Abstract
Both budding yeast Saccharomyces cerevisiae and fission yeast Schizosaccahromyces pombe have been very popular organisms used for biological research with eukaryotes for many decades. Judging from the fission yeast Schizosaccharomyces japonicus DNA sequence determined 2 years ago, this species is evolutionarily very much unrelated to the commonly used yeasts for research. Indicating evolutionary divergence, the S. japonicus makes 8-spored asci and mitosis occurs with a partial breakdown of nuclear membrane whereas the other yeasts make 4-spored asci and cells divide without nuclear breakdown. The commonly used yeast species exhibit a generation time between 1.5 and 2.0 hr, and their genetic cross takes a period of more than 7 working d. As described here, a generation time of only 63 min and meiotic analysis completed in just 2.5 d, the S. japonicus fission yeast is predicted to become a choice organism for future research on the biology of eukaryotes.
Keywords: Schizosaccharomyces japonicus, fission yeast, fast growing, rapid meiotic analysis, organism conducive for research
In the 1970 edition of the Molecular Biology of the Gene, Dr. James D. Watson (Watson 1970) remarked, “even if our primary interest is the human cell, this may be the time for many biologists to work with organisms like yeast.” Examples abound whereby basic research in biology has been fueled by studies introducing new organisms and/or new techniques for research. Such developments have permitted the investigators to ponder new questions not possible to address by the research of previously exploited organisms and techniques. The popularization of the roundworm research to study the development of multicellular organisms by Brenner (2003) and the development of genetic engineering by a DNA-mediated transformation procedure of Saccharomyces (Sc.) cerevisiae by Fink and others (Hicks et al. 1979; Hinnen et al. 1978) are just two such prominent examples that have sped the progress of biological research in the last four decades. Here I describe extremely advantageous features that are bound to help popularize biological research with Schizosaccharomyces (S.) japonicus, the fission yeast whose genome sequence was determined two years ago (Rhind et al. 2011).
The budding yeast Sc. cerevisiae and fission yeast Schizosaccahromyces (S.) pombe are inexpensive model eukaryotic organisms used for biological research. Notably, most research areas of yeast could not be investigated in a timely manner in higher eucaryotes. Because mechanisms of many biological pathways are evolutionarily conserved in eukaryotes, prominent among them being the cell cycle (Hartwell 2002; Nurse 2002), these two organisms have led the way to defining new principles of eukaryotic biology. The knowledge gained with model organisms has been subsequently applied to fuel investigations of higher organisms, such as mammals. We recently published a study on S. japonicus variety versatilis in which the process of asymmetric cell division through the DNA strand-specific imprinting/segregation mechanism was described (Yu et al. 2013). During the study with this highly diverged yeast, compared with his experience working with the S. pombe and Sc. cerevisiae yeasts, this author noted important features highly conducive for conducting research with S. japonicus. These features, which are not presently appreciated by yeast researchers at large, are described herein. Because this species is not well represented in the literature, presently its potential has not been recognized.
Materials and Methods
Strains and culture conditions
The genotype of strains used is listed in Table 1. Yeast extract agar (YEA) medium was used for cultures growth; Pombe minimal adenine (PMA) medium was used to induce meiosis and sporulation; octad analyses were carried out according to the previously described procedures used for research with S. pombe cultures (Moreno et al. 1991).
Table 1. S. japonicus strains genotype.
| Strain | Iod | Genotype | Source |
|---|---|---|---|
| Sjk2 | + | mat1-P, mat2/3Δ | Yu et al. (2013) |
| Sjk3 | + | mat1-M, mat2/3Δ | Yu et al. (2013) |
| Sjk10 | + | mat1-P, mat2/3Δ, ade6-dam-E, ura4-D3 | This study |
| Sjk17 | + | h+, mat-2017, mrc1Δ::Nat | This study |
| Sjk19 | + | mat1-M, mat2/3Δ, mrc1Δ::Nat | This study |
| NIG6701 | - | h+, mat-2017, mrc1Δ::Nat | Furuya and Niki (2012) |
Iod indicates iodine vapor−staining colony phenotype when cells of opposite mating type are mated for meiosis and sporulation. The symbol Δ indicated deletion of the gene. The mat2/3Δ donor loci−deleted strains were derived from Sjk4, which is homothallic for the mating-type switching phenomenon (Yu et al. 2013). Nat is a Nourseothricin gene cassette that replaced the mrc1 gene in the genome; it confers the Nourseothricin antibiotic resistance to yeast. The ade6-damE and ura4-D3 markers were derived from a stock provided by Dr. H. Niki.
