Abstract
Parathyroid hormone-related protein (PTHrP)(1–36) increases lumbar spine (LS) bone mineral density (BMD), acting as an anabolic agent when injected intermittently, but has not been directly compared to parathyroid hormone (PTH)(1–34). We performed a three month, randomized, prospective study in 105 postmenopausal women with low bone density or osteoporosis comparing daily subcutaneous injections of PTHrP(1–36) to PTH(1–34). Thirty-five women were randomized to each of three groups: PTHrP(1–36) 400 μg/d; PTHrP(1–36) 600 μg/d; and PTH(1–34) 20 μg/d. The primary outcomes measures were changes in amino-terminal telopeptides of procollagen 1 (PINP) and carboxy-terminal telopeptides of collagen 1 (CTX). Secondary measures included safety parameters, 1,25(OH)2vitamin D and BMD. The increase in bone resorption (CTX) by PTH(1–34) (92%) (p<0.005) was greater than for PTHrP(1–36) (30%) (p<0.05). PTH(1–34) also increased bone formation (PINP) (171%) (p<0.0005) more than either dose of PTHrP(1–36) (46 & 87%). The increase in PINP was earlier (day 15) and greater than the increase in CTX for all three groups. LS BMD increased equivalently in each group (p<0.05 for all). Total hip (TH) and femoral neck (FN) BMD increased equivalently in each group but were only significant for the two doses of PTHrP(1–36) (p<0.05) at the TH, and for PTHrP(1–36) 400 (p<0.05) at the FN. PTHrP(1–36) 400 induced mild, transient (day 15) hypercalcemia. PTHrP(1–36) 600 required a dose reduction for hypercalcemia in three subjects. PTH(1–34) was not associated with hypercalcemia. Each peptide induced a marked biphasic increase in 1,25(OH)2D. Adverse events (AE) were similar among the three groups. This study demonstrates that PTHrP(1–36) and PTH(1–34) cause similar increases in LS BMD. PTHrP(1–36) also increased hip BMD. PTH(1–34) induced greater changes in bone turnover than PTHrP(1–36). PTHrP(1–36) was associated with mild transient hypercalcemia. Longer term studies using lower doses of PTHrP(1–36) are needed to define both the optimal dose and full clinical benefits of PTHrP.
Keywords: Osteoporosis, PTH, PTHrP, Anabolic, Bone Turnover, DXA
Introduction
Osteoporosis affects some 11 million people in the US (1). As the population ages, the prevalence of osteoporosis and the incidence of osteoporotic fracture are increasing. Most current therapies for osteoporosis are anti-resorptive: they inhibit osteoclastic bone resorption, but do not foster the development of new bone. While widely used and important, the effect on non-vertebral fracture is only 20–30% leaving a significant percent of fracture risk unprotected (2–8). Therefore, there is a need for a skeletal anabolic therapy that can further increase bone mineral density (BMD) and quality, while reducing risk for osteoporotic fractures through stimulation of new bone growth, that is safe, well tolerated, and that might achieve a greater effect than the antiresoprtives.
Despite widespread consensus on this goal, teriparatide {PTH(1–34)} is the only skeletal anabolic drug available in the US (9–12). PTH(1–34) is effective and safe, and has been clinically available for more than a decade (13). However, its use is limited in some patients by mild hypercalcemia, muscle cramps, and other occasional adverse events (13,14).
Parathyroid hormone-related protein (1–36) {PTHrP(1–36)} (15) and synthetic PTHrP analogues (16) have shown promise as novel skeletal anabolic agents. Like PTH(1–34), when infused continuously, PTHrP(1–36) is catabolic for the skeleton (17,18). However, as with PTH(1–34), when injected intermittently (e.g., once per day), PTHrP(1–36) displays marked skeletal anabolic characteristics in humans and rodents (15,19).
We have explored the efficacy and safety of daily subcutaneous administration of PTHrP(1–36) in preliminary studies, and have shown that it is safe, effective and well tolerated when given for up to three months (15,20,21). In these prior studies, PTHrP(1–36) appeared to differ from PTH(1–34) in two important ways. First, PTHrP(1–36) was not associated with hypercalcemia at therapeutic doses (400 μg/d/day). Second, it did not activate markers of bone resorption at clinically relevant doses (15,21). Thus, unlike PTH(1–34), PTHrP(1–36) appeared to be a “pure anabolic” skeletal agent. Encouraged by these results, we directly compared PTHrP(1–36) to PTH(1–34) in a three-month, randomized clinical trial. We hypothesized that PTHrP(1–36) would be less resorptive than PTH(1–34), yet retain anabolic efficacy without significant side effects. The primary outcome measures were bone turnover markers, with secondary outcome measures being safety, 1,25 dihydroxyvitamin D {1,25(OH)2D} and changes in BMD.
Material and Methods
Study Subjects
Healthy post-menopausal women aged 45–75 were recruited from the greater Pittsburgh area over a two year period. Subjects were recruited through newspaper advertisements, flyers posted in the University of Pittsburgh Medical Center and Endocrinology Clinic and, predominantly, by direct mailings. Informed consent, approved by the University of Pittsburgh IRB, was obtained from each subject. The trial was registered in advance on Clinical Trials.gov (NCT0853723). 105 subjects were randomized with equal allocation to one of three treatment groups (n=35 each): daily subcutaneous PTHrP(1–36) 400 ug/d; PTHrP(1–36) 600 ug/d; or, PTH(1–34) 20 ug/d (Fig 1). Treatment assignments were generated using permuted block randomization with random block sizes of three or six. No pre-randomization stratification was employed. The dose of PTH(1–34) is the current FDA approved dose. The doses of PTHrP(1–36), 400 ug/d and 600 ug/d were selected based on their safety and efficacy in prior trials (15, 20, 21). Inclusion required a lumbar spine (LS), total hip (TH) or femoral neck (FN) bone densitometry (DXA) T-score of between −2.0 and −4.0, with at least two vertebrae evaluable by DXA. Exclusions included: being African-American, smoking, BMI>30, use of bisphosphonates within the prior six months or for more than one month within the past two years; use of any estrogen, raloxifene or calcitonin in the prior year; use of PTHrP(1–36), PTH or PTH analog within the past year or previously for more than 21 months; fracture within the prior 12 months; significant active cardiac, vascular, renal (creatinine >1.5 mg/dl), pulmonary, hepatic, hematologic (hematocrit <34%), rheumatologic or endocrine disorder; any medication that could interfere with skeletal turnover; or a serum calcium >10.5 mg/dl, 25(OH)D <20 ng/ml, or an intact PTH >65 pg/ml at screening.
