Abstract
The pharmacokinetics of levetiracetam were determined prospectively in 18 neonates with seizures. Neonates were found to have lower clearance, higher volume of distribution, and a longer half-life as compared with older children and adults. Mild somnolence was the only adverse effect.
Neonatal seizures are a common problem in the first month of life. Phenobarbital is still the most frequently used medication to treat neonatal seizures,1 despite evidence that it causes neuronal apoptosis in animal models2 and may have long-term adverse effects on neurodevelopment.3 Levetiracetam (Keppra; UCB Pharma Inc, Smyrna, Georgia) is an anticonvulsant medication with a good safety and efficacy profile in adults and older children.4-6 Levetiracetam has linear pharmacokinetics, is mainly excreted unchanged by the kidneys, and is metabolized via enzymatic hydrolysis by a plasma esterase. Case series suggest that levetiracetam may be safe in the treatment of neonatal seizures,7-11 but no pharmacokinetic studies have been performed in this population. The purpose of this study was to determine the pharmacokinetics of levetiracetam and to gather preliminary safety data in neonates with seizures.
Methods
The institutional review boards at Cincinnati Children's Hospital Medical Center and Good Samaritan Hospital approved the protocol and written informed consent was obtained from the legal guardian of all subjects. We prospectively enrolled infants ≤30 days of age and ≥32 weeks gestational age with seizures treated with levetiracetam who were admitted to the neonatal intensive care unit. Exclusion criteria included birth weight <2000 g and known creatinine level ≥2.0 mg/dL. Consent was obtained for the blood draws before the levetiracetam dose was given. All subjects received at least 20 mg/kg of phenobarbital before receiving levetiracetam.
The levetiracetam dose was determined by the clinician prescribing the drug. Blood sampling was conducted with a D-optimal sparse sampling design with 3 samples collected in each patient. Patients were divided in 3 groups to obtain informative time points in the entire dosing interval. Levetiracetam concentrations were determined by using a validated liquid chromatography-electrospray tandem mass spectrometry assay.12
Non-linear mixed effects modeling (NONMEM, version 7.1, ICON Dev Soln, Ellicott City, Maryland) was used to perform the pharmacokinetic analyses. Individual Bayesian pharmacokinetic parameter estimates were calculated with MW/Pharm software (version 3.60, MediWare, Gronigen, The Netherlands). SAS software (version 9.2, SAS Institute, Cary, North Carolina) was used to analyze associations between demographic and pharmacokinetic parameters (Appendix; available at www.jpeds.com). Safety assessments included physical examination before and 24 hours after the loading dose of levetiracetam and closely monitoring vital signs after the loading dose for 24 hours. Adverse events were identified by means of bedside nurse reports and medical record review.
Results
A total of 21 infants who received levetiracetam for clinical seizure control, electrographic seizure control, or both were screened for the study from October 2008 to May 2010, and 19 of these infants were enrolled. The two patients who were not enrolled received levetiracetam before consent could be obtained. One subject was excluded because of a laboratory error. Pharmacokinetic data included 54 levetiracetam measurements from 18 subjects. Patient characteristics are summarized in Table I. The initial loading doses ranged from 14.4 to 39.9 mg/kg.
Table I.
Demographic information and pharmacokinetic parameters.
| Parameter | n = 18 | % of total |
|---|---|---|
| Sex | ||
| Male | 10 | 56 |
| Female | 8 | 44 |
| Race | ||
| African-American | 7 | 39 |
| Caucasian | 11 | 61 |
| Cause of seizures | ||
| HIE | 6 | 33 |
| Other* | 12 | 67 |
| Total body cooling for HIE during study period | 1 | <1 |
| Median | Range | |
|
| ||
| GA at birth (weeks) | 38 + 6 | 35 + 2-41 |
| Postnatal age (days) | 2 | 0-32 |
| Weight at time of dosing (kg) | 3.5 | 2.0-4.4 |
| Creatinine level at time of dosing | 0.7 | 0.2-1.6 |
| Clearance (mL/min/kg) | 1.21 | 0.47-2.89 |
| Volume of distribution (L/kg) | 0.89 | 0.37-1.26 |
| Half-life (hours) | 8.9 | 3.2-13.3 |
HIE, hypoxic-ischemic encephalopathy; GA, gestational age.
Other causes of seizures included ornithine transcarbamylase deficiency, hemimegalencephaly, cortical dysplasia, perinatal stroke, herpes simplex virus, meningitis, birth trauma, kernicterus, and unknown (4 patients).
