Abstract
Since intracellular proteins involved in carcinogenesis have been shown to provoke autoantibody responses, autoantibodies can be used as probes in immunoproteomics to isolate, identify, and characterize potential tumor-associated antigens (TAAs). Once a TAA is identified, several approaches will be used to comprehensively characterize and validate the identified TAA/anti-TAA systems that are potential biomarkers in certain types of cancer. Our ultimate goal is to establish rigorous criteria for designation of an autoantibody to a TAA as a cancer biomarker, examine candidate TAAs for sensitivity and specificity of anti-TAA antibody response, and further develop customized TAA arrays that can be used to enhance anti-TAA antibody detection in cancer. This review will mainly focus on the recent advances in our studies using immunoproteomic approach to identify and characterize TAAs as biomarkers in cancer.
Keywords: autoantibody, tumor-associated antigen, immunoproteomics, immunodiagnosis, cancer
1. Introduction
Cancer has long been recognized as a multi-step process, which involves not only genetic changes conferring growth advantage but also factors that disrupt regulation of growth and differentiation [1–3]. It is possible that some of these factors could be identified and their functions evaluated with the aid of autoantibodies arising during tumorigenesis. Although the mechanisms leading to autoantibody production in cancer patients are not completely understood, emerging evidence indicates that most tumor-associated antigens (TAAs) are cellular proteins whose aberrant regulation of function could be linked to malignancy [4]. Several approaches are currently available for the identification of TAAs in cancer. One of the approaches is the utilization of serum antibodies from cancer patients to immunoscreen cDNA expression library to identify TAAs in cancer, and some of these identified TAAs may have potential diagnostic values in cancer diagnosis. Another approach involving the use of a proteome-based methodology, which is generally named as immunoproteomics, has been recently implemented in our laboratory for the identification of TAAs in cancer [5–7]. The practical utility of these approaches remains to be established with the proviso that efforts should be made to identify tumor-associated from tumor-irrelevant antigens. This review will mainly focus on the recent advances in our studies using immunoproteomic approach to identify and characterize TAAs as biomarkers in cancer.
2. Tumor-associated antigens (TAAs) and anti-TAA autoantibodies
As described above, many studies have demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens called TAAs [8, 9]. The types of cellular proteins that induce these autoantibody responses are quite varied and include the tumor suppressor p53 [10, 11], p16 [12], oncogene products such as c-Myc [13], HER-2/neu [14], and CIP2A/p90 [15, 16], proteins that protect mRNAs from degradation such as IMPs [17], onconeural antigens [18], differentiation-antigens such as tyrosinase, cancer/testis antigens [19], and anti-apoptotic proteins such as survivin [20] and LEDGF [21], etc. The different factors leading to the increased production of such autoantibodies are not completely understood. However, available data show that many of the target antigens are cellular proteins like p53 whose aberrant regulation or overexpression could lead to tumorigenesis [10, 11]. A highly informative study showed that in lung tumors containing several types of p53 gene mutations, including missense, stop codon and frameshift mutations, only the missense p53 mutations, with overexpression of a protein that altered function and increased the protein stability, correlated with autoantibody production [22]. In the case of the mRNA binding protein p62, a fetal protein absent in adult tissues, the presence of autoantibodies relates to abnormal expression of p62 in tumor cells [23]. The immune systems of certain cancer patients appear to sense these aberrant tumor-associated proteins as foreign antigens and have the capability to respond by producing autoantibodies which we generally called anti-TAA antibodies [24]. Thus, these ant-TAA antibodies might be regarded as reporters identifying aberrant de novo or dysregulated cellular mechanisms in tumorigenesis [4, 8, 9]. In recent years, the potential utility of TAAs and ant-TAA antibody systems as early cancer biomarker tools to monitor therapeutic outcomes or as indicators of disease prognosis has been extensively explored.