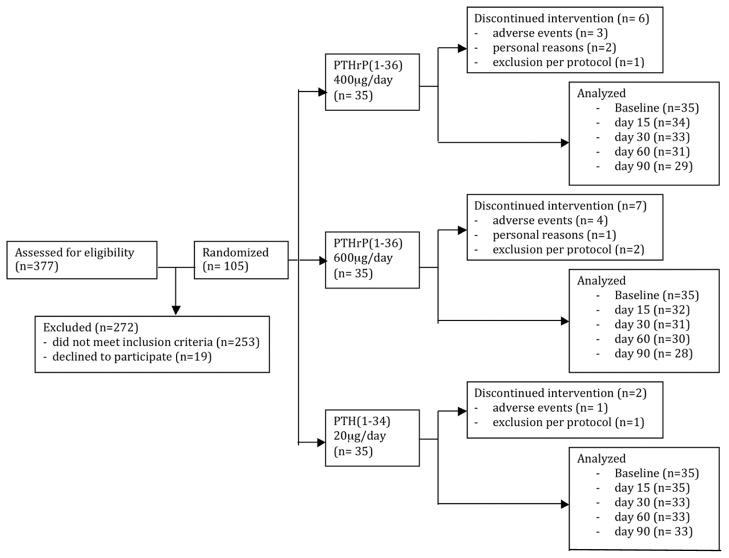
Figure 1. Study Flow Diagram.
377 subjects were screened. 272 subjects were excluded: 197 failed to meet DXA inclusion criteria; 10 were ineligible at the screening visit because of exclusion criteria in their medical history; and, 5 withdrew after signing the consent. Among the remaining subjects, an additional 37 failed screening blood tests, 8 had medical exclusions, and an additional 16 withdrew voluntarily after qualifying by DXA and labs. A total of 105 were randomized equally to the three study groups (n=35 per group). A total of 90 subjects completed the 90 day trial [PTHrP(1–36) 400 ug/d, n=29; PTHrP (1–36) 600 ug/d, n= 28; PTH (1–34) 20 ug/d, n=33].
Preparation and Administration of PTHrP(1–36) and PTH(1–34)
Subjects in the PTH(1–34) group were given Forteo® (Lilly Inc, Indianapolis, IN) pens. PTHrP(1–36) was synthesized using solid-phase f-moc chemistry in a cGMP-certified facility (NeoMPS, Strasbourg, FR) as approved by the Food and Drug Administration (FDA) (IND #49175). PTHrP(1–36) was aliquotted and packaged in individual dose vials at the University of Pittsburgh GMP facility and assessed for purity, sterility, amino acid content and bioactivity, as previously described (15,19–26). Individual vials of lyophylized PTHrP(1–36) were stored at −80° until dispensed, then in a home freezer, and were reconstituted with normal saline just prior to administration.
Study Design
This was an outpatient, 90-day comparison trial in which subjects were aware of the drug they received, but not the dose, if receiving PTHrP(1–36). The primary endpoints were changes in amino-terminal telopeptides of procollagen 1 (PINP) and carboxy-terminal telopeptides of collagen 1 (CTX). Secondary outcome measurements included safety parameters (serum total and ionized calcium, phosphorous, creatinine, urine calcium and phosphorous, blood pressure and pulse), 1,25(OH)2D, and BMD changes at the LS, TH, FN and forearm. All subjects underwent screening labs which were analyzed at the time of the screening visit (serum calcium, intact PTH, 25OHD, and hematocrit) and a screening/baseline DXA. Eligible subjects were randomized to study drug within 60 days. Subjects were instructed in self-injection at the baseline visit, and seen for follow-up at day 15, 30, 60 and 90 for blood and urine collection, physical exam, assessment of adverse events (AE’s), drug compliance by diary and unused vials/doses, and an update in medications, medical history, and calcium intake. Calcium intake was assessed based on a food frequency questionnaire assessing weekly consumption of 22 dairy and non-dairy high and moderate calcium-containing foods (15, 27). In addition, all subjects were studied on the clinical research unit for six hours after the last dose of study drug, as described in Results. A second DXA scan was obtained at day 90. Subjects collected a 24 hour urine for calcium, creatinine and phosphorous at baseline and on day 90; second morning voids were collected at each visit. Subjects were asked to continue their usual intake of calcium and vitamin D throughout the study, unless adjustments were needed due to hypercalcemia. No additional calcium or vitamin D supplements were given.
Hypercalcemia Algorithm
Prior to initiation of the study, the following algorithm was planned, and was followed during the study. Mild hypercalcemia (serum calcium >10.5 mg/dl and <11.0 mg/dl) would be confirmed by repeating the test within 48 hours, and, if confirmed, would be addressed by decreasing calcium intake and repeating labs in seven days. For more significant hypercalcemia (calcium = 11.1–11.9 mg/d), study drug would be held and the lab repeated within 48 hours. If the repeat lab were still in the same range, the drug dose would be reduced by a 50% for those randomized to receive PTHrP(1–36) and discontinued in those receiving PTH(1–34). Labs would be repeated in seven days and study drug would be discontinued in any PTHrP(1–36) subject if the serum calcium remained >11.0 mg/dl after dose reduction. If serum calcium were ≥12.0 mg/d, calcium supplements and study drug would be withheld and labs repeated in 48 hours, and the above protocol followed.
Serum and Urine Biochemistries
Serum and urine were analyzed immediately after collection at each visit in the Clinical Chemistry Laboratory at the University of Pittsburgh Medical Center for total and ionized serum calcium, phosphorus, albumin, creatinine, and spot urine calcium, phosphorus, and creatinine. The fractional excretion of calcium (FECa) and tubular maximum for phosphorous (TmP/GFR) were calculated as previously described (26). Intact PTH and 25(OH)D were measured by University of Pittsburgh laboratories over an 18 month period from 2/17/2010 to 10/28/2011 as follows: iPTH (Advia Centaur, Seimens, Deerfield IL, CV = 10.0%); and, 25(OH)D (liquid chromatography-tandem mass spectrometry, Waters Corp, Milford MA, CV D2 = 6.5%, and D3 = 8.9%). The laboratory participates in the CAP external vitamin D survey (28). Samples for all other measurements were frozen at the time of collection and stored at −80° for batch analysis at the completion of the study. 1,25(OH)2D was measured at Heartland Laboratory, Ames, IA by Dr. Ronald Horst using a competitive RIA assay as previously described (29) (inter-assay CV=12.6%, intra-assay CV = 9.8%). Cross reactivity with 25(OH)D is less than 0.01%. Markers of bone turnover were measured in a single batch as follows: PINP (Orion Diagnostics RIA, Espoo, Finland, intra-assay CV = 3.1%, inter-assay CV = 4.8%), and CTX (Crosslaps ELISA, Nordic Bioscience Diagnostics, Herlev, Denmark, intra-assay CV = 7.7%, inter-assay CV =7.5%)
Bone Densitometry
All subjects underwent a screening DXA of the AP LS, right and left hip, and non-dominant forearm using a GE Lunar iDXA by the same trained study personnel. For those who qualified, the screening scan was used as the baseline measurement and a repeat scan was done at completion of the study for those who completed the entire 90 days. All scans were reviewed in a blinded fashion by a single investigator at the completion of the study and were unblinded by a different investigator after all blinded data were entered into the study database.