Pharmacokinetic Modeling
A two-compartment model with first order elimination provided the best fit to the data. Significant co-variates determined in the univariate analysis were weight, postmenstrual age, serum creatinine level, and creatinine clearance. After multivariate analysis, only weight and creatinine clearance remained in the final model. Some unexplained variability remained in the model (32%-43%), which may have been caused by the small number of subjects.
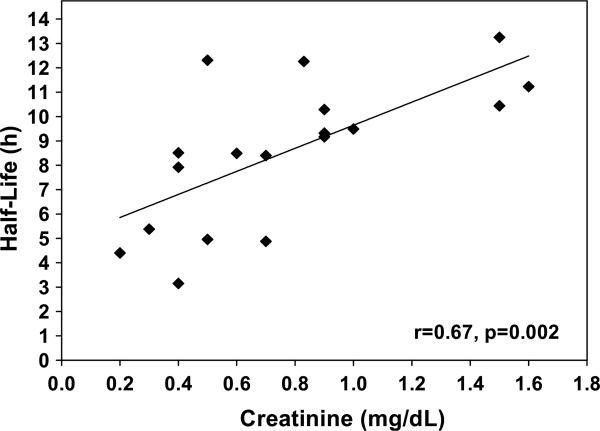
The parameter estimates from the base model were entered into MW/Pharm to determine the pharmacokinetic parameters for each individual subject (Table I). The median maximum drug concentration as predicted with the model was 39.8 mg/L (range, 14.8-91.9 mg/L). The highest measured concentration was 87.6 mg/L, 1 hour after a 30-mg/kg dose. There was a significant linear relationship between serum creatinine level and half-life (Figure 1, Pearson correlation r = 0.67, P = .0002). Linear relationships were also found between serum creatinine level and levetiracetam clearance (r = −0.53, P = .02) and creatinine clearance and levetiracetam clearance (r = 0.55, P = .003).
Figure 1.
Levetiracetam half-life versus serum creatinine.
Safety and Tolerability
Levetiracetam was well tolerated in this population. No changes in vital signs or laboratory parameters were observed. Several infants were noted to be somnolent in the 24 hours after levetiracetam administration. One patient with overwhelming herpes simplex virus infection died, and one patient with severe hypoxic ischemic encephalopathy died, both after withdrawal of support. These deaths were deemed not related to levetiracetam.
Discussion
Levetiracetam pharmacokinetics were different in this population of neonates with seizures than in adults and older children. The half-life is 6 to 8 hours in adults4 and 5 to 7 hours in older children.12,13 We found the median half-life to be 8.9 hours, as expected on the basis of the lower clearance of levetiracetam in neonates. The volume of distribution (Vd) is 0.5 to 0.7 L/kg in adults14 and 0.6 to 0.7 L/kg in older children.12,13 The median Vd in our study was 0.89 L/kg. Because neonates have higher body water content than adults and children and levetiracetam distributes in parallel with total body water, we would expect the Vd to be higher in neonates.
Total body clearance of levetiracetam is 0.96 mL/min/kg in adults4 and 1.43 to 1.46 mL/min/kg in children.12,13 We found the clearance in neonates to be 1.21 mL/min/kg. Clearance was lower in neonates than predicted with allometric scaling (clearance in neonates predicted with the three-fourths power model15 = 6.8 mL/min, actual clearance of neonates in this study = 4.2 mL/min), so we concluded that the lower clearance of levetiracetam in this population is caused by a lower GFR and possibly by lower plasma esterase activity. In support of this conclusion, a significant linear relationship was found between serum creatinine level and levetiracetam clearance. We found this relationship despite most infants being 0 to 2 days of age, when serum creatinine levels reflect maternal serum creatinine level and not the infant's GFR.
On the basis of the higher volume of distribution in neonates, the loading dose on a milligram per kilogram basis should be higher than in older children and adults. Because of the reduced clearance in neonates, the dosing interval may need to be extended, at least until the GFR matures. Therefore, a twice-daily dosing schedule may be more appropriate than a 3-times daily schedule in the first several weeks of life.
Infants in this study were closely monitored for 24 hours after the loading dose of levetiracetam. Two subjects died after withdrawal of support, but these deaths were not considered related to levetiracetam. Somnolence was the only adverse event recorded in subjects 24 hours after the dose. The somnolence did not interfere with feeding or cause subjects to require increased respiratory support. In older children, the main adverse events seen with levetiracetam are somnolence, nervousness, dizziness, and irritability.4,5,16
We conclude that the pharmacokinetics of levetiracetam differ in neonates compared with children and adults. Limitations of our study include the small sample size with high interindividual variability. Future studies should be conducted to confirm safety and establish efficacy in this population.
