Abstract
Objective
The pathophysiology of the eating disorder anorexia nervosa remains obscure, but structural brain alterations could be functionally important biomarkers. Here we assessed taste pleasantness and reward sensitivity in relation to brain structure, which might be related to food avoidance commonly seen in eating disorders.
Method
We used structural magnetic resonance brain imaging to study gray and white matter volumes in individuals with restricting type currently ill (n = 19) or recovered-anorexia nervosa (n = 24), bulimia nervosa (n= 19) and healthy control women (n=24).
Results
All eating disorder groups showed increased gray matter volume of the medial orbitofrontal cortex (gyrus rectus). Manually tracing confirmed larger gyrus rectus volume, and predicted taste pleasantness across all groups. The analyses also indicated other morphological differences between diagnostic categories: Ill and recovered-anorexia nervosa had increased right, while bulimia nervosa had increased left antero-ventral insula gray matter volumes compared to controls. Furthermore, dorsal striatum volumes were reduced in recovered-anorexia and bulimia nervosa, and predicted sensitivity to reward in the eating disorder groups. The eating disorder groups also showed reduced white matter in right temporal and parietal areas when compared to healthy controls. Notably, the results held when controlling for a range of covariates (e.g., age, depression, anxiety, medications).
Conclusion
Brain structure in medial orbitofrontal cortex, insula and striatum is altered in eating disorders and suggests altered brain circuitry that has been associated with taste pleasantness and reward value.
Introduction
Restricting type anorexia nervosa is a severe eating disorder associated with malnutrition, underweight and high mortality. It is distinct from bulimia nervosa, which is characterized by regular binge eating and purging episodes but normal weight. Both eating disorders usually begin during adolescence, occur most commonly in females, and aggregate in families (1).
Previously, functional brain imaging has implicated striatum, insula, anterior cingulate, amygdala and orbitofrontal cortex in eating disorders (2). The underlying mechanisms for those alterations are unclear, but brain gray and white matter might be directly related to altered brain function and behavior (3).
Research on brain structure in eating disorders is inconsistent. Early studies suggested reduced total gray and white matter volume, while studies after recovery found reduced or normal total brain tissue volumes (4). For the study of regionally specific volume alterations, brain analysis methods have become available that allow automated whole brain comparison reducing bias (4). A systematic review of those studies (4) found 8 such studies in adults, with an additional study published since (5). Those studies suggested reduced gray matter volume in anorexia nervosa in insula, frontal operculum, occipital, medial temporal or cingulate cortex, while one recent study found increased gray matter volume in dorsolateral prefrontal cortex (6-9). After short-term recovery anorexia nervosa showed reduced gray matter in insula, striatum, occipital, frontal and parietal cortex (9), but brain tissue seems to increase with weight gain (10), and was normal after long-term recovery (11). The few studies in bulimia nervosa suggested normal or increased localized gray matter volume in orbitofrontal cortex and striatum (5, 7). These variable results may reflect the heterogeneity of approaches as only some studies corrected for age or overall brain volumes, some studies distinguished restricting from binge eating/purging anorexia nervosa while others did not, and the effects of comorbid diagnoses or medication were often not directly taken into account.
Ill and recovered-anorexia nervosa show increased eating concerns, as do bulimia nervosa individuals between binge episodes (12). Importantly, affective value attributed to food stimuli, as well as food avoidance (13), have been associated with medial orbitofrontal cortex function (14). Furthermore, orbitofrontal cortex function has been directly associated with taste pleasantness (15), which could have implications for sensory specific satiety in eating disorders and being quickly over-stimulated by a food type. Orbitofrontal function has been repeatedly associated with brain pathology in anorexia and bulimia nervosa including food valence ratings (14, 16, 17) and could be a key area of brain pathology in eating disorders. On the other hand, brain reward function indicated opposite response in striatum and insula in anorexia and bulimia nervosa (18, 19) and those regions might therefore distinguish eating disorder groups.
Methodological problems in eating disorders brain research can include inaccurate brain alignment or separation of gray and white matter due to brain shapes that do not conform with standard brain templates. Whole brain structural studies in eating disorders most commonly used voxel based morphometry (VBM) and statistical parametric mapping (SPM) 5 software (http://www.fil.ion.ucl.ac.uk/spm/), which analyze gray and white matter probability across the entire brain. Recently VBM8 was developed to address shortcomings of the previous program. It uses a new image registration algorithm, a template based on the individual study population without relying on standard template assumptions, and shows improved gray-white matter separation compared to previous VBM versions (20, 21).