Statistics
Sample Size Estimate
The sample size for this study was determined considering the expected effects of PTHrP(1–36) (combined doses) relative to PTH(1–34) on the primary endpoints of PINP and CTX after three months of treatment relative to baseline using previously published data (9,10,21) as well as an anticipated attrition rate. Using these data, an equivalence test of means for the percentage change in PINP and CTX using two one-sided tests applied to data from the parallel group design with a sample size of 31 in the PTH(1–34) group and 62 in the combined PTHrP(1–36) group achieved 80% power at 2.5% significant level for two-sided hypothesis testing (adjusted for the hypothesis testing at two key endpoints). To conservatively adjust for an 11% attrition over the three months, 35 subjects per group (105 total) were enrolled to have at least 93 complete the study.
Data Analysis
Data were analyzed using SAS (version 9.3, SAS Institute, Inc., Cary, NC). Data were first screened to identify any data anomalies (e.g. outliers, missing data, etc.) prior to the main analyses. Groups were compared using either F-tests via the one-way analysis of variance or the Kruskal-Wallis procedure using exact methods for continuous variables and contingency table analyses with chi-square tests of independence or Fisher exact tests for categorical variables.
An “intent-to-treat” approach was the primary method for handling protocol deviations when addressing research aims during data analysis. Since the study endpoints of interest (PINP and CTX) were measured longitudinally, repeated measures analyses via linear mixed modeling were used to investigate the effect of PTHrP(1–36) versus PTH(1–34). Models included fixed effects for the within-subjects factor of time and the between-subjects factor of treatment and their two way interaction.
A similar analysis strategy was also used to examine the effect of PTHrP(1–36) and PTH(1–34) on the secondary study. For BMD, percentage change scores from baseline to 3 months were computed and compared among three groups using either F-tests via the one-way analysis of variance (parametric) or the Kruskal-Wallis (nonparametric) procedure using exact methods.
Results
Demographics
The baseline demographics of the 105 subjects are shown in Table 1. There were no differences in age, height, weight, BMI, or calcium intake. As per protocol, all subjects were vitamin D-replete at screening. However, there was a small difference in baseline 25(OH)D levels among the three groups (Table 1). There was no difference in baseline PTH among the groups.
Table 1.
Baseline Demographics
| PTHrP(1–36) 400ug (n=35) | PTHrP(1–36) 600ug (n=35) | PTH(1–34) 20ug (n=35) | p value | ||||
|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | ||
| Age (years) | 60.6 | 0.9 | 61.2 | 0.9 | 62.4 | 0.9 | ns |
| Height (cm) | 162.6 | 1.1 | 159.8 | 1.2 | 160.8 | 1 | ns |
| Weight (kg) | 62.4 | 1.5 | 63.4 | 1.5 | 63.3 | 1.2 | ns |
| BMI (kg/m2) | 23.6 | 0.4 | 24.8 | 0.5 | 24.5 | 0.5 | ns |
| Diet Ca (mg/d) | 815.5 | 65.5 | 761.2 | 60.4 | 716.1 | 57.0 | |
| Ca Suppl (mg/d) | 594.3 | 91.8 | 567.7 | 94.3 | 553.4 | 87.1 | |
| Total CA intake (mg/d) | 1409.7 | 107.3 | 1328.9 | 110.5 | 1269.5 | 95.7 | ns |
| PTH (pg/ml) | 31.6 | 2.1 | 31.3 | 2 | 37.2 | 2.4 | ns |
| 25OHD (ng/ml) | 40.3 | 2.1 | 39.4 | 1.7 | 34 | 1.8 | 0.04 |
| PINP (ng/ml) | 63.7 | 3.4 | 78.7 | 10.6 | 54.2 | 2.6 | ns |
| CTX (ng/l) | 0.54 | 0.044 | 0.71 | 0.078 | 0.53 | 0.046 | ns |
| Lumbar Spine (T-score) | −2.04 | 0.15 | −2.01 | 0.16 | −2.17 | 0.17 | ns |
| Total Hip (T-score) | −1.8 | 0.1 | −1.76 | 0.09 | −1.86 | 0.08 | ns |
| Fem Neck (T-score) | −2.14 | 0.08 | −2.14 | 0.08 | −2.28 | 0.08 | ns |
| Total Forearm (T- score) | −2.44 | 0.19 | −2.32 | 0.18 | −2.62 | 0.16 | ns |
Effects on Markers of Bone Turnover
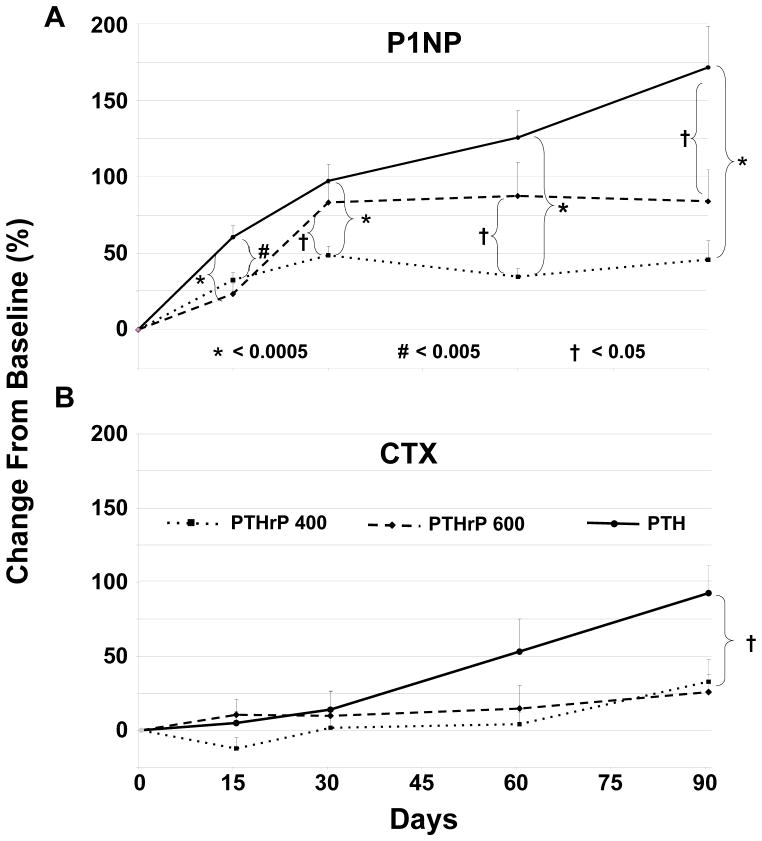
There were no differences among the groups in terms of baseline bone formation, as measured by PINP, or bone resorption, as measured by CTX (Table 1). There was an early (day 15) and significant (p<0.0005) increase in PINP in all three groups, which was sustained through the 90 days of the study (Fig 2A). The increase in PINP in the PTH(1–34) group was significantly greater than both doses of PTHrP(1–36) at all times, and by completion of the study was two- to four-fold greater than the response to the PTHrP(1–36) 600 or 400 ug/d doses, respectively. In contrast, the increases in CTX occurred later [day 60 for PTH(1–34) and day 90 for both PTHrP (1–36) groups] and were less robust than the changes in PINP (Fig 2B). The change at day 90 was three-fold greater for the PTH(1–34) group than either of the PTHrP(1–36) (p<0.05) groups, which were not different from each other.
