached after appropriate discussion.
Statistical analysis
Chi-square or Fisher’s exact tests were utilized to determine the significance of associations between the pathologic findings and categorical variables. For multivariable analysis, a logistic regression model was applied to identify correlations between pathologic response and clinicopathological variables. A receiver-operating characteristic curve was constructed to define the cutoff value for the various continuous variables that were correlated with predicting pathologic tumor response. SPSS (v 16.0; IBM Corporation, Armonk, NY, USA) was implemented for all statistical calculations. In all tests, a two-sided P < 0.05 was considered to indicate significance.
Results
Clinicopathologic variables and pathologic tumor response
Pathological nonresponse (tumor regression grade [TRG] 3) was observed in 61.7% (29/47) of patients, and pathological response (TRG 1–2) in 38.3% (18/47) of patients. The chemotherapy efficiency was 38.3%. On univariate analysis, both tumor size (P = 0.01) and lymph metastasis (P = 0.043) were found to be related with patient chemotherapy response (Table 1).
Table 1.
Relationship between patient characteristics and chemo-response
| Variable | Pathologic tumor response
|
P value | |
|---|---|---|---|
| No response | Response | ||
| Age | |||
| ≤60 | 16 (34.0%) | 10 (21.3%) | |
| >60 | 13 (27.7%) | 8 (17.0%) | 0.609 |
| Sex | |||
| Male | 18 (38.3%) | 14 (29.8%) | |
| Female | 11 (23.4%) | 4 (8.5%) | 0.213 |
| Tumor size | |||
| ≤4.25 cm | 21 (44.7%) | 6 (12.7%) | |
| >4.25 cm | 8 (17.1%) | 12 (25.5%) | 0.010 |
| Tumor differentiation | |||
| Differentiated | 8 (17.0%) | 8 (17.0%) | |
| Undifferentiated | 21 (44.7%) | 10 (21.3%) | 0.192 |
| Tumor location | |||
| Upper | 6 (12.8%) | 4 (8.5%) | |
| Middle and lower | 23 (48.9%) | 12 (25.5%) | |
| Diffuse type | 0 (0.0%) | 2 (4.3%) | 0.176 |
| Serosa invasion | |||
| Yes | 22 (46.8%) | 16 (34.0%) | |
| No | 7 (14.9%) | 2 (4.3%) | 0.239 |
| Lymph metastasis | |||
| Yes | 14 (29.8%) | 14 (29.8%) | |
| No | 15 (31.9%) | 4 (8.5%) | 0.043 |
Lin28 expression predicting pathologic tumor response
The average Lin28 expression histoscore was 0.53 and the range was 0.05–1.65 (Figure 1). Compared with patients who had a lower Lin28 histoscore (0.423 ± 0.059), patients with a higher Lin28 histoscore (0.695 ± 0.112) seemed to have a worse pathological response to chemotherapy (TRG 1–2; Figure 2).
Figure 1.
Lin28 Immunohistochemical staining of gastroscope tissues in gastric patients (A–C) Lin28 expression are strong positive in representative slide (A, B, C is separately shown at 100×, 200×, 400× magnification). (D–F) Lin28 expression are almost negative in representative slide (D, E, F is separately shown at 100×, 200×, 400× magnification).
Figure 2.
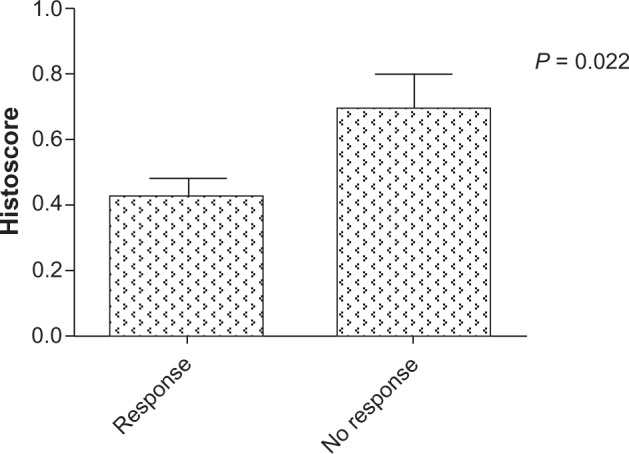
Lin28 average histoscore between two groups which divide patients with chemo-response. Patients who had better chemotherapy pathological response (TRG2–3), have lower level of Lin28 histoscore.