Cytology
Cells pictures were taken with ZeissAxioobserver Z1 with LD plan nesofluar microscope.
Results and Discussion
The S. japonicus var. versatilis genetic cross completed in 60 hr
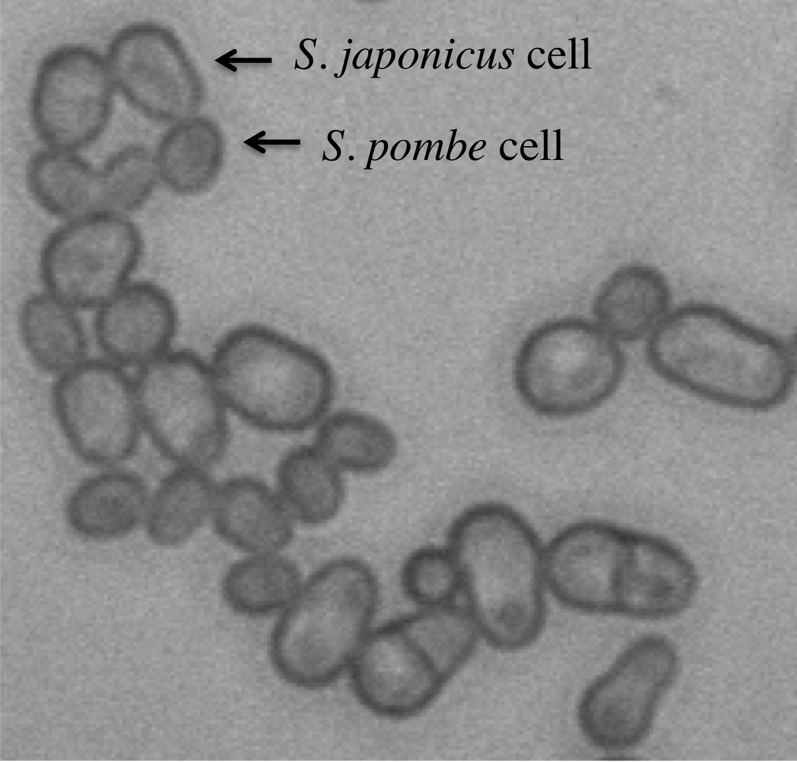
The S. japonicus organism consists of haploid cells, genome comprising of three chromosomes, and DNA content of 11.5 Mb (Rhind et al. 2011); these features are similar to those of S. pombe species. The cell size of S. japonicus is several folds bigger than that of S. pombe (Figure 1).
Figure 1.
Size differences of S. japonicus var. versatilis and S. pombe cells. The larger cells of S. japonicus and the smaller ones of S. pombe are indicated.