Acknowledgments
We would like to thank Katie Holland for her help with electroencephalography monitoring and interpretation (National Institutes of Health grant R01NS062756) and Steven J. Soldin, PhD, FACB, FCACB, for measuring the levetiracetam concentrations in his lab (NIH grant M01-RR13297 and receives equipment, but not funding, from ABSiex.
Supported by the National Institutes of Health (grants 5T32AR007594-15 to C.S. and 5K24HD050387 and 5U10HD037249 to A.V.).
Glossary
- CL
Clearance
- CPR
Creatinine production rate
- GA
Gestational age
- IPRED
Individual-predicted value
- OFV
Objective function value
- PMA
Post-menstrual age
- PNA
Post-natal age
- PRED
Population-predicted value
- V1
Volume of distribution in the central compartment
- GFR
Glomerular filtration rate
- Vd
Volume of distribution
Appendix
Methods
Population Pharmacokinetic Analysis
During model development, the data were assessed for fit to structural models, including one- or two-compartment models. Models were evaluated and selected on the basis of goodness of fit and stability. Further assessment and comparison used the likelihood ratio test and reviewed changes in the objective function value (OFV) between models. Improvement in model fit was determined with χ2 distribution with one degree of freedom (ΔOFV <3.84 = P < .05).
During model development, these diagnostic plots were used to visually assess model fit: observed (DV) versus population-predicted (PRED) or individual-predicted (IPRED) values. Plots of residuals and weighted residuals versus time or PRED were also examined.
A two-compartment model with first-order elimination was chosen as the base model on the basis of visual inspection of the diagnostic plots and successful minimization of the OFV. The model included parameters for clearance (CL), intercompartmental clearance, volume of distribution in the central compartment (V1), and volume of distribution in the peripheral compartment (V2). The modeling added random effects for the PK parameters to account for differences and similarities between individuals and observations. The terms for variability in the model included intra- and inter-individual variability and residual (unexplained) variability. Estimations of inter-individual and residual variability were assessed for evidence of model over-parameterization.1 Inter-individual variability was assessed with exponential (equation 1) variability models:
| (1) |
in which CLi is LEV clearance of the ith individual, θpop is the population value for LEV clearance, and η in the inter-individual random effect with mean 0 and variance ω2.
The residual (unexplained) variability error models evaluated with a combined additive and constant coefficient of variation error model for LEV (equation 2):
| (2) |
in which Y is the observed and IPRED is the individual-predicted plasma concentrations. εaddX, and εpropX are the residual unexplained variability terms for the additive and proportional error models.
Co-Variate Analysis
Patient characteristics investigated in the base model included weight, gestational age (GA), postnatal age (PNA), postmenstrual age (PMA), sex, race, and creatinine level at time of dosing. PMA was calculated as GA plus PNA at birth in completed weeks. Specific to the occurrence and treatment of seizures in neonates, these co-variates were considered for inclusion: hypoxic-ischemic encephalopathy not cause of seizures or hypoxic-ischemic encephalopathy main cause of seizures and being cooled (therapeutic hypothermia for hypoxic-ischemic encephalopathy) or not being cooled at time of levetiracetam dose. Models were developed to further evaluate the influence of creatinine and renal maturation on levetiracetam clearance. PMA can be used as a predictor for calculating glomerular filtration rate (GFR). It has previously been shown that GFR matures during infancy. An adult rate of 6 L/h/70 kg is estimated by 6 months PNA.2 Creatinine concentration decreases in the first few days of life. Estimates for creatinine clearance can be determined by using PMA to predict a creatinine production rate (CPR) with the inclusion of a scaling constant for age (agek; equation 3):
| (3) |
in which 516 μmol/h is the assumed CPR in a 70-kg, 40-year-old male patient, and CLcr is the estimated creatinine clearance.
An exploratory analysis was used to look for relationships between the PK parameters and co-variates by visually inspecting plots of the empirical Bayesian (posthoc) estimates of individual parameters from the base model against co-variate values. Allometric scaling was applied to CL and V in the model and standardized to a body weight of 70 kg. Weight was also investigated as a priori and centered on the population mean.3 After the initial analysis, statistically significant co-variates (P < .04) were included in the model by using a forward stepwise inclusion approach and added into the model until there was no further decrease in OFV. Co-variates were subsequently removed from the model with a backward stepwise approach.
