Abstract
Hypoxia-inducible factors (HIFs) are oxygen-sensitive transcription factors that mediate cellular adaptation to hypoxia. Depending on the type of injury, activation of HIF signaling in renal cells may be renoprotective or promote fibrosis. Isoe and colleagues demonstrate that hyperglycemia activates HIF-1 in mesangial cells via carbohydrate response element binding protein (ChREBP), thus providing a novel link between alterations in systemic glucose homeostasis and HIF-regulated gene expression.
Key mediators of cellular adaptation to hypoxia are hypoxia-inducible factors (HIFs), basic helix-loop-helix transcription factors that consist of an oxygen-sensitive α-subunit and a constitutively expressed (β-subunit, also known as the aryl hydrocarbon receptor nuclear translocator (ARNT). HIFs regulate energy metabolism, angiogenesis, erythropoiesis, cellular differentiation, extracellular matrix turnover, and other biological processes, primarily by transactivation of oxygen-sensitive genes following binding to specific regulatory DNA sequences, so-called hypoxia-response elements. Increased HIF expression has been found in animal models of chronic kidney disease (CKD) and in renal biopsy material from patients with diabetic nephropathy and other forms of renal disease.1–3 Relative hypoxia, which is the major stimulus that causes HIF activation, can be detected in CKD tissues irrespective of etiology and is thought to result from a combination of structural and functional changes that include decreased peritubular blood flow associated with glomerular injury, capillary rarefaction, vasoconstriction, luminal narrowing of atherosclerotic vessels, increased oxygen demand from hyper-filtration and tubular hypertrophy, limited oxygen diffusion as a consequence of extracellular matrix expansion, and renal anemia.1 HIF activation in this setting has been shown to promote fibrogenesis and to negatively impact disease outcome on the basis of cell type-specific gene ablation studies.2 There are several possible mechanisms by which HIF may exert its profibrotic effect. These include direct transcriptional regulation of gene products that control extracellular matrix turnover; functional cooperation with transforming growth factor-β1, a potent profibrotic factor; promotion of epithelial to mesenchymal transition; and modulation of renal inflammation. In contrast to HIFs' profibrotic role in renal epithelial cells, there is experimental evidence in animal models that systemic administration of cobalt chloride, possibly through HIF activation, is beneficial in diabetic nephropathy and other forms of CKD.4,5
Although hypoxia is a major stimulus for HIF activation, stabilization of the oxygen-sensitive HIF α-subunit can occur in the absence of significant hypoxia. This notion has significant implications for the pathogenesis of renal injury, as activation of HIF responses could modulate disease activity at an early stage, when tissue oxygenation is not impaired. Classic examples of oxygen-independent HIF activation are inherited renal cancer syndromes, which are associated with genetic defects that result in an inability to degrade HIF-α—for example, von Hippel – Lindau disease and hereditary leiomyomatosis and renal cancer syndrome (HLRCC).6 Normoxic HIF stabilization can also be achieved pharmacologically by inhibition of 2-oxoglutarate-dependent dioxygenases (prolyl-4–hydroxylase domain (PHD) proteins), which function as intracellular oxygen sensors. PHDs hydroxylate specific proline residues within the oxygen-dependent degradation domain of HIF-α thus enabling interaction with the von Hippel – Lindau – E3 ubiquitin ligase complex, which in turn targets HIF-α for proteasomal degradation (reviewed by Kaelin and Ratcliffe7). Several signaling molecules, some with key roles in the pathogenesis of CKD, have been shown to induce stabilization of HIF-α under normoxia. These include nitric oxide (NO), reactive oxygen species (ROS), tumor necrosis factor-α, interleukin-1, angiotensin II, and growth factors such as epidermal growth factor and insulin and insulin-like growth factors, which either inhibit HIF prolyl-hydroxylation directly or indirectly (for example, NO and ROS are direct inhibitors) or increase HIF-α levels through phos-phatidylinositol-3-kinase-dependent mechanisms (growth factors).