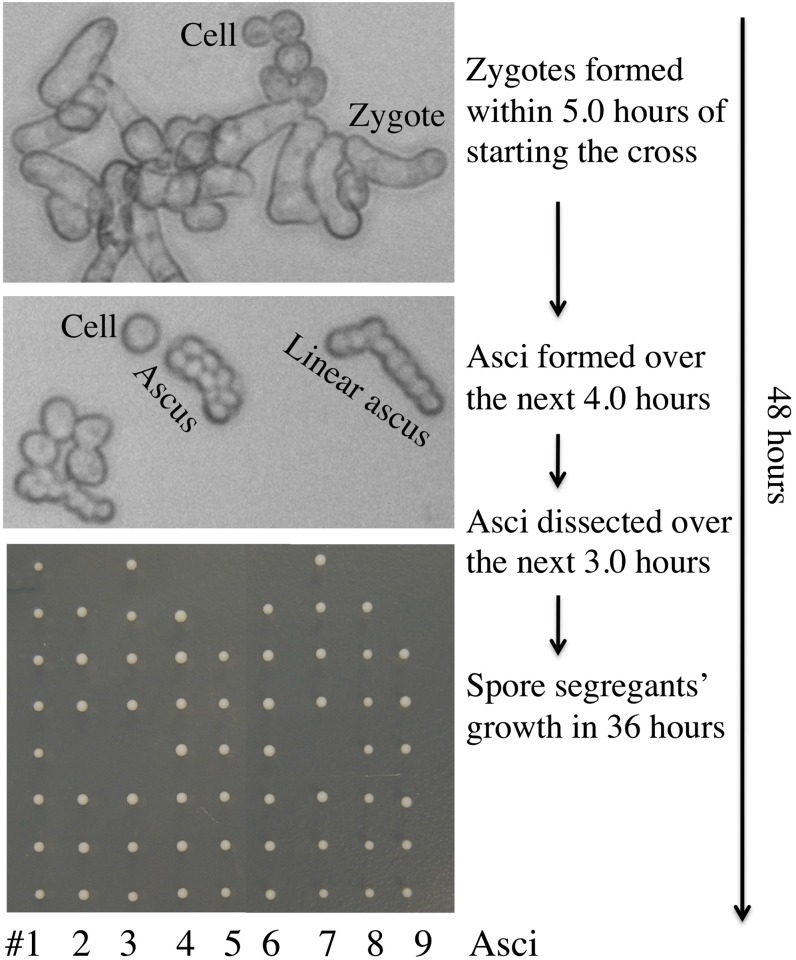
Like S. pombe (Klar 2007), S. japonicus cells alternate between two cell types, called P (plus)and M (minus), which are controlled by the mat1-P and mat1-M alleles, respectively, of the mating-type (mat1) locus (Furuya and Niki 2009; Rhind et al. 2011; Yu et al. 2013). When cells of opposite mating types, strains Sjk2 (mat1-P, mat2/3Δ, iod+; see Table 1 for a description of the complete genotype) and Sjk3 (mat1-M, mat2/3Δ, iod+), were mixed on the mating/sporulation medium used for the S. pombe research, S. japonicus var. versatilis cells formed zygotes by mating within 5 hr and produced matured 8-spored asci over the next 4 hr at the 30° incubation temperature we used to induce mating, meiosis and sporulation (Figure 2). The asci were dissected by micromanipulation in the next 3 hr. After dissection, the spore segregants grew into colonies in only 36 hr with the size suitable for genotype determination. Thereafter, we determined the genotype of the segregants in the next 12 hr, including their mating type, by replica plating to nutritional dropout medium (to be described in the section The japonicas and versatilis varieties are genetically compatible). Thus, meiotic analysis was completed in a mere 60-hr period from the time the cross was started (Figure 2). Because sporulation of versatilis occurs at 30° in 9 hr and the asci start to fall apart in the following couple of hours, for logistical reasons we slowed the process by sporulating cultures at 25°. This way, a genetic cross started late in the afternoon was found to be perfectly poised for ascus dissection as first thing the following morning.
Figure 2.
A timeline of the formation of spore segregants by the Sjk2 and Sjk3 heterothallic strains cross. The colonies of segregants of each one of nine asci, arranged in a vertical row, are displayed in the bottom panel.