Figure 2. Bone Turnover Markers in the Three Groups.
The three groups are indicated by the patterns shown in the legend within the figure. Bars indicate SEM, and the *, # and † symbols refer to statistical significance comparing the three groups as described in Panel A. Results are presented as percent change from baseline. A. The bone formation marker, PINP, increased in all three groups by day 15 (p<0.0005), and was sustained throughout the 90 days. The increase in the PTH (1–34) group was significantly greater than for both doses of PTHrP(1–36), as shown by the brackets in the panel. B. Bone resorption as assessed using CTX increased later than the increase in PINP, at day 60 in the PTH(1–34) group and at day 90 in the PTHrP(1–36) groups. The change at day 90 was significantly greater in the PTH(1–34) group compared to both PTHrP(1–36) groups (p<0.05), which were not different from each other. Baseline values were not different among the three groups (Table 1).
Effects on Bone Mineral Density
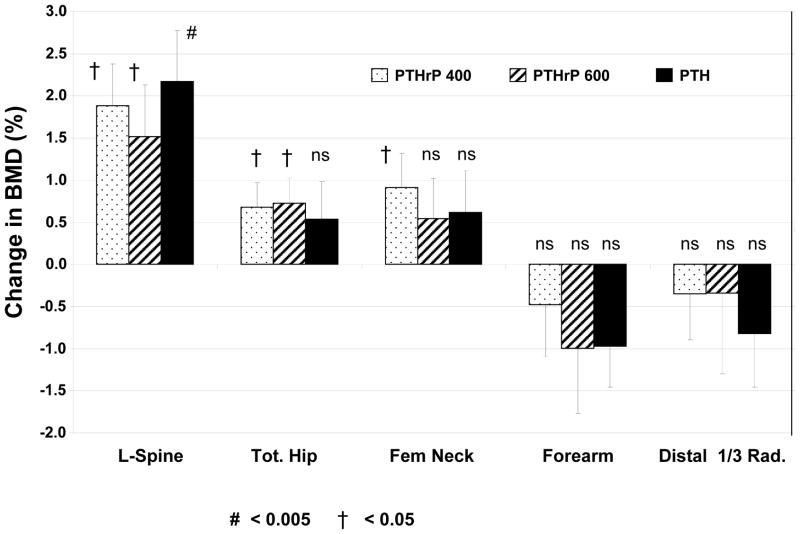
The baseline BMD values were not different between groups at any site among the three groups (Table 1). As per study design, the subjects had baseline BMD T-scores that were below −2.0. There was no significant difference in BMD change among the three groups at any site. LS BMD increased equivalently and significantly by day 90 in all three groups (p<0.05 compared to baseline for all) (Fig 3). TH and FN BMD increased equivalently in all three groups but was significant only for the two PTHrP(1–36) groups at the TH (p<0.05 vs baseline), and for the PTHrP(1–36) 400 group at the FN (p<0.05 vs baseline). There was no significant change in the forearm BMD in any group.
Figure 3. Changes in Bone Mineral Density in the Three Groups.
The three groups are indicated by the patterns shown in the legend within the figure. There was no significant difference in BMD change between groups at any site. Bars indicate SEM, and the # and † symbols refer to statistical significance compared baseline values as described in the Figure. LS BMD increased equivalently and significantly by day 90 in all three groups. TH and FN BMD increased equivalently in all three groups, but was significant only for the two PTHrP(1–36) groups (p<0.05 vs baseline) at the TH, and for the PTHrP(1–36) 400 group at the FN (p<0.05 vs baseline). There was no significant change in the forearm BMD in any group. Results are presented as percent change from baseline. Baseline values are shown in Table 1.
Effects on Serum and Urine Minerals
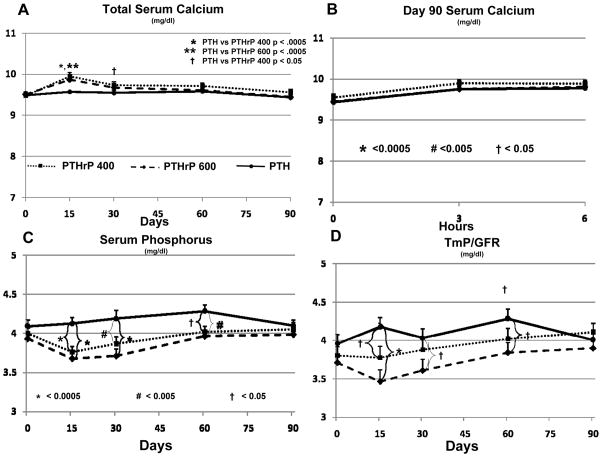
There were no significant differences in baseline values for serum total, phosphorous, creatinine or urine calcium, phosphorous, creatinine, FECa or TmP/GFR (Fig 4, 5). PTHrP(1–36) 400 ug/d induced a small but significant increase in serum calcium on days 15 and 30 (Fig 4A). The serum calcium was significantly greater in the PTHrP(1–36) 400 group when compared to the PTH(1–34) group at day 15 (p<0.0005) and day 30 (p<0.05) but did not differ from the PTHrP(1–36) 600 group. This increase was significant on both day 15 (mean = 9.95 mg/dl) and day 30 (mean = 9.73 mg/dl) compared to baseline (mean = 9.48 mg/dl) (p<0.0005 for both). Hypercalcemia (serum calcium >10.5 mg/dl) occurred in ten subjects in the PTHrP (1–36) 400 group, but normalized spontaneously (n=2), with lowering calcium intake to 500 mg/day (n=4), by holding study drug for 48 hours (n=4) or both (n=2) and then resuming the same drug dose (Suppl Table 1). No subject in this group required a dose reduction, and none was discontinued due to hypercalcemia.
Figure 4. Mineral Metabolism in the Three Groups.