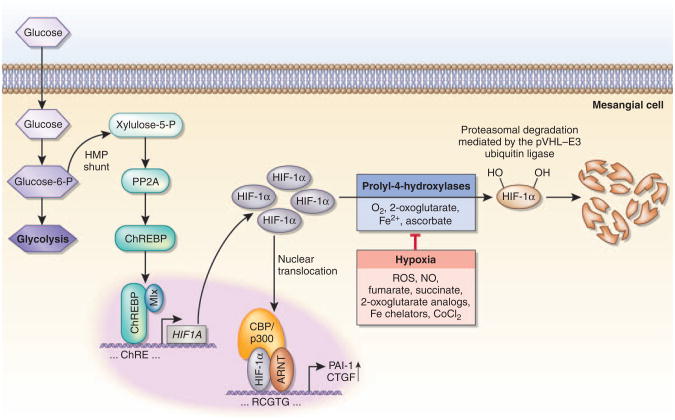
Given the wide range of HIF-regulated biological functions, normoxic stabilization of HIF-α is likely to modulate disease activity at an early stage before the occurrence of significant hypoxia. An example of this is the stabilization of glomerular HIF-1α in the early phases of diabetic nephropathy in db/db mice.8 Isoe and colleagues9 (this issue) now identify a carbohydrate response element (ChRE) in the proximal HIF1A promoter that regulates HIF1A transcription in mesangial cells in response to glucose in vitro and in vivo (Figure 1). Exposure of mesangial cells to high-glucose culture conditions or to hyperglycemia in vivo resulted in increased HIF1A (and also EPAS1/HIF2A) transcription and HIF-1α stabilization, which appeared to be independent of PHD activity. This response, which is cell type-specific, as HIF1A transcription cannot be induced in renal tubular epithelial cells, depends on the presence of carbohydrate response element binding protein (ChREBP), which binds to the HIF-ChRE localized between nucleotides –254 and –243. ChREBP is a glucose-responsive basic helix-loop-helix transcription factor of approximately 100 kDa, also termed MondoB or Williams–Beuren syndrome critical region gene 14 (WBSCR14), that dimerizes with Max-like protein X (Mlx) to transactivate glucose-sensitive genes, such as liver-type pyruvate kinase, fatty acid synthase, and acetyl-CoA carboxylase 1. In the liver, ChREBP functions as a regulator of de novo lipid synthesis in response to elevated serum glucose, irrespective of insulin levels. Its nuclear translocation is regulated by the formation of xylulose-5-phosphate and protein phosphatase 2A activity (reviewed by Uyeda and Repa10) (Figure 1).
Figure 1. Glucose regulates HIF1A transcription via ChREBP in mesangial cells.
Under normoxia, HIF-1α is hydroxylated by prolyl-4-hydroxylases and targeted for proteasomal degradation by the pVHL–E3 ubiquitin ligase complex. When prolyl-4-hydroxylation is inhibited—for example, in the absence of molecular oxygen—HIF-1α is not degraded and translocates to the nucleus, where it heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1α/ARNT heterodimers bind to the HIF consensus binding site, RCGTG, followed by transactivation of target genes. Nitric oxide, reactive oxygen species, the Krebs cycle metabolites succinate and fumarate, cobalt chloride, and iron chelators such as desferrioxamine inhibit HIF prolyl-4-hydroxylation in the presence of oxygen. In normoxic mesangial cells, hyperglycemia results in increased HIF-1α protein levels and increased expression of HIF-regulated genes (for example, PAI-1 and CTGF) irrespective of oxygen levels. Increased glucose flux results in the conversion of glucose-6-phosphate to xylulose-5-phosphate by the hexose monophosphate shunt pathway. Xylulose-5-phosphate activates protein phosphatase 2A, which in turn dephosphorylates carbohydrate response element binding protein (ChREBP), allowing for its nuclear translocation. In mesangial cells ChREBP binds to the proximal HIF1A promoter, stimulating its transcription. The proposed mechanism for ChREBP activation in mesangial cells is based on studies with hepatocytes. CBP, CREB-binding protein; CoCl2, cobalt chloride; CTGF, connective tissue growth factor; Fe2+, ferrous iron; HMP, hexose monophosphate; Mlx, Max-like protein X; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; PP2A, protein phosphatase 2A; ROS, reactive oxygen species.
A recent report indicates that ChREBP in pancreatic islet β-cells is a negative regulator of ARNT, the constitutively expressed β-subunit of HIF heterodimers.11 This finding predicts an impairment of HIF-mediated transcriptional responses under high-glucose conditions. Whether ARNT is also glucose-regulated in mesangial cells has not been investigated. Isoe and colleagues show that several genes known to be HIF targets, including vascular endothelial growth factor (VEGF), plasminogen activator inhibitor-1 (PAI-1), connective tissue growth factor (CTGF), glucose transporter 1 (GLUT-1), hexokinase 2, and adrenomedullin, are upregulated in normoxic mesangial cells when cultured in high-glucose-containing medium.9 Although the authors do not formally demonstrate that HIF-1 binds to the respective hypoxia-response elements using chromatin immunoprecipitation analysis, they found that interfering RNA directed against HIF1A reduced CTGF and PAI-1 expression to control levels (expression levels under normal glucose conditions). ChREBP knock-down phenocopied HIF-1α knock-down, supporting the notion that the ChREBP – HIF axis is responsible for the upregulation of these genes under high-glucose conditions. It is important to mention here that CTGF and PAI-1 are strong promoters of fibrosis, and further studies using genetic models are needed to investigate to what degree hyperglycemic activation of HIF-1 in mesangial cells contributes to the development of diabetic glomerulopathy and matrix deposition.