The japonicus and versatilis varieties are genetically compatible
An extremely useful, powerful and simple procedure employed in research with S. pombe consists of staining sporulated colonies by exposing them to iodine vapors for two minutes (Bresch et al. 1968). The ascospores synthesize the starch-like compound, but the mitotically multiplying cells do not. As a consequence, sporulating colonies stain black with this procedure but the vegetatively growing colonies remain unstained. Likewise, the sporulated colonies of the S. japonicus var. versatilis stock that we employed for our cell differentiation study stained with iodine vapors (Yu et al. 2013). By exploiting this procedure, we recently described isolation of switching-incapable (designated “heterothallic”) mutants of S. japonicus var. versatilis. These were isolated simply by screening for non-staining phenotype of spontaneously generated mutant derivatives with the iodine-exposure procedure. These mutants, strains Sjk2 and Sjk3, resulted from spontaneous deletion of both donor mat2 and mat3 loci from Sjk4 (homothallic), which are used as the source of genetic information for switching mat1 through recombination (Yu et al. 2013). A handful of recent publications have described research with another variety, named S. japonicus var. japonicus. Notably, the japonicus variety sporulated colonies do not stain with the iodine procedure (Furuya and Niki 2009). Lacking the ability to stain, isolation of heterothallic mutants from S. japonicus var. japonicus stock necessitated the determination of the mating type of approximately 20,000 mutagenized colonies, assaying them individually by microscopic analysis (Furuya and Niki 2009). This highly useful staining procedure is also not applicable for research with the Sc. cerevisiae yeast.
We crossed heterothallic strain Sjk3, our strain of the versatilis variety, with the NIG6701 (mat1-P, mat-2701, mrc1Δ::Nat, iod−) strain of the japonicus variety. Fortunately, these two varieties were compatible in meiotic intercrosses (Figure 3). We recently reported a >99.9% DNA sequence identity of the mat2 and mat3 genes of versatilis (Yu et al. 2013) with those of the japonicus variety (Rhind et al. 2011). Thus, it is not surprising to discover that the versatilis and japonicus varieties are genetically fertile when crossed with each other.
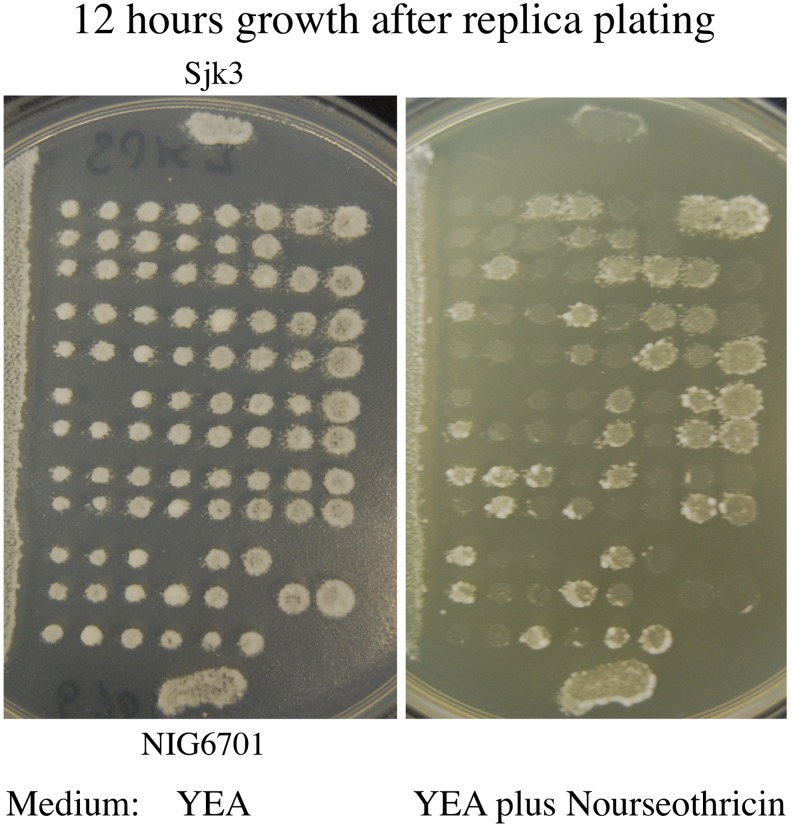
Figure 3.