The three groups are indicated by the patterns shown in the legend within Panel A. Bars indicate SEM, and the *, # and † symbols refer to statistical significance as described in Panel B. A. There was a mild but statistically significant increase in serum calcium as compared to baseline in the PTHrP(1–36) groups, but not the PTH(1–34) group. The serum calcium was significantly greater in the PTHrP(1–36) 400 group when compared to the PTH(1–34) group at day 15 and day 30, but did not differ from the PTHrP(1–36) 600 group. This increase was significant on both day 15 and day 30 compared to baseline (p<0.0005 for both. The mean serum calcium increased on day 15 in the PTHrP(1–36) 600 group and was significantly greater than the PTH(1–34) group at this time point but no different from the other PTHrP (1–36) group. The mean serum calcium in the PTHrP(1–36) 600 group at day 15 was also significantly increased compared to baseline (p<0.0005) B. Serum calcium increased transiently and comparably in all three groups at 3 and 6 hours after the last dose of PTH(1–34) or PTHrP(1–36) on Day 90. C and D Serum phosphorus was minimally but significantly higher in the PTH(1–34) group compared to the PTHrP(1–36) groups on days 15, 30, and 60, but not different at baseline nor day 90. Similarly the renal phosphorus threshold was minimally, but significantly, higher in the PTH(1–34) group compared to both PTHrP(1–36) groups on days 15, and compared to the PTHrP(1–36) 600 group on day 30, and 60, but not different at baseline or day 90.
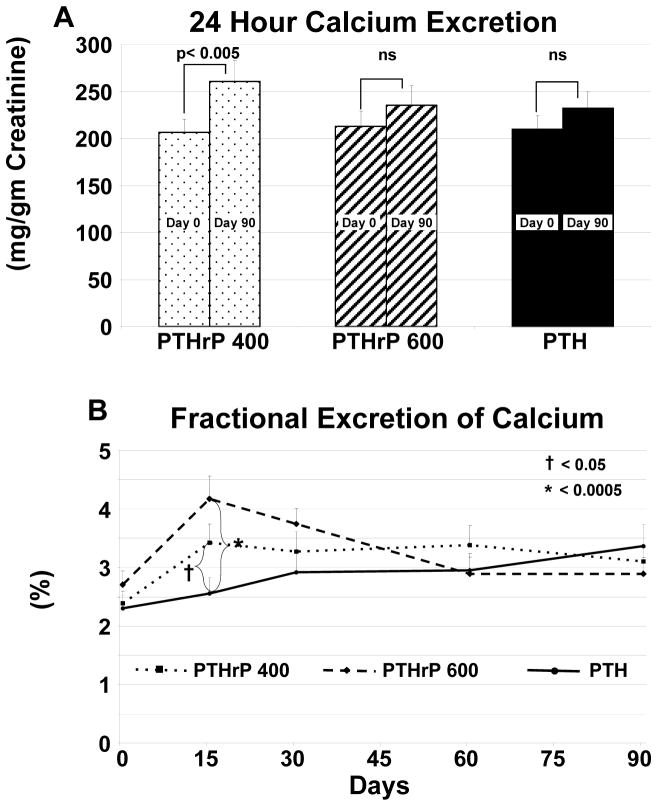
Figure 5. Urinary Calcium Excretion.
The three groups are indicated by the patterns shown in the legend within the figure. Bars indicate SEM, and the *, # and † symbols refer to statistical significance as described below Panel A and in Methods. A. There was no difference in 24-hour urine calcium excretion among the three groups at baseline or on Day 90. There was a significant increase in the PTHrP(1–36) 400 ug/d group on day 90 compared to baseline and an upward trend in the other two groups, which was not significant from baseline. B. Fractional excretion of calcium increased early (day 15) in the two PTHrP(1–36) groups, and rose to a lesser extent and more gradually in the PTH(1–34) group. FECa was significantly greater in the PTHrP(1–36) 400 and 600 groups only on day 15 compared to the PTH (1–34). FECa remained elevated in the PTHrP(1–36) 400 ug/d group compared to baseline (p<0.005) throughout the study, and in the PTHrP(1–36) 600 ug/d group only at day 15 and 30 (p<0.05). FECa increased in the PTH(1–34) group at day 90 (p <0.005).
The mean serum calcium also increased on day 15 in the PTHrP(1–36) 600 group and was significantly greater than the PTH(1–34) group at this time point (p<0.05), but no different from the other PTHrP(1–36) group (Fig 4A). The mean serum calcium in the PTHrP(1–36) 600 group at day 15 (mean = 9.87 mg/dl) was also significantly increased compared to baseline (mean = 9.51 mg/dl) (p<0.0005) (Fig 4A). Eight subjects in this group also developed hypercalcemia, requiring a decrease in drug dose to 300 μg/d in three subjects. The serum calcium normalized in the remaining five with a lower calcium diet (n=5) in combination with holding study drug for 48 hours (n = 2) (Suppl Table 1). No subject was terminated due to hypercalcemia. There was no significant change in serum calcium, nor any hypercalcemia in the group receiving PTH(1–34). Similar changes were seen for ionized calcium (S Fig 1).
Of the 105 subjects enrolled, 90 completed the study. All were observed on the clinical research unit after the final drug dose and had labs assessed at 0, 3 and 6 hours post-dose (Fig 4B). Total serum calcium was not different at baseline. There was a small but significant increase from 0 to 3 hours and 0 to 6 hours post-dose for all three groups (Fig 4B) (p < 0.0005) but no difference among the three groups. Similar changes were seen for ionized calcium (S Fig 1).
Serum phosphorus and TmP/GFR were minimally but significantly higher in the PTH(1–34) group compared to the PTHrP(1–36) groups on days 15, 30, and 60, but not different at baseline or day 90 (Fig 4 C, D). Creatinine (not shown) was not different at baseline among the groups, and did not change substantially during the study.
There was no difference in 24-hour urine calcium excretion at baseline or on day 90 among the three groups (Fig 5A). There was a small but significant increase from day 0 to day 90 in the PTHrP(1–36) 400 ug/d group (p<0.005), and a non-significant upward trend in the other two groups. FECa, which was not different at baseline among the three groups, increased early (day 15) in the two PTHrP(1–36) groups and rose to a lesser extent and more gradually in the PTH(1–34) group. FECa was significantly greater in the PTHrP(1–36) 400 and 600 groups on day 15 compared to the PTH (1–34) group at this time point (p<0.05, and p<0.0005 respectively) (Fig 5B). There were no differences between the two PTHrP(1–36) groups at any time point, and no differences from PTH(1–34) except at day 15. FECa remained elevated in the PTHrP (1–36) 400 group compared to baseline (p<0.005) throughout the study and in the PTHrP(1–36) 600 ug/d group only at day 15 and 30 (p<0.05). FECa increased in the PTH(1–34) group at day 90 (p <0.005).