Using receiver-operating characteristic analysis, a Lin28 histoscore cutoff level of 0.325 was found. The sensitivity and specificity of this cutoff value in predicting tumor regression were 88.9% and 58.6%, respectively. Consequently, the correlation between Lin28 histoscores (≤0.325 and >0.325) and pathological response were investigated by Chi-square statistical analysis. We found that high Lin28 expression levels were more frequent in nonresponse patients (P = 0.001; Table 2).
Table 2.
Chi-square statistical analysis to identify Lin28 expression and pathologic tumor response
| Lin28 histoscore | Pathologic tumor response
|
P value | |
|---|---|---|---|
| No Response | Response | ||
| ≤0.325 | 16 (34.0%) | 2 (4.3%) | |
| >0.325 | 13 (27.7%) | 16 (34.0%) | 0.001 |
In multivariate analysis, controlling for other variables studied on univariate analysis, Lin28 expression was found to be an independent predictive factor for pathologic tumor regression response (P < 0.05) and tumor size (P < 0.05; Table 3).
Table 3.
Multivariate logistic analysis to identify Lin28 and other variables
| Variable | Exp | 95% CI | P value |
|---|---|---|---|
| Lin28 histoscore (≤0.325 vs >0.325) | 13.334 | 2.095~84.871 | 0.006 |
| Tumor size (≤4.25 cm vs >4.25 cm) | 4.809 | 1.056~21.904 | 0.042 |
| Lymph metastasis (yes vs no) | 4.739 | 0.955~23.529 | 0.057 |
Abbreviations: CI, confidence interval; Exp, expectations.
Discussion
Neoadjuvant chemotherapy is an important adjunctive treatment to increase the radical resection rate in gastric cancer and prolong the overall survival (OS) of gastric cancer patients.14 However, as chemotherapy is less efficient due to tumor heterogeneity, the screening of a factor which could predict the chemosensitivity of a gastric cancer patient to neoadjuvant chemotherapy is particularly important. Neoadjuvant chemotherapy is a good platform that provides a chance to filter tumor molecular markers for predicting the efficacy of chemotherapy for gastric cancer.15
Tumor regression grading reportedly has prognostic value in patients with various cancers after preoperative chemoradiotherapy, such as rectal,16 breast,17 and gastric.11 Lin28 is a highly conserved RNA-binding protein that has emerged as a modulator of the processing of microRNAs (eg, let-7, miR-107, miR-143, and miR-200c).18 Lin28 recruits terminal uridylyltransferase 4 (TUT4) to pre-let-7 by recognizing a tetra-nucleotide sequence motif (GGAG) in the terminal loop. In turn, TUT4 adds an oligo uridine tail to the pre-let-7, which blocks Dicer processing.18 This role for Lin28 has important implications for our mechanistic understanding of pluripotency, the timing of development, and oncogenesis.20 “Let-7” is a family of small noncoding RNAs regulating the expression of many genes that control important cellular activities. Loss of let-7 increases resistance to certain chemotherapeutic drugs and radiation.21 In agreement with our result, Lv et al found that Lin28 expression is a possible mechanism of chemoresistance in breast cancer and that overexpression of Lin28 effectively induced p21 and Rb expression and inhibited let-7 miRNA.13 Meanwhile, Liu et al12 revealed that a low level of let-7i is associated with chemotherapy resistance and shorter OS in gastric cancer patients treated with neoadjuvant chemotherapy. Besides, accumulating data have proved the double negative feedback between Lin28 and let-7.19 The Lin28/let-7 pathway plays an important role in cancer progress.22 Consistent with this observation, the study presented here showed a relationship between Lin28 and the chemotherapy response of gastric cancer patients. This indicates that Lin28 might also play a role in gastric cancer patient’s chemoresistance by downregulating let-7. However, although the lin28 histoscore cutoff value of 0.325 was found to differentiate pathologic nonresponse and response, the sensitivity and specificity were still low, which might be due to our small sample size. Moreover, the potential molecular mechanism of how Lin28 mediated chemoresistance needs to be further studied in vitro.