Octad analysis of heterothallic strains of Sjk3 (mat1-M, mat2/3Δ; variety versatilis) crossed with NIG6701 (h+, mrc1Δ::Nat; variety japonicus). Patches of cells from both parental stocks were spotted on agar surface to compare their phenotype with those of segregants of their cross. Here, segregants from individual asci were spotted in horizontal rows. The picture shows 12-hr growth after replica plating on the indicated medium.
Analysis of this cross demonstrated that the Natr drug resistance marker segregated by the expected four drug-resistant: four drug-sensitive patterns in each octad (Figure 3). Moreover, we were able to score this marker in just 12 hr of growth of replicates on the nourseothricin antibiotic-containing medium (Figure 3). However, scoring mat1 alleles by replica-plating segregants to plates containing lawns of our mating-type tester strains, SJk2 and SjK3, with the aforementioned iodine-staining procedure, was only partially successful. This is a simple replica-plating procedure commonly used to score the mat1 marker of S. pombe crosses (Bresch et al. 1968). In our cross between the versatilis and japonicus varieties, some segregants showed iodine-positive responses while others did not. Therefore, we determined the mat1 marker of segregants by mating them to our mating-type testers, Sjk2 and Sjk3, and microscopically observing their ability to form zygotes. The expected 4 mat1-P: 4 mat1-M segregation pattern was found in all 12 octads we analyzed (Figure 3).
The iodine-nonstaining phenotype of japonicus variety changed to the staining phenotype by crossing with the versatilis variety
Interestingly, the mating mix of the cross did not exhibit an iodine-positive staining phenotype despite containing plenty of ascospores. Therefore we conclude that the iodine-negative staining genotype (designated iod-) of japonicus variety is dominant to the iodine-positive genotype (iod+) of versatilis variety. However, from the cross we were able to choose segregants that showed iodine-positive reactions; in subsequent crosses with versatilis strains, their iodine-positive feature was propagated through mitosis and meiosis. For example, Sjk17 (a segregant of the cross discussed above; h+, mat-2017, mrc1Δ::Nat, iod+) and Sjk3 cross produced 38 Parental ditype: 7 Nonparental ditype: 39 Tetratype asci for segregation of the mat1 and mrc1 pair of genetic markers, a result indicating loose genetic linkage between them. Notably, the mat1 marker in this cross was recorded by the replica-plating and iodine-staining procedure employed for research with S. pombe.
Nuclei orientation is indeterminate in the S. japonicus meiosis
Interestingly, the two spores at each end of the ascus are “sisters” in the linear four-spored asci of the S. pombe fission yeast (Leupold 1950), whereas they are nonsisters in both Saccharomycodes ludwigii (Winge and Laustsen 1939; Yamazaki et al. 1976) and Sc. cerevisiae (Hawthorne 1955) budding yeasts. This difference results from two different types of nuclei distribution in a species-specific manner after second nuclear division in meiosis within the ascus. The two spindles of second meiotic division are arranged in a line in S. pombe but they lie parallel to each other in the long axis of ascogenous cells of S. ludwigii and S. cerevisiae. A minority of yeast asci has spores arranged in a linear array, called linear asci. The nuclei distribution can be determined genetically by analyzing segregation pattern of centromere-linked markers of spore segregants extracted from the linear asci. We likewise defined the nuclear location behavior during S. japonicus meiosis, although this yeast, in comparison to other yeasts, produces 8-spored asci due to a subsequent mitotic division of each of the four meiotic nuclei. Because S. japonicus cells mate at the cellular pole, nearly 20% of S. japonicus zygotes produce discernible linear asci (see one example of it in Figure 3). We dissected linear asci from a cross of Sjk10 (mat1-P, mat2/3Δ, ade6) with Sjk19 (mat1-M, mat2/3Δ) strains. This cross was designed to segregate four unlinked markers, of which, ade6 (on chromosome 2) and mat1 (on chromosome 1) markers are tightly linked to their respective centromeres (Furuya and Niki 2009; Rhind et al. 2011; Yu et al. 2013). The asci were carefully dissected and the spores were collected and analyzed in the order they had been originally arranged in each ascus. To analyze the data, we compared the genetic composition of four pairs of segregants derived from each ascus such that each pair represented adjoining spores in the ascus. Among the nine linear asci analyzed, 21 among 36 pairs were discordant for alleles of at least one among the four markers; two of them were centromere linked (Table 2). On the basis of these data we conclude that members of a pair do not always derive from sister nuclei and that the nuclei orientation during meiosis in the elongated ascogenous cell is indeterminate. Thus, S. japonicus arranges nuclear products in meiosis in an unordered fashion, a pattern very different from those of the three unrelated yeast species quoted above.