Effects on 1,25(OH)2D
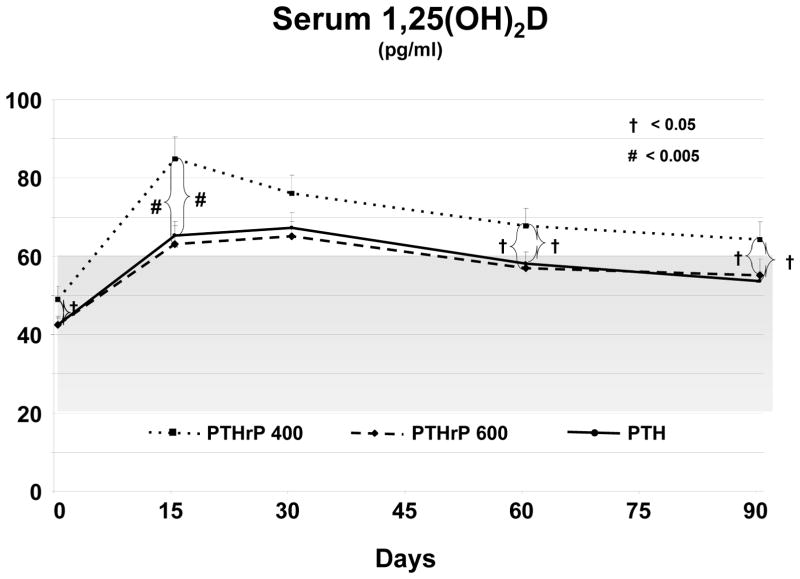
There was a marginally significant difference in the baseline 1,25(OH)2D values among the three groups (p <0.05) (Fig 6). After entering the study, all three groups experienced a sustained and significant increase in 1,25(OH)2D, which began at day 15 and was greatest for PTHrP(1–36) 400 ug/d group compared to the PTHrP (1–36) 600 and PTH (1–34) group on days 15, 60 and 90. (Fig 6).
Figure 6. Changes in 1,25(OH)2D in the Three Groups.
The three groups are indicated by the patterns shown in the legend within the figure. Bars indicate SEM, and the *, # and † symbols refer to statistical significance as described in the figure and in Methods. The shaded grey area indicates the normal range for 1,25(OH)2D. There was a marginally significant difference in the baseline 1,25(OH)2D values among the three groups. Serum 1,25(OH)2D rose substantially in each of the three groups by day 15 and remained greater than baseline throughout the 90 day study. The increase was greatest in the PTHrP(1–36) 400 group which was significantly greater than the PTHrP (1–36) 600 and PTH (1–34) groups on days 15, 60, and 90.
Adverse Events
There were no serious adverse events. Mild or moderate adverse events (AE) were similar among the three groups (Table 2). In spite of this, there were a greater number of terminations in the two PTHrP(1–36) groups compared to the PTH(1–34) group. In the PTHrP(1–36) 400 ug/d group, there were six terminations: three for AEs, one for a study exclusion (traumatic fracture) and two for personal reasons. In the PTHrP(1–36) 600 ug/d group, there were seven terminations: four for AEs, two for study exclusions (traumatic fracture, glucocorticoids) and one for personal reasons. Two PTH(1–34) subjects terminated early, one for AE’s and one for a study exclusion (pacemaker placement). There was no association between termination for AE’s and hypercalcemia.
Table 2.
Adverse Events
| PTHrP(1–36) 400ug (n=35) | PTHrP(1–36) 600ug (n=35) | PTH(1–34) 20ug (n=35) | p value | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Dermatologic | 19 | (54%) | 23 | (66%) | 14 | (40%) | ns |
| Musculoskeletal | 7 | (20%) | 8 | (23%) | 10 | (29%) | ns |
| Neurologic | 11 | (31%) | 14 | (40%) | 9 | (26%) | ns |
| Psychological | 5 | (14%) | 11 | (31%) | 12 | (34%) | ns |
| Gastrointestinal | 13 | (37%) | 12 | (34%) | 11 | (31%) | ns |
| Infection | 4 | (11%) | 4 | (11%) | 3 | (9%) | ns |
| Cardiovascular | 0 | (0%) | 4 | (11%) | 2 | (6%) | ns |
| Genitourinary | 3 | (9%) | 0 | (0%) | 2 | (6%) | ns |
| Other | 17 | (49%) | 15 | (43%) | 20 | (57%) | ns |
| Total Adverse Events | 79 | 91 | 83 | ||||
Discussion
This report describes the first head-to-head comparison of PTH(1–34) versus PTHrP(1–36) for the treatment of low bone density and osteoporosis. The principal observations are that both peptides stimulate bone formation; both peptides performed similarly with respect to changes in spine BMD; PTHrP(1–36) generated less bone resorption but paradoxically was associated with more frequent hypercalcemia, albeit mild and transient; and, both peptides stimulated remarkable increases in 1,25(OH)2D. Adverse event profiles were modest and comparable.
The primary endpoints of the study were changes in bone turnover markers. PTH(1–34) induced an early (15 days) and robust (171%) increase in bone formation as assessed using PINP, accompanied by a later (60 days), but still robust (92%), increase in bone resorption as assessed using CTX. This pattern has been observed in many prior studies with PTH(1–34) (9,10). In contrast, as also described previously (21), PTHrP(1–36) resulted in lesser increases in PINP (46–87%), but also minimal increases in CTX (25–30%). The increases in BMD described here and previously demonstrate that although PTH(1–34) increases both resorption as well as formation, the net overall effect of PTH(1–34) is anabolic (9, 10, 14, 30). The picture revealed by PTHrP(1–36) suggests lesser effects on bone formation as well as lesser effects on resorption, also resulting in a net anabolic effect (15). This net anabolic effect also led to increases in BMD at the spine that were comparable to those observed for PTH(1–34). Since these studies were limited for regulatory reasons to three months, it is remains uncertain whether longer studies might reveal a more pronounced effect of PTHrP(1–36) on resorption. Thus, from an overall efficacy standpoint, PTHrP(1–36) induced similar effects to PTH(1–34) on BMD, with a suggestion that PTHrP(1–36) may yield advantages at certain sites such as hip and femoral neck.
In contrast to these beneficial effects, PTHrP(1–36) induced hypercalcemia more frequently than PTH(1–34), which was associated with little or no hypercalcemia in this study. This was a surprising observation for the 400 ug/d PTHrP(1–36) dose, because hypercalcemia had not been observed in prior studies using this dose (15, 20, 21). We attribute this to differences in potency of the PTHrP(1–36) preparation used in the current study, which was synthesized using f-moc chemistry, as compared to our prior studies where the PTHrP(1–36) was synthesized using t-boc chemistry (15, 20, 21). Thus, it is possible that the PTHrP(1–36) used in the current study might have been more potent than that used in prior studies. This difference must have been small, because it was not detected in the adenylyl cyclase bioassay or amino acid analyses used to assess peptide potency and amount, and because the hypercalcemia was modest and, in most cases, self-limited. Nonetheless, the occurrence of hypercalcemia would make monitoring of serum calcium a requirement if PTHrP(1–36) would be used at this dose in future studies. On the other hand, lower doses and/or less frequent dosing of PTHrP(1–36) might eliminate the occurrence of hypercalcemia, a hypothesis that would be attractive to assess in future studies.