Interest in the use of anti-TAA antibodies as serological markers for cancer diagnosis derives from the recognition that these antibodies are generally absent, or present in very low titers, in normal individuals and in non-cancer conditions [8, 9, 25]. Their persistence and stability in the serum of cancer patients is an advantage over other potential markers, including the TAAs themselves, which are released by tumors but rapidly degrade or are cleared after circulating in the serum for a limited time [24]. Furthermore, the widespread availability of methods and reagents to detect serum autoantibodies facilitates their characterization in cancer patients and assay development. However, in contrast to autoimmune diseases, where the presence of a particular autoantibody may have diagnostic value, cancer-associated autoantibodies, when evaluated individually, have little diagnostic value primarily because of their low frequency. We have observed that this drawback can be overcome by using mini-arrays of carefully selected TAAs, and that different types of cancer may require different TAA arrays to achieve the sensitivity and specificity required to make immunodiagnosis a feasible adjunct to tumor diagnosis [26].
3. Immunoproteomic approach in the identification of TAAs
The methods which we have used in the identification of putative TAAs has involved initially examining the sera from cancer patients using extracts of tissue culture cells as source of antigens in Western blotting and by indirect immunofluorescence on whole cells. With these two techniques, we identify sera which have high-titer fluorescent staining or strong signals to cell extracts on Western blotting and subsequently use the serum antibodies either in isolating cDNA clones from cDNA expression libraries or in immunoproteomics to identify tumor-associated proteins. Using the approach of immunoscreening cDNA expression libraries, several novel TAAs including HCC1 [27], SG2NA [28], CENP-F [29], p62/IMP2 [30] and p90/CIP2A [15, 16] have been identified. Several novel as well as previously defined tumor antigens have been also identified with autoantibodies from patients with different types of cancer [31] using a methodology called SEREX (serological analysis of recombination cDNA expression libraries) [32], which is essentially a modification of our previous approach [27–30]. Immunoscreening of cDNA libraries with serum antibodies for identifications of autoantigens is a well-established method and has been used not only to identify TAAs but also antigens in autoimmune diseases [33]. This methodology was the basis of the methods described in SEREX with the difference that cDNA expression libraries constructed from autologous patient tumor were used as substrate in immunoscreening. Subsequent reports using the SEREX technique have shown that the TAAs identified are no different from standard methods using cDNA expression libraries from cell lines derived from different sources, so that there did not appear to be any advantage to using cDNA libraries from autologous patients.
In the past decade, the proteomic approach has been extensively implemented for identifying tumor-associated proteins in cancer patients [34,35]. Compared to the approach of immunoscreening cDNA expression libraries, which we have previously used, the immunoproteomic technology allows individual screening of a large number of sera, as well as determination of a large number of cancer-related antigens. The immunoproteomic approach can also distinguish isoforms and the detection of autoantibodies directed against post-translational modifications (PTMs) of specific targets. It is well known that mRNA levels do not necessarily correlate with corresponding protein abundance [36]. Additional complexity of protein is conferred by PTMs including phosphorylation, acetylation, and glycosylation, as well as protein cleavage [37]. These modifications may not reflect any change at the mRNA level but play important roles in protein stability, activity and functions. Intracellular proteins may also participate in the transformation of a healthy cell into a neoplastic cell. Therefore, protein levels may be more accessible and relevant to therapeutic targets than mRNA levels.
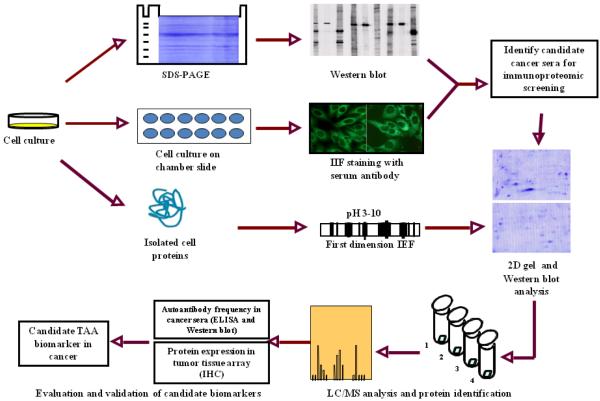
A brief description of the immunoproteomic approach we have used to identify and characterize TAAs is shown in Fig. 1. Briefly, the sera from cancer patients were initially examined using extracts of tissue culture cells as the source of antigens in western blot and by indirect immunofluorescence (IIF) on whole cells. With these two techniques, we identified sera that have high-titer fluorescent staining or strong signals to cell extracts on western blot and subsequently used the antibodies in these sera as probes in a proteomic approach to isolate potential TAAs. Cell extract of cultured cancer cells was applied onto the first dimension isoelectrofocusing gel (1D-IEF), and subsequently loaded onto the second-dimension gel (2D-SDS-PAGE). The protein was transferred to the nitrocellulose membrane or visualized by Coomassie blue staining (or silver staining). After immunoblotting with cancer sera and normal human sera (as controls), a number of protein spots of interest were excised from the 2D gels, digested by trypsin, and subsequently analyzed by mass spectrometry (MS). In subsequent studies, we will use several approaches such as enzyme-linked immunosorbent assay (ELISA), western blot and immunohistochemistry (IHC) with tissue arrays to comprehensively characterize and validate the identified tumor-associated antigen-antibody systems that are potentially useful in cancer immunodiagnosis, and then evaluate the sensitivity and specificity of different antigen-antibody systems as markers in certain type of cancer for further developing “TAA array” systems for cancer diagnosis, prediction, and for following the response of patients to treatment.
Figure 1.
Schematic representation of identification and validation of TAAs using immunoproteomic approach.
A potential pitfall in using an immunoproteomic approach is that most likely several potential TAAs will not be detected, because of various limitations of two-dimensional gel electrophoresis (2-DE) followed by in-gel trypsin digestion [38,39]. First, highly hydrophobic, very large, or highly post-translationally modified (i.e., glycosylated, phosphorylated) proteins are difficult to be resolved in the 2-DE. Secondly, in-gel digestion of proteins resolved by 2-DE results in very low recovery of peptides. In solving the problem, we suggest to use an immunoprecipitation approach and in-solution digestion of potential TAAs. Briefly, purified serum IgG from cancer patients or normal individuals will be labeled with Sulfo-NHS-biotin (Pierce Inc.) under physiological conditions to preserve antibody activity. The cancer cell lysate will be incubated with biotinylated cancer serum IgG (or with normal human serum IgG, as a control). Then, the cancer cell protein/cancer serum biotin-IgG complexes will be immunoprecipitated with streptavidin-sepharose; and after extensive washing, the specifically bound cancer cell proteins will be eluted with 1 M propionic acid and immediately lyophilized. Cancer cell proteins will be digested with trypsin and identified by LC-MS/MS analysis. Recently, this approach has been successfully used in our laboratory.
4. Potential TAAs identified in cancer and other disease with immunoproteomics
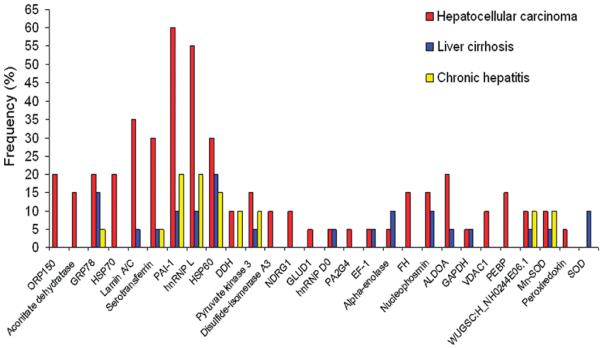
Recent several years, we have used immunoproteomic approach to extensively screen sera from patients with certain types of cancer such as hepatocellular carcinoma (HCC) and esophageal squamous cell carcinoma (ESCC), and sera from patients with pre-cancer condition such as liver fibrosis to identify and characterize the potential TAAs [5–7]. Here, we will briefly review the work we have done in HCC [5] and ESCC [6]. In the initial study, we have applied an immunoproteomic approach to screen sera from patients with HCC and pre-HCC conditions such as liver cirrhosis and chronic hepatitis as well as sera from normal individuals, and identified 28 HCC-associated tumor antigens [5]. In order to distinguish HCC-related and non-HCC-related proteins, the frequency of 28 identified proteins with sera from different conditions were analyzed. As shown in Fig. 2, 17 were reactive not only to serum antibodies in HCC but also in pre-HCC conditions, indicating these proteins might not be appropriate TAA markers in HCC detection, and 11 were only reactive with serum antibodies in HCC but not with antibodies in pre-HCC condition, suggesting these proteins could be potential TAA markers in HCC. In the further analysis, two representative proteins, heat shock protein 60 (HSP60) and heat shock protein 70 (HSP70), were selected as examples for the validation purpose. The results from the immunoassay were consistent with the data from proteomic analysis, supporting our hypothesis that proteins identified with autoantibodies that have appeared in pre-cancer conditions may be not appropriate to use as TAA markers in cancer detection.
Figure 2.
Frequency of immunoreactive proteins identified by autoantibodies in sera from patients with HCC and pre-HCC conditions. Of 28 identified proteins, seventeen proteins such as GRP78, sterotransferrin, HSP60 and so on were identified by antibodies in sera from patients with either HCC, liver cirrhosis or chronic hepatitis, and 11 proteins such as ORP150, aconitate dehydratase, HSP70 and so on were only identified by antibodies in sera from patients with HCC.
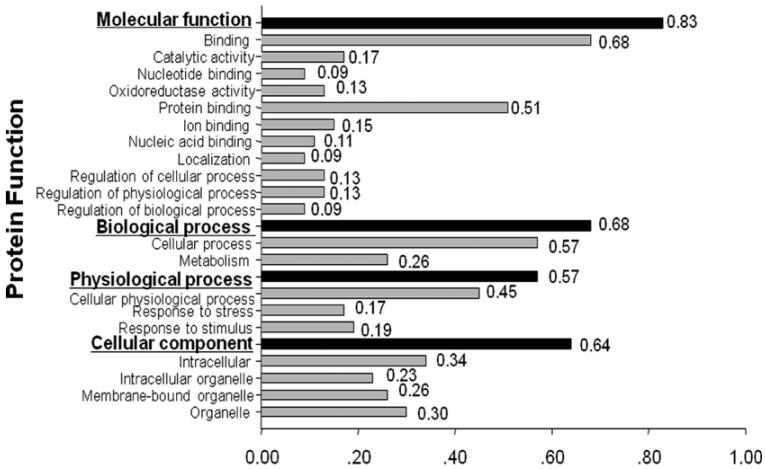
More recently, immunoproteomic approach was also used to identify and characterize cancer-associated proteins in ESCC [6]. Of 104 protein spots with different expression levels found on 2DE, 47 proteins were eventually identified by MALDI-TOF-MS. Among these identified proteins, 33 proteins including HSP70, high-mobility group box-1 (HMGB1), proteasome activator subunit 1 (PSME1), manganese superoxide dismutase (MnSOD), peroxiredoxin-1 (PRDX1) and keratin 13 (KRT13), and so on were over-expressed, and 14 proteins including cystatin B (CSTB), tropomyosin 2 (TPM2), annexin 1 (ANX1), transgelin (TAGLN), keratin 19 (KRT19), and stratifin (SFN), and so on were down-expressed in ESCC. In the subsequent study, the gene ontology (GO) analysis using Goblet algorithm and searching sequences against TrEMBL and Swiss-Prot databases (http://www.expasy.ch/sprot/) was performed to explore the function of these identified proteins. As shown in Fig. 3, the 47 identified proteins were categorized in four groups including molecular function, biological process, physiological process, and cellular component. Proteins in each group were further categorized in different subgroups. For example, proteins in the group of molecular function were divided into 11 subgroups such as catalytic activity, nucleotide binding, oxidoreductase activity, and so on. In order to understand the cancer association of these identified proteins, a literature search was conducted with PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). Based on the literature search, 33 of the 47 identified proteins have been reported clearly relating to cancer.
Figure 3.
Functional categorization of identified proteins in ESCC. Forty-seven identified proteins were functionally categorized in four groups based on gene ontology (GO) annotation terms and searching sequences against the TrEMBL and Swiss-Prot databases. Four functional groups include molecular function, biological process, physiological process and cellular component. Proteins in each group were further categorized in different sub-groups.
4. A concern in using immunoproteomics to identify TAAs as biomarkers in cancer immunodiagnosis
A major issue in the field of cancer immunodiagnosis is the definition of what constitutes a TAA. It is erroneous to include all cellular antigens identified by autoantibodies in cancer sera as TAAs since some autoantibodies may exist in conditions that pre-date malignancy. This was particularly evident in several studies of subjects with HCC where serial serum samples were available several years before malignancy when these subjects had conditions such as chronic hepatitis and liver cirrhosis. In cases where the novel antigen-antibody systems were characterized, many antigens turned out to be cellular components that have been described to be aberrantly expressed in cancer.
As described in above section, we have used immunoproteomic approach to identify a group of HCC- and ESCC-related proteins. Whether all these identified proteins can be used as TAAs in cancer immunodiagnosis remains to be investigated. In the further characterization of these identified proteins, we found that not all these proteins can induce autoantibody response in cancer. As shown in Table 1, we have further evaluated 11 proteins identified in ESCC, and found that only 4 proteins including HSP70 (heat shock protein 70), GSTO1 (glutathione S transferase omega 1), FKBP12 (FK506 binding protein 1A) and PRDX1 (peroxiredoxin 1) can induce significantly higher antibody response in ESCC patients compared to normal individuals (unpublished data). These preliminary data strongly suggest that not all proteins identified in cancer with immunoproteomics can be used as TAA biomarkers, and only some of these proteins can induce antibody responses, which could be potential TAAs in cancer immunodiagnosis. Failing to recognize the likelihood of pre-malignancy circulating antibodies would result in the inclusion of many antigens erroneously as TAAs, especially if serum drawn at one time point from a cancer subject was used to characterize the antigens since this might include both cancer-related and unrelated antigens.
Table 1.
Frequency of autoantibody to 11 identified proteins in human ESCC sera by ELISA
| Identified proteins | ESCC | NHS | p |
|---|---|---|---|
| Heat Shock Protein 70 (HSP70) | 39.1% (27/69) | 1.3% (1/76) | <0.01 |
| Glutathione S-transferase omega 1 (GSTO1) | 35.8% (24/67) | 1.1% (1/90) | <0.01 |
| FK506 Binding Protein 1A (FKBP12) | 35.8% (24/67) | 1.1% (1/90) | <0.01 |
| Peroxiredoxin 1 (PRDX1) | 11.9% (8/67) | 1.1% (1/90) | <0.01 |
| Profilin1 | 7.5% (5/67) | 1.1% (1/90) | >0.05 |
| High-Mobility Group Box-1 (HMGB1) | 7.2% (5/69) | 1.3% (1/76) | >0.05 |
| Chloride Intracellular Channel 1(CLIC1) | 6.0% (4/67) | 3.3% (3/90) | >0.05 |
| Annexin 1 (ANX1) | 4.5% (3/67) | 3.3% (3/90) | >0.05 |
| Bip Protein | 3.0% (2/67) | 3.3% (3/90) | >0.05 |
| Glyoxalase 1 (GLO1) | 3.0% (2/67) | 0.0% (0/90) | >0.05 |
| Cytokeratin 19 (KRT19) | 1.5% (1/67) | 1.1% (1/90) | >0.05 |
Cutoff value: Mean+3SD of normal group
Abbreviation: ESCC, esophageal squamous cell carcinoma; NHS, normal human sera
5. Conclusion and Perspectives
With the application of cancer immunoproteomics in TAA identification and cancer immunodiagnosis, our efforts was aimed at increasing both the sensitivity and specificity of anti-TAA antibodies as biomarkers in cancer by expanding TAA array to include antigens which might be more selectively associated with cancer and not with non-cancer conditions. In cancer, the major task ahead is the continuing identification of TAAs, and the challenging problem is the separation of tumor-associated from non-tumor-associated antigens, because autoantibodies to other cellular antigens can be present before appearance of new antibodies occurring with malignancy [40–42]. In our experience, it has been necessary to validate a candidate TAA by testing not only with cancer sera but also with pre-cancer sera when available and with sera from other autoimmune disorders. Our previous studies have demonstrated that a panel of recombinant TAAs could enhance the sensitivity and specificity of autoantibody detection in cancer [26, 43–46]. It will be expected that the further investigation on other identified proteins from these studies may help us to define more proteins as candidate TAAs for the formulation of a more cancer-specific TAA array in immunodiagnosis of certain types of cancer. A mini-array protein chip of multiple TAAs can be also developed and evaluated as a novel non-invasive approach for early detection of cancer. The molecular identification and characterization of TAAs in cancer will also contribute to our understanding of their role in malignant transformation, thereby providing attractive candidates for early diagnosis and targeted therapies.
Take-home messages.
Antigenic changes in cancer cells can be recognized by the immune system of patients themselves and presented as autoantibody responses to proteins involved in malignant transformation. These autoantibodies, which have been called “reporters” from the immune system, can be used as probes in immunoproteomics to isolate, identify, and characterize potential tumor-associated antigens (TAAs).
A proteome-based methodology, which is generally named as immunoproteomics, has been recently implemented for the identification of TAAs in cancer. A large number of so-called cancer-associated proteins were identified.
Our data demonstrates that not all proteins identified in cancer with immunoproteomics can be used as TAA biomarkers, and only some of these proteins can induce antibody responses, which could be potential TAAs in cancer immunodiagnosis.
The practical utility of the immunoproteomic approach remains to be established with the proviso that efforts should be made to identify tumor-associated from tumor-irrelevant antigens.
Acknowledgments
We thank Dr. Eng M. Tan (The Scripps Research Institute, La Jolla, California, USA) for his support. This work was supported in part by NIH grants (5SC1CA166016, 5G12MD007592), and also by a grant from National Natural Science Foundation of China (81172086).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends genet. 1993;9:138–41. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- (2).Sarasin A. An overview of the mechanisms of mutagenesis and carcinogenesis. Mutat Res. 2003;544:99–106. doi: 10.1016/j.mrrev.2003.06.024. [DOI] [PubMed] [Google Scholar]
- (3).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- (4).Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Looi KS, Nakayasu ES, Diaz RA, Tan EM, Almeida IC, Zhang JY. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J Proteome Res. 2008;7:4004–12. doi: 10.1021/pr800273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zhang J, Wang K, Zhang J, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10:2863–72. doi: 10.1021/pr200141c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Peng B, Huang X, Nakayasu ES, Petersen JR, Qiu S, Almeida IC, Zhang JY. Using Immunoproteomics to Identify Alpha-enolase as an Autoantigen in Liver Fibrosis. J Proteome Res. 2013 Mar 15; doi: 10.1021/pr3011342. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10:321–8. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Soussi T. p53 antibodies in the sera of patients with various types of cancer. A review. Cancer Res. 2000;60:1777–88. [PubMed] [Google Scholar]
- (11).Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer. 1982;30:403–8. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- (12).Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16(5):1105–10. [PubMed] [Google Scholar]
- (13).Yamamoto A, Shimizu E, Takeuchi E, Houchi H, Doi H, Bando H, Ogura T, Sone S. Infrequent presence of anti-c-Myc antibodies and absence of c-Myc oncoprotein in sera from lung cancer patients. Oncology. 1999;56:129–33. doi: 10.1159/000011953. [DOI] [PubMed] [Google Scholar]
- (14).Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–7. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- (15).Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- (16).Soo Hoo L, Zhang JY, Chan EKL. Cloning and characterization of a novel 90kDa `companion' auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–15. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- (17).Liu W, Peng B, Lu Y, Xu W, Qian W, Zhang JY. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun Rev. 2011;10(6):331–5. doi: 10.1016/j.autrev.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Keene JD. Why is Hu where? Shuttling of early response gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–92. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- (21).Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, et al. Antinuclear autoantibodies in PCa: Immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62:14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- (22).Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–74. [PubMed] [Google Scholar]
- (23).Lu M, Nakamura RM, Dent ED, Zhang JY, Nielsen FC, Christiansen J, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159:945–53. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–33. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Old LJ, Chen YT. New paths in human cancer serology. J. Exp. Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- (27).Imai H, Chan EKL, Kiyosawa K, Fu XD, Tan EM. Novel nuclear antoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J Clin Invest. 1993;92:2419–26. doi: 10.1172/JCI116848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Landberg G, Tan EM. Characterization of a DNA-binding nuclear autoantigen mainly associated with S phase and G2 cells. Exp Cell Res. 1994;212:255–61. doi: 10.1006/excr.1994.1141. [DOI] [PubMed] [Google Scholar]
- (29).Casiano CA, Landberg G, Ochs R, Tan EM. Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci. 1993;106:1045–56. doi: 10.1242/jcs.106.4.1045. [DOI] [PubMed] [Google Scholar]
- (30).Zhang JY, Chan EKL, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J. Exp. Med. 1999;189:1101–10. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chambers JC, Keene JD. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci USA. 1985;82:2115–9. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Le Naour F, Brichory F, Beretta L, Hanash SM. Identification of tumor-associated antigens using proteomics. Technology in Cancer Research & Treatment. 2002;1:257–262. doi: 10.1177/153303460200100406. [DOI] [PubMed] [Google Scholar]
- (35).Le Naour F, Brichory F, Misek DE, Brechot C, Hanash SM, Beretta L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol Cell Proteomics. 2002;1:197–203. doi: 10.1074/mcp.m100029-mcp200. [DOI] [PubMed] [Google Scholar]
- (36).Ginestier C, Charafe-Jauffret E, Bertucci F, Eisinger F, Geneix J, Bechlian D, et al. Distinct and complementary information provided by use of tissue and DNA microarrays in the study of breast tumor markers. Am J Pathol. 2002;161:1223–33. doi: 10.1016/S0002-9440(10)64399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Tyers M, Mann M. From genomics to proteomics. Nature. 2003;422:193–7. doi: 10.1038/nature01510. [DOI] [PubMed] [Google Scholar]
- (38).Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc Natl Acad Sci U S A. 2000;97:9390–5. doi: 10.1073/pnas.160270797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Iwadate Y. Clinical proteomics in cancer research-promises and limitations of current two-dimensional gel electrophoresis. Curr Med Chem. 2008;15:2393–400. doi: 10.2174/092986708785909102. [DOI] [PubMed] [Google Scholar]
- (40).Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71:26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- (41).Imai H, Kiyosawa K, Chan EKL, Tan EM. Autoantibodies in viral hepatitis related hepatocellular carcinoma. Intervirology. 1993;35:73–85. doi: 10.1159/000150297. [DOI] [PubMed] [Google Scholar]
- (42).Zhang JY, Zhu W, Imai H, Kiyosawa K, Chan EKL, Tan EM. De-novo humoral immune responses to cancer-associated autoantigens during transition from chronic liver disease to hepatocellular carcinoma. Clin Exp Immunol. 2001;125:3–9. doi: 10.1046/j.1365-2249.2001.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Koziol JA, Zhang JY, Casiano CA, Peng XX, Shi FD, Feng AC, et al. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin Cancer Res. 2003;9:5120–6. [PubMed] [Google Scholar]
- (44).Shi FD, Zhang JY, Liu D, Rearden A, Elliot M, Nachtsheim D, et al. Preferential humoral immune response in prostate cancer to cellular proteins p90 and p62 in a panel of tumor-associated antigens. Prostate. 2005;63:252–8. doi: 10.1002/pros.20181. [DOI] [PubMed] [Google Scholar]
- (45).Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EKL. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J. Hepatol. 2007;46:107–14. doi: 10.1016/j.jhep.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chen Y, Zhou Y, Qiu S, Wang K, Liu S, Peng XX, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289:32–9. doi: 10.1016/j.canlet.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]