Table 2. Marker segregation in the linear asci of Sjk10 × Sjk19 cross.
| Ascus | Spore | mat1 | ade6 | ura4 | mrc1 | Ascus | Spore | mat1 | ade6 | ura4 | mrc1 | Ascus | Spore | mat1 | ade6 | ura4 | mrc1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A | M | – | – | – | 2 | A | P | + | + | + | 3 | A | M | – | + | – |
| B | M | – | – | – | B | P | + | – | – | B | M | – | + | – | |||
| C | P | + | + | – | C | P | + | + | + | C | M | – | – | – | |||
| D | M | – | + | + | D | P | + | – | – | D | P | + | – | + | |||
| E | P | + | + | – | E | M | – | + | – | E | M | – | – | – | |||
| F | M | – | + | + | F | M | – | – | + | F | P | + | – | + | |||
| G | P | + | – | + | G | M | – | – | + | G | P | + | + | + | |||
| H | P | + | – | + | H | M | – | + | – | H | P | + | + | + | |||
| 4 | A | M | + | + | – | 5 | A | M | + | + | + | 6 | A | P | – | – | + |
| B | M | + | – | + | B | P | – | + | + | B | P | – | – | + | |||
| C | M | + | + | – | C | M | + | + | + | C | P | – | – | + | |||
| D | P | – | – | + | D | M | + | – | – | D | P | – | – | + | |||
| E | M | + | – | + | E | P | – | + | + | E | M | + | + | – | |||
| F | P | – | – | + | F | P | – | – | – | F | M | + | + | – | |||
| G | P | – | + | – | G | M | + | – | – | G | M | + | + | – | |||
| H | P | – | + | – | H | P | – | – | – | H | M | + | + | – | |||
| 7 | A | M | + | – | + | 8 | A | P | – | + | – | 9 | A | P | – | + | – |
| B | M | + | – | + | B | M | + | – | + | B | P | – | + | – | |||
| C | M | + | – | + | C | P | – | + | + | C | P | – | + | + | |||
| D | M | + | – | + | D | M | + | – | – | D | P | – | + | + | |||
| E | P | – | + | – | E | P | – | + | + | E | M | + | – | + | |||
| F | P | – | + | – | F | M | + | – | – | F | M | + | – | – | |||
| G | P | – | + | – | G | M | + | – | + | G | M | + | – | + | |||
| H | P | – | + | – | H | P | – | + | – | H | M | + | – | – |
Data of nine asci are displayed. The letters A to H indicate spore segregants of an ascus. Spores shown in bold letters were dead. The dead spore’s genotype was deduced from a segregant that did not have a matching sister spore genotype because each meiotic product undergoes mitosis to produce sister spores with identical genotype.