The increase in circulating 1,25(OH)2D in response to PTH(1–34) in the setting of osteoporosis treatment has been observed by Cosman et al (31), but for PTHrP(1–36) is novel. We presume that the increases in 1,25(OH)2D, in conjunction with increases in bone resorption, in PTH(1–34)-treated subjects leads to the hypercalcemia that has been well described in subjects treated with PTH(1–34) in prior series (13). PTHrP(1–36) is a much weaker agonist of renal 1,25(OH)2D synthesis than PTH(1–34) (17). Nonetheless, we observed that PTHrP(1–36) also increased 1,25(OH)2D, albeit at doses much higher than those of PTH(1–34). Since PTH and PTHrP indirectly enhance intestinal calcium absorption via the increase in 1,25(OH)2D, this may reduce the requirement for oral calcium supplementation in subjects with osteoporosis treated with PTH(1–34) or PTHrP(1–36). The increases in 1,25(OH)2D may also contribute to the skeletal anabolic effects of both peptides.
With regard to adverse events, the results were similar with PTHrP(1–36) and PTH(1–34), with the exception of the more frequent occurrence of hypercalcemia in the PTHrP(1–36) groups. The hypercalcemia was mild, occurred principally at the 15–30 day time point, and then declined or disappeared spontaneously or with a reduction in calcium intake in most cases. Etiologically, it is unlikely that bone resorption caused hypercalcemia, since CTX was not increased at the 15–30 day time point when hypercalcemia occurred. Similarly, it is unlikely that excessive renal calcium reabsorption could have been responsible, since fractional calcium excretion did not decline, but rather increased. In addition, systemic renal effects of PTHrP(1–36) are short lived (23) and would not explain why the serum calcium was still increased 24 hours post-dose, long after the dose of PTHrP(1–36) would have been cleared. Thus, it is likely that the PTHrP(1–36)-associated hypercalcemia was related to excessive intestinal calcium absorption. In support of this possibility, the peak in serum calcium at 15–30 days correlated temporally with an increase in 1,25(OH)2D. Also, reducing calcium intake led to normalization of serum calcium in seven of 15 hypercalcemic subjects, supporting the “intestinal hyperabsorption absorption” argument. However, this hypothesis is not entirely satisfactory, since the serum 1,25(OH)2D increased in the PTH(1–34) group as well, but they did not become hypercalcemic. Additionally the hypercalcemia was more prominent in subjects receiving the 600 ug/d PTHrP(1–36) dose, yet 1,25(OH)2D values were higher in those receiving 400 ug/d. One might speculate that the less robust increase in bone formation at day 15 in the PTHrP(1–36) groups compared to the PTH group, despite similar increases in 1,25(OH)2D, also contributed to the hypercalcemia.
This study has limitations. First, the increments in BMD in this study were smaller than those reported in many studies focusing on PTH or PTHrP. For example, the LS changes in BMD for PTH and PTHrP at three months are commonly in the 3–5% range (9, 15), yet in the current study, were in the 2% range. This most likely reflects the decision to enroll subjects with low bone density or osteoporosis. This decision was intentional, and is common in more recent osteoporosis clinical trials in order to reduce the risk of fracture in subjects in the experimental arms, and to enhance study subject recruitment (32).
Second, while it was randomized, this study was not double-blinded: investigators were blinded while interpreting DXA scans, but it was not possible to completely blind the subjects, since the subjects receiving PTH(1–34) used the “pen” purchased from the manufacturer of the PTH(1–34), while there was no analogous “pen” for administering PTHrP(1–36). It is unlikely that this influenced the outcome measures, however, since the three groups were randomly assigned and were demographically well matched, and since the main outcomes (bone turnover markers, BMD and other biochemical measures) are not subject to manipulation by the study subjects.
Third, there was no placebo group in this study. This design was also intentional, since adding a fourth study group would have delayed recruiting and increased cost, and since both PTH(1–34) and PTHrP(1–36) have been compared amply to placebo previously (13, 15).
Lastly, subjects in all three groups had higher than anticipated calcium intake and baseline vitamin D levels. This reflects in part the fact that subjects with vitamin D deficiency were excluded from the study. It is also compatible with a participant bias such that women who volunteer for a current osteoporosis study may be more likely than in the past to use calcium supplements. Therefore, these results may not apply to less calcium and vitamin D replete population.
Overall, PTHrP(1–36) appears to be similar to PTH(1–34) in terms of efficacy, safety and tolerability in patients with postmenopausal low bone density or osteoporosis. Future studies with PTHrP(1–36) are needed to determine the optimal doses, dosing intervals, and duration of use to optimize efficacy while eliminating the incidence of hypercalcemia. In addition, it may be of interest to compare PTHrP(1–36) with non-injectable forms of PTH under development, to define its role as a potential future therapy for osteoporosis.
Supplementary Material
Acknowledgments
The authors would like to thank the members of our DSMB including Drs. Elizabeth Shane, Susan Greenspan, David Roodman and Steven Wisniewski, and the staff of the University of Pittsburgh CTRC and CTSI for their outstanding support with this study. We also acknowledge Ronald Horst PhD for his assistance in performing the vitamin D assays. This work was funded by NIH grants R-01 DK 073039 and DK 51081, and CTSA UL1 RR024153 and M-01 RR000056.