Conclusion
To our knowledge, this is the first study to have investigated the role of Lin28 in predicting chemosensitivity in gastric cancer patients treated with neoadjuvant chemotherapy. Our findings indicate that lin28 could serve as a predictive biomarker for neoadjuvant chemotherapy in patients with gastric cancer, warranting further study.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (grant no 81101659/H1609, 81101659), Natural Science Foundation of Zhejiang Province (grant numbers Y2110073 and Q13H160024), Science and Health Care Foundation of Zhejiang Province (grant no 2011KYA086), and the Program for Innovative Research Team in Zhejiang Province (grant no 2010R50046).
Footnotes
Disclosure
Other than the grants outlined in the “Acknowledgments” section, the authors declare no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Napieralski R, Ott K, Kremer M, Specht K, Vogelsang H, Becker K, Müller M, Lordick F, et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res. 2005;11:3025–3031. doi: 10.1158/1078-0432.CCR-04-1605. [DOI] [PubMed] [Google Scholar]
- 3.Hirakawa M, Sato Y, Ohnuma H, Takayama T, Sagawa T, Nobuoka T, Harada K, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013;71:789–797. doi: 10.1007/s00280-013-2073-5. [DOI] [PubMed] [Google Scholar]
- 4.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88(5):637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 5.Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3(6):719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan SR, Powers JT, Einhorn W, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41(7):843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y, Chen Y, Ito H, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Xu C, Shen J, Xie S, Jiang Z, Huang L, Wang L. Positive expression of Lin28 is correlated with poor survival in gastric carcinoma. Med Oncol. 2013;30(1):382. doi: 10.1007/s12032-012-0382-x. [DOI] [PubMed] [Google Scholar]
- 9.Oh JS, Kim JJ, Byun JY, Kim IA. Lin28-let7 modulates radiosensitivity of human cancer cells with activation of K-Ras. Int J Radiat Oncol Biol Phys. 2010;76(1):5–8. doi: 10.1016/j.ijrobp.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang LB, Teng RY, Jiang ZN, et al. Clinicopathologic variables predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol. 2012;105(3):293–296. doi: 10.1002/jso.22085. [DOI] [PubMed] [Google Scholar]
- 11.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98(7):1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Qian T, Tang L, Wang J, Yang H, Ren J. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J Surg Oncol. 2012;10:225. doi: 10.1186/1477-7819-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teo K, Gemmell L, Mukherjee R, Traynor P, Edwards J. Bad expression influences time to androgen escape in prostate cancer. BJU Int. 2007;100(3):691–696. doi: 10.1111/j.1464-410X.2007.07001.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;(3):CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16(44):5621–5628. doi: 10.3748/wjg.v16.i44.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saigusa S, Tanaka K, Toiyama Y, et al. Gene expression profiles of tumor regression grade in locally advanced rectal cancer after neoadjuvant chemoradiotherapy. Oncol Rep. 2012;28(3):855–861. doi: 10.3892/or.2012.1863. [DOI] [PubMed] [Google Scholar]
- 17.Lv K, Liu L, Wang L, et al. Lin28 mediates paclitaxel resistance by modulating p21, Rb and Let-7a miRNA in breast cancer cells. PLoS One. 2012;7(7):e40008. doi: 10.1371/journal.pone.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo I, Joo C, Kim YK, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Lin X, Zhong X, Kaur S, Li N, Liang S, Lassus H, et al. Double-negative feedback loop between reprogramming factor LIN28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010 Nov 15;70(22):9463–9472. doi: 10.1158/0008-5472.CAN-10-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140(4):445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17(1):F19–F36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- 22.Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012;22(9):474–482. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]