The variety versatilis grows very fast
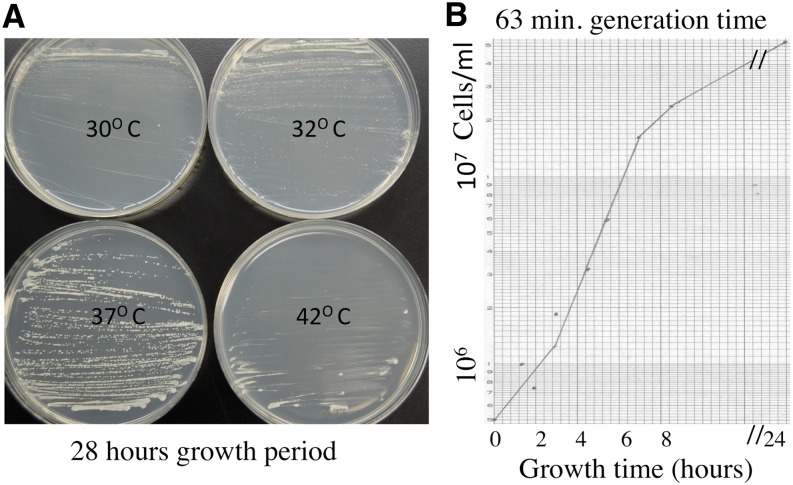
S. Japonicus colonies grew best at 37° among indicated temperatures, which we tested (Figure 4A). Individual cells grew into colonies visible to the naked eye after growth for 28 hr. Supporting this fast colony-growth phenotype, cultures grew in liquid growth medium with a generation time of only 63 min (Figure 4B) compared with between 1.5 and 2.0 hr required by S. pombe and S. cerevisiae. For comparison, we derived generation time of 93 min from the previously published growth curve of variety japonicus (Rhind et al. 2011). Thus, versatilis cultures grow significantly faster than those of japonicus variety.
Figure 4.
Growth of the Sjk2 strain. (A) The effect of temperature on the growth of Sjk2 colonies. The single-cell colony isolation procedure was performed on YEA medium-containing plates incubated at the indicated temperatures. The picture of plates was taken after 28 hr of growth. (B) The growth curve. The culture was grown in 50 mL of YEA broth medium contained in a 250-mL flask. The flask was shaken at 37° for aeration. The generation time calculated from the exponential phase of growth curve was 63 min.
The reason for the iodine-negative phenotype of the japonicus variety remains to be determined in future studies. In our cross of the versalitis and japonicus varieties presented above, 12.3% segregants showed iodine-positive response. This result is consistent with the idea that japonicus variety’s iod− phenotype is due to at least two unlinked genes difference between the species. Nonetheless, as demonstrated here, the vesatilis and japonicus varieties can be intercrossed and strains with iodine-positive genetic constitutions can be easily derived through genetic crosses. Because of the feasibility of intercrossing these two varieties, recently developed research tools consisting of DNA-mediated transformation (Aoki et al. 2010), gene deletions and drug markers inserted at 500 Kb apart in the entire genome of the japonicus variety (Furuya et al. 2012) could be used for research with the S. japonicus organism. The material generated for research with the japonicus variety should be combined by genetic crosses with the highly useful iodine-positive trait of the versatilis variety for future research with the S. japonicus organism. Already, the japonicus variety has been exploited as an organism to define the mechanism of dimorphic transition from yeast to hyphal growth, which is often associated with pathogenicity in some fungi (Furuya and Niki 2012); S. japonicus is not a human pathogen (Furuya and Niki 2009).
Because there is only 55% identity at the amino acid level between 1:1 orthologs of S. japonicus proteins (an organism with the 44% GC content) with those of S. pombe (an organism with the 36% GC content), these organisms have diverged far apart in evolution (Rhind et al. 2011). Indicating evolutionary divergence, we found that the S. pombe cells do not mate with S. japonicus cells. Both S. pombe and Sc. cerevisiae produce 4-spored asci and go through mitosis without nuclear breakdown. In comparison, S. japonicus produces 8-spored asci (Figure 2), and it goes through mitosis with a partial nuclear membrane breakdown (Robinow and Hyams 1989). Investigations with evolutionarily distinct organisms have been the key to discovering conserved mechanisms of biological processes. Both S. pombe and Sc. cerevisiae grow with a generation time between 1.5 and 2.0 hr and accomplishing their genetic cross requires a period of more than a week. In comparison with those popular research organisms, S. japonicus var. versatilis grew with a generation time of only 63 min (Figure 4B) and the meiotic analysis of a genetic cross was completed in just 2.5 days time (Figures 2 and 3). This author has more than 40 years of experience working on the biology of both Sc. cerevisiae (Klar 2010) and S. pombe (Klar 2007). On the basis of previous experience working with other yeasts and because of features described here that are deemed very much conducive for research, one can predict that S. japonicus species would become a popular experimental organism in the near future.