Isoe et al.9 make the important observation that high glucose induces mesangial VEGF expression through presumed HIF-1 transactivation (the role of HIF-1 in its glucose-mediated induction is not directly examined), which not only has significant implications for the pathogenesis of diabetic nephropathy, but is also important for understanding HIF trans-activation function in the setting of diabetes, which appears to be cell type-dependent. In contrast, at least in the context of hypoxia, HIF-mediated VEGF induction in certain diabetic tissues has been shown to be impaired or absent. For example, dermal fibroblasts from diabetic patients fail to induce VEGF under hypoxia, resulting in poor wound healing, and reduced tubulointerstitial VEGF expression was found in renal biopsy material from patients with diabetic nephropathy and evidence of capillary rarefaction, which is associated with the presence of relative hypoxia.12,13 The inability to induce VEGF under hypoxic conditions in these settings may involve glucose-dependent alterations of HIF-α protein stability14 or may result from a covalent, methylglyoxal-based modification of p300, a coactivator of HIF that interacts with the C-terminal transactivation domain, resulting in reduced transcriptional activity and decreased VEGF levels.12 Importantly, HIF-1α itself was found to be modified by methylglyoxal, which reduces its ability to dimerize with ARNT, thereby negatively impacting HIF signaling.15 Given these findings, it is likely that the mesangial HIF transcriptional complex is also subject to similar hyperglycemia-induced protein modifications. Whether these changes affect the pattern of HIF-dependent glomerular gene expression, and to what degree they impact the pathogenesis of diabetic renal disease, remain to be defined. Interestingly, PHD3, a known HIF-1 target, does not respond to high glucose.9 PHD3 is a HIF prolyl-4-hydroxylase that is highly hypoxia-inducible and is involved in targeting HIF-α for degradation following reoxygenation.7 The unresponsiveness of PHD3 to high glucose indicates that hyperglycemia-induced expression of HIF targets is likely to involve additional regulatory mechanisms that modulate HIF responses in a gene-dependent manner.
In summary, the study by Isoe and colleagues9 provides novel insights into the regulation of HIF signaling in mesangial cells exposed to hyperglycemia and links systemic glucose homeostasis directly to HIF-dependent gene expression via ChREBP. The study also nicely illustrates that activation of HIF signaling can occur in the absence of significant hypoxia. Although a time course was not presented, the study by Isoe and colleagues9 furthermore suggests that HIF activation can occur at an early stage during disease evolution before overt diabetic nephropathy is present. The pathogenetic role of early mesangial HIF activation in this setting, however, remains to be investigated. With regard to therapeutic intervention, inhibition of HIF for the treatment of diabetic nephropathy may not be feasible, given its role in the regulation of a wide spectrum of hypoxia responses, which include renal erythropoietin production and activation of certain renoprotective pathways. A more viable approach could be the pharmacologic targeting of specific HIF-regulated gene products that have key roles in the pathogenesis of diabetic nephropathy. There is certainly need for detailed genetic studies in rodents that examine cell type- and context-dependent functions of HIF. These studies will ultimately tell whether and to what degree glucose-induced activation of HIF signaling in mesangial cells contributes to the pathogenesis of diabetic nephropathy.
Acknowledgments
V.H.H. is supported by the Krick-Brooks Chair in Nephrology and by grants from the National Institutes of Health.
Footnotes
Disclosure: The author declared no competing interests.
References
- 1.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 2.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neusser MA, Lindenmeyer MT, Moll AG, et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am J Pathol. 2010;176:594–607. doi: 10.2353/ajpath.2010.090268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtomo S, Nangaku M, Izuhara Y, et al. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Matsumoto M, Inagi R, et al. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 2005;68:2714–2725. doi: 10.1111/j.1523-1755.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 6.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Makino H, Miyamoto Y, Sawai K, et al. Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes. 2006;55:2747–2756. doi: 10.2337/db05-1683. [DOI] [PubMed] [Google Scholar]
- 9.Isoe T, Makino Y, Mizumoto K, et al. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 2010;78:48–59. doi: 10.1038/ki.2010.99. [DOI] [PubMed] [Google Scholar]
- 10.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Noordeen NA, Khera TK, Sun G, et al. Carbohydrate-responsive element-binding protein (ChREBP) is a negative regulator of ARNT/HIF-1beta gene expression in pancreatic islet beta-cells. Diabetes. 2010;59:153–160. doi: 10.2337/db08-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA. 2009;106:13505–13510. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 14.Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceradini DJ, Yao D, Grogan RH, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]