Model Evaluation
The population PK model was evaluated with a non-parametric re-sampling bootstrap method to assess model accuracy and stability.4 PDX-Pop software (Pediatrix, Sunshine, Florida) was used to generate 1000 bootstrap runs generated by random sampling by using the original dataset. Standard errors for the estimated population parameters and random effects error models were also assessed. Empirical Bayesian estimates for the predicted concentrations were obtained by using the posthoc option inNONMEM (University of California, San Fransisco, California). The final model was further evaluated by generating visual predictive checks.
Results
The base model provided these estimates: CL, 0.16 L/h; V1, 1.2 L; Q, 4.8 L/h; and V2, 1.4 L. Inter-individual variability was described by using an exponential model, and residual variability was evaluated with a combined additive and constant co-efficient error model. The model estimate inter-individual variability of 57.7 and 58.1 CV% for clearance and volume of distribution in the central compartment, respectively. The variability for inter-compartmental clearance was fixed at 31.6% CV. An estimate of 48.9 CV% was determined for volume of distribution in the peripheral compartment. Residual variability was 26.4 CV% for the proportional error and 0.13 μg/ml SD for the additive error.
Various co-variates were tested in the model. The principal criteria for evaluating each co-variate were based on statistically significant minimization of the objective function value. The most significant co-variates determined in the univariate analysis were weight, PMA, and creatinine clearance. Plots of the post hoc values of clearance and volume of distribution versus co-variates from the final model were inspected. In comparison with the plots from the base model, there is an improved relationship between clearance and volume of distribution with weight, creatinine clearance, and PMA. Sex, race, hypoxic-ischemic encephalopathy as cause of seizures, and being cooled did not change the OFV. PNA, GA, and length did not provide statistically significant minimization of the OFV. There was evidence of some influence of edema on influencing CL and V1, but the change was not statistically significant. After a forward and backward multivariate analysis, only weight on V1 and creatinine clearance on CL remained in the final model.
The results of the final co-variate model are presented in final parameter estimates and the 95% CI in comparison with the results of the bootstrapping (Table II). The final co-variate model was clearance = 0.097 × creatinine clearance0.004, volume of distribution = 1.96 × weight3.83, intercompartmental clearance = 1.1, and volume of distribution in the peripheral compartment = 0.89. The change in inter-individual variability and residual error estimates between the base and final co-variate model showed a decrease in variability for all parameters except V2.
Table II. Summary of parameter estimates for the final pharmacokinetic model of levetiracetam.
| Final model | Bootstrap n = 1000 | ||
|---|---|---|---|
| Parameter | Estimate | Median | 95%CI |
| CL (L/h) | 0.097 | 0.093 | 0.06-0.15 |
| V1 (L) | 1.96 | 1.89 | 1.83-1.95 |
| Q (L/h) | 1.1 | 0.98 | 0.92-1.04 |
| V2 (L) | 0.89 | 0.69 | 0.63-0.75 |
| CL θ5 creatinine CL | 0.04 | 0.067 | 0.01-0.13 |
| V1 θ6 weight | 3.83 | 3.67 | 3.61-3.73 |
| Inter-individual variability | CV% | ||
| CL | 43.2 | 0.187 | 0.13-0.25 |
| V1 | 37.5 | 0.141 | 0.08-0.2 |
| Q (fixed) | 31.6 | 0.12 | 0.06-0.18 |
| V2 | 114 | 1.1 | 1.04-1.16 |
| Residual variability | |||
| Proportional error (CV%) | 24.1 | 0.07 | 0.01-0.13 |
| Additive error (SD) | 0.028 | 0.11 | 0.05-0.17 |
Q, intercompartmental clearance; V1, volume of distribution in the central compartment; V2, volume of distribution in the peripheral compartment.