Acknowledgments
I thank Michael Bonaduce for providing technical support and Dr. C. Cagliero for helping to take photographs of yeast cells. Dr. H. Niki (National Institute of Genetics, Japan) is thanked for providing strains of japonicus variety. The Intramural Research Program of the National Cancer Institute, National Institutes of Health, supports this research.
Footnotes
Communicating editor: S. L. Forsburg
Literature Cited
- Aoki K., Nakajima R., Furuya K., Niki H., 2010. Novel episomal vectors and a highly efficient transformation procedure for the fission yeast Schizosaccharomyces japonicus. Yeast 27: 1049–1060. [DOI] [PubMed] [Google Scholar]
- Brenner S., 2003. Noble lecture. Nature’s gift to science. Biosci. Rep. 23: 225–237. [DOI] [PubMed] [Google Scholar]
- Bresch C., Muller G., Egel R., 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102: 301–306. [DOI] [PubMed] [Google Scholar]
- Furuya K., Niki H., 2009. Isolation of heterothallic haploid and auxotrophic mutants of Schizosaccharomyces japonicus. Yeast 26: 221–233. [DOI] [PubMed] [Google Scholar]
- Furuya K., Niki H., 2012. Hyphal differentiation induced via a DNA damage checkpoint-dependent pathway engaged in crosstalk with nutrient stress signaling in Schizosaccharomyces japonicus. Curr. Genet. 58: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K., Aoki K., Niki H., 2012. Construction of an insertion marker collection of Sz. japonicus (IMACS) for genetic mapping and a fosmid library covering its genome. Yeast 29: 241–249. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., 2002. Nobel Lecture. Yeast and cancer. Biosci. Rep. 22: 373–394. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., 1955. The use of linear asci for chromosome mapping in Saccharomyces. Genetics 1955: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Hinnen A., Fink G. R., 1979. Properties of yeast transformation. Cold Spring Harb. Symp. Quant. Biol. 43: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R., 1978. Transformation of yeast. Proc. Natl. Acad. Sci. USA 75: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J. S., 2007. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu. Rev. Genet. 41: 213–236. [DOI] [PubMed] [Google Scholar]
- Klar A. J. S., 2010. The yeast mating-type switching mechanism: a memoir. Genetics 186: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold U., 1950. The inheritance of homothally and heterothally in Schizosaccharomyces pombe. C. R. Trav. Lab. Carlsberg., Ser. Physiol. 24: 381–480. [Google Scholar]
- Moreno S., Klar A., Nurse P., 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Nurse P., 2002. Cyclin dependent kinases and cell cycle control (nobel lecture). ChemBioChem 3: 596–603. [DOI] [PubMed] [Google Scholar]
- Rhind N., Chen Z., Yassour M., Thompson D. A., Haas B. J., et al. , 2011. Comparative functional genomics of the fission yeasts. Science 332: 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow C. F., Hyams J., 1989. General Cytology of Fission Yeast. Academic Press, New York, NY. [Google Scholar]
- Watson J. D., 1970. Molecular Biology of the Gene. W. A. Benjamin Inc., New York, NY. [Google Scholar]
- Winge O., Laustsen O., 1939. Saccharomycodes ludwigii Hansen, a balanced heterozygote. Compt. Rend. Trav. Lab. Carlsberg Ser. Physiol 22: 357–370. [Google Scholar]
- Yamazaki T., Ohara Y., Oshima Y., 1976. Rare occurrence of the tetratype tetrads in Saccahromyces ludwigii. J. Bacteriol. 125: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Bonaduce M. J., Klar A. J., 2013. Defining the epigenetic mechanism of asymmetric cell division of Schizosaccharomyces japonicus yeast. Genetics 193: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]