In this study we wanted to compare ill with recovered-anorexia nervosa to identify potential trait alterations in the disorder, but also compare anorexia and bulimia nervosa in order to identify brain alterations across eating disorders. We expected the orbitofrontal cortex to show common abnormality across all eating disorder groups and possibly related to hedonic taste perception (14, 17), while we expected insula and striatum structures to differentiate the eating disorder types (4, 18).
Method
Subjects
Nineteen restricting-type anorexia, 20 bulimia nervosa, 24 recovered restricting-type anorexia nervosa and 24 age-matched healthy control women participated in the study. Anorexia and bulimia nervosa participants were recruited from the Children's Hospital Colorado and the Eating Disorders Center of Denver. The study was approved by the Colorado Multiple Institutional Review Board. Anorexia and bulimia individuals were within 1-2 weeks of closely supervised inpatient or partial hospitalization treatment, and followed the program meal plan to avoid acute effects of starvation and dehydration (see Supplemental Material). Control and recovered-anorexia nervosa women were recruited through local advertisements. Participants were administered the structured clinical interview for DSM-IV diagnoses (1) by a doctoral level interviewer. Recovered-anorexia women had a history of restricting-type anorexia but had for at least one year normal weight for height, menstrual cycle, exercise, and food intake. All participants were right-handed, without history of head trauma, neurological disease, major medical illness, psychotic or substance use disorder. Thirteen controls, 1 anorexia, 5 bulimia, and 7 recovered-anorexia women took birth control pills. After complete description of the study, participants’ written informed consent was obtained.
Behavioral Measures
Participants completed as described previously (18) the Eating Disorder Inventory-3, Temperament and Character Inventory, Spielberger State and Trait Anxiety Inventory, Beck Depression Inventory-II, Revised Sensitivity to Reward and Punishment Questionnaire, and a taste perception test (prior to brain imaging, subjects rated 1 Molar sucrose solution for sweetness and pleasantness on 9-point Likert scales; see Supplemental Material).
MRI acquisition
Structural brain images were acquired on a GE Signa 3T scanner, axial three-dimensional T-1 weighted magnetization-prepared rapid acquisition gradient echo (spoiled gradient recall, SPGR, field of view 22 cm, flip angle 10°, slice thickness 1.2 mm, scan matrix 256x256, TR 10 msec, TE 3 msec, voxel size 1.2 mm3).
Image analysis
Images were manually aligned on the anterior-posterior commissure line. Preprocessing of T1-weighted images was performed using SPM VBM8 toolbox (http://dbm.neuro.unijena.de/vbm/download/) in Matlab R2009b, 7.9.0 (MathWorks, Natick, MA, USA). VBM8 brain segmentation (see Supplemental Material) does not require a priori tissue probabilities information. After segmentation of T1/SPGR images into 3 pure tissue classes: gray matter, white matter, and cerebral spinal fluid, two additional mixed tissue classes (gray matter-white matter and gray matter-cerebral spinal fluid) are estimated using Partial Volume Effects. The result is an estimation of fraction of pure tissue type present in every voxel. Images were smoothed to an 8-mm full-width at half-maximum Gaussian kernel. Non-linear modulated data were used in the analyses. Images were normalized to MNI space using high-dimensional diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL).
Total intracranial volume (global tissue volume) was obtained by adding up gray and white matter and cerebral spinal fluid volumes from the tissue class images in native space using the VBM8 toolbox (gray matter+white matter+cerebrospinal fluid=total intracranial volume).
To confirm orbitofrontal gyrus rectus volume VBM results left gyrus rectus gray matter was traced using mricron (MES, blind to diagnosis) (http://www.mccauslandcenter.sc.edu/mricro/mricron/) from the most inferior orbitofrontal brain slice to the level of the inferior rostral sulcus as superior border to include the functionally connected agranular and dysgranular layers, and between olfactory sulcus as the lateral and medial longitudinal fissure as medial boundary (22).
Statistical analysis
A general linear model whole-brain analysis was used (SPM8), the model comprising a factorial design with diagnosis as a factor with 4 levels (controls, anorexia, bulimia and recovered-anorexia nervosa) and age and total intracranial volume as covariates, as well as use of anti-psychotic or SSRI medication and comorbid depression or anxiety which were each assigned a 0 or 1 coding presence of absence. Initially, a voxel-wise F-test was performed, threshold p<0.001 uncorrected, extent threshold >50 voxels to include functionally relevant brain structures such as insula taste area or orbitofrontal cortex. We used SPM8 AAL-atlas defined anatomical regions (orbitofrontal cortex, insula, caudate, putamen) for small volume correction (family-wise error corrected p<0.05). Gray matter regional volumes that reached significance within the anatomical region were extracted using MarsBar for post hoc analysis. Similarly, significant white