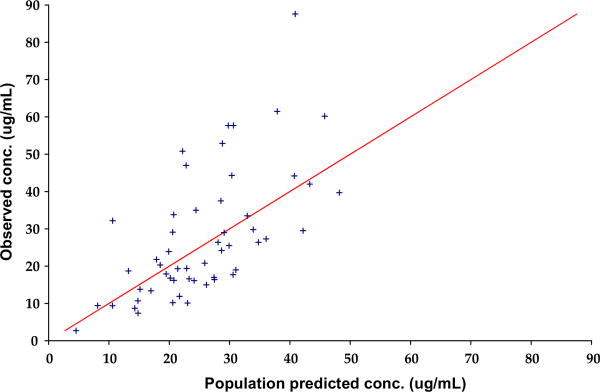
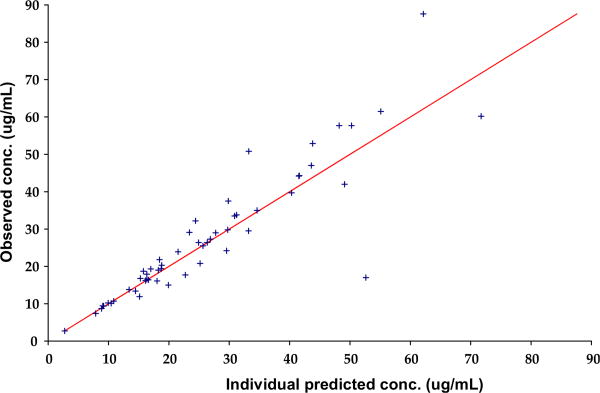
Diagnostic plots for the final co-variate model showed that there was some improvement in the DV versus PRED concentration plot (Figure 2; available at www.jpeds.com), with data clustering more closely around the line of identity in the final co-variate model compared with the base model. This was also reflected in the DV versus IPRED concentration plot (Figure 3; available at www.jpeds.com). The parameter estimates and bootstrap for the final co-variate model are provided in Table II, showing reasonable robustness and stability within the final co-variate model.
Figure 2.
Observed levetiracetam concentration versus population-predicted levetiracetam concentration.
Figure 3.
Observed levetiracetam concentration versus individual predicted levetiracetam concentration.
References
1. Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 1993; 21:735-50.
2. Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 2006; 165:819-29.
3. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303-32.
4. Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 1997; 37:486-95.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures—effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364–9. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 4.Grosso S, Cordelli DM, Franzoni E, Coppola G, Capovilla G, Zamponi N, et al. Efficacy and safety of levetiracetam in infants and young children with refractory epilepsy. Seizure. 2007;16:345–50. doi: 10.1016/j.seizure.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Grosso S, Franzoni E, Coppola G, Iannetti P, Verrotti A, Cordelli DM, et al. Efficacy and safety of levetiracetam: an add-on trial in children with refractory epilepsy. Seizure. 2005;14:248–53. doi: 10.1016/j.seizure.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Glauser TA, Pellock JM, Bebin EM, Fountain NB, Ritter FJ, Jensen CM, et al. Efficacy and safety of levetiracetam in children with partial seizures: an open-label trial. Epilepsia. 2002;43:518–24. doi: 10.1046/j.1528-1157.2002.13101.x. [DOI] [PubMed] [Google Scholar]
- 7.Ng YT, Hastriter EV, Cardenas JF, Khoury EM, Chapman KE. Intravenous levetiracetam in children with seizures: a prospective safety study. J Child Neurol. 2010;25:551–5. doi: 10.1177/0883073809348795. [DOI] [PubMed] [Google Scholar]
- 8.Shoemaker MT, Rotenberg JS. Levetiracetam for the treatment of neonatal seizures. J Child Neurol. 2007;22:95–8. doi: 10.1177/0883073807299973. [DOI] [PubMed] [Google Scholar]
- 9.Furwentsches A, Bussmann C, Ramantani G, Ebinger F, Philippi H, Poschl J, et al. Levetiracetam in the treatment of neonatal seizures: a pilot study. Seizure. 2010;19:185–9. doi: 10.1016/j.seizure.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramantani G, Ikonomidou C, Walter B, Rating D, Dinger J. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol. 2011;15:1–7. doi: 10.1016/j.ejpn.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Guo T, Oswald LM, Mendu DR, Soldin SJ. Determination of levetiracetam in human plasma/serum/saliva by liquid chromatography-electrospray tandem mass spectrometry. Clin Chim Acta. 2007;375:115–8. doi: 10.1016/j.cca.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42:1574–9. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 13.Glauser TA, Mitchell WG, Weinstock A, Bebin M, Chen D, Coupez R, et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia. 2007;48:1117–22. doi: 10.1111/j.1528-1167.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 14.Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43:707–24. doi: 10.2165/00003088-200443110-00002. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 16.Pina-Garza JE, Nordli DR, Jr, Rating D, Yang H, Schiemann-Delgado J, Duncan B, et al. Adjunctive levetiracetam in infants and young children with refractory partial-onset seizures. Epilepsia. 2009;50:1141–9. doi: 10.1111/j.1528-1167.2008.01981.x. [DOI] [PubMed] [Google Scholar]