Footnotes
Registered with Clinical Trials: NCT0853723
Supplemental data has been included
Author Disclosure
Author Disclosure Summary: MA, LK, EM, CCO, RMC, MBT, AL, SMS, CMG, AB, and AGO have no conflicts of interest. MJH is a consultant for NPS. JAC is a consultant for Merck and Novartis. AFS is a member of Osteotrophin LLC.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. Epub 2006/12/06. [DOI] [PubMed] [Google Scholar]
- 2.Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC, 3rd, Yanover MJ, Mysiw WJ, Kohse L, Rao MB, Steiger P, Richmond B, Chesnut CH., 3rd Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323(2):73–9. doi: 10.1056/NEJM199007123230201. Epub 1990/07/12. [DOI] [PubMed] [Google Scholar]
- 3.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333(22):1437–43. doi: 10.1056/NEJM199511303332201. Epub 1995/11/30. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. Epub 1996/12/07. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82. doi: 10.1001/jama.280.24.2077. Epub 1999/01/06. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. Epub 1999/10/12. [DOI] [PubMed] [Google Scholar]
- 7.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344. Epub 1999/10/20. [DOI] [PubMed] [Google Scholar]
- 8.Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, Abu Elnour NO, Erwin PJ, Hazem A, Puhan MA, Li T, Montori VM. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80. doi: 10.1210/jc.2011-3060. Epub 2012/04/03. [DOI] [PubMed] [Google Scholar]
- 9.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–8. doi: 10.1001/archinte.165.15.1762. Epub 2005/08/10. [DOI] [PubMed] [Google Scholar]
- 10.Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Koukoulis GN, Efstathiadou Z, Kita M, Avramidis A. Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: a randomised trial. Int J Clin Pract. 2008;62(6):919–24. doi: 10.1111/j.1742-1241.2008.01768.x. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 11.Dempster DW, Zhou H, Recker RR, Brown JP, Bolognese MA, Recknor CP, Kendler DL, Lewiecki EM, Hanley DA, Rao DS, Miller PD, Woodson GC, 3rd, Lindsay R, Binkley N, Wan X, Ruff VA, Janos B, Taylor KA. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97(8):2799–808. doi: 10.1210/jc.2012-1262. Epub 2012/06/16. [DOI] [PubMed] [Google Scholar]
- 12.Han SL, Wan SL. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials. Int J Clin Pract. 2012;66(2):199–209. doi: 10.1111/j.1742-1241.2011.02837.x. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 13.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. Epub 2001/05/11. [DOI] [PubMed] [Google Scholar]
- 14.Capriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: A decade of experience. J Bone Miner Res. 2012;27(12):2419–28. doi: 10.1002/jbmr.1800. Epub 2012/11/21. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocana A, Stewart AF. Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88(2):569–75. doi: 10.1210/jc.2002-021122. Epub 2003/02/08. [DOI] [PubMed] [Google Scholar]
- 16.Hattersley G, Bilezikian J, Guerriero J, Kumar P, Zanchetta J, Lyttle CR, O’Dea LSL, editors. Bone anabolic efficacy and safety of BA 058, a novel analog of hPTHrP: results from a phase 2 clinical trial in postmenopausal women with osteoporosis. Endocrine Reviews.Endo 2012, the Endocrine Society’s 94th annual meeting and expo; 2012; Houston, TX. 2012. [Google Scholar]
- 17.Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocana A, Bisello A, Hollis BW, Rosen CJ, Wysolmerski JJ, Dann P, Gundberg C, Stewart AF. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res. 2005;20(10):1792–803. doi: 10.1359/JBMR.050602. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz MJ, Tedesco MB, Sereika SM, Prebehala L, Gundberg CM, Hollis BW, Bisello A, Garcia-Ocana A, Carneiro RM, Stewart AF. A 7-day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J Bone Miner Res. 2011;26(9):2287–97. doi: 10.1002/jbmr.415. Epub 2011/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart AF, Cain RL, Burr DB, Jacob D, Turner CH, Hock JM. Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1–34, parathyroid hormone-related protein 1–36, and SDZ-parathyroid hormone 893. J Bone Miner Res. 2000;15(8):1517–25. doi: 10.1359/jbmr.2000.15.8.1517. Epub 2000/08/10. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz MJ, Tedesco MB, Sereika SM, Garcia-Ocana A, Bisello A, Hollis BW, Gundberg C, Stewart AF. Safety and tolerability of subcutaneous PTHrP(1–36) in healthy human volunteers: a dose escalation study. Osteoporos Int. 2006;17(2):225–30. doi: 10.1007/s00198-005-1976-3. Epub 2005/09/10. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz MJ, Tedesco MB, Garcia-Ocana A, Sereika SM, Prebehala L, Bisello A, Hollis BW, Gundberg CM, Stewart AF. Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: defining the maximal tolerable dose. J Clin Endocrinol Metab. 2010;95(3):1279–87. doi: 10.1210/jc.2009-0233. Epub 2010/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry JG, Mitnick M, Dann PR, Stewart AF. Parathyroid hormone-related protein-(1–36) is biologically active when administered subcutaneously to humans. J Clin Endocrinol Metab. 1997;82(3):900–6. doi: 10.1210/jcem.82.3.3811. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin H, Gundberg C, Mitnick M, Stewart AF. Dissociation of bone formation from resorption during 2-week treatment with human parathyroid hormone-related peptide-(1–36) in humans: potential as an anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 1998;83(8):2786–91. doi: 10.1210/jcem.83.8.5047. Epub 1998/08/26. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AF, Mangin M, Wu T, Goumas D, Insogna KL, Burtis WJ, Broadus AE. Synthetic human parathyroid hormone-like protein stimulates bone resorption and causes hypercalcemia in rats. J Clin Invest. 1988;81(2):596–600. doi: 10.1172/JCI113358. Epub 1988/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everhart-Caye M, Inzucchi SE, Guinness-Henry J, Mitnick MA, Stewart AF. Parathyroid hormone (PTH)-related protein(1–36) is equipotent to PTH(1–34) in humans. J Clin Endocrinol Metab. 1996;81(1):199–208. doi: 10.1210/jcem.81.1.8550752. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 26.Syed MA, Horwitz MJ, Tedesco MB, Garcia-Ocana A, Wisniewski SR, Stewart AF. Parathyroid hormone-related protein-(1--36) stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancy. J Clin Endocrinol Metab. 2001;86(4):1525–31. doi: 10.1210/jcem.86.4.7406. Epub 2001/04/12. [DOI] [PubMed] [Google Scholar]
- 27.Rizzoli R, Greenspan SL, Bone G, 3rd, Schnitzer TJ, Watts NB, Adami S, Foldes AJ, Roux C, Levine MA, Uebelhart B, Santora AC, 2nd, Kaur A, Peverly CA, Orloff JJ Alendronate Once-Weekly Study Group. Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res. 2002 Nov;17(11):1988–96. doi: 10.1359/jbmr.2002.17.11.1988. [DOI] [PubMed] [Google Scholar]
- 28.Vogeser M, Kyriatsoulis A, Huber E, Kobold U. Candidate reference method for the quantification of circulating 25-hydroxyvitamin D3 by liquid chromatography-tandem mass spectrometry. Clin Chem. 2004;50(8):1415–7. doi: 10.1373/clinchem.2004.031831. Epub 2004/07/28. [DOI] [PubMed] [Google Scholar]
- 29.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42(4):586–92. Epub 1996/04/01. [PubMed] [Google Scholar]
- 30.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20(6):962–70. doi: 10.1359/JBMR.050105. Epub 2005/05/11. [DOI] [PubMed] [Google Scholar]
- 31.Cosman F, Dawson-Hughes B, Wan X, Krege JH. Changes in vitamin D metabolites during teriparatide teatment. Bone. 2012;50:1368–71. doi: 10.1016/j.bone.2012.02.635. Epub 2012 Mar 9. [DOI] [PubMed] [Google Scholar]
- 32.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–45. doi: 10.1210/jc.2009-1703. Epub 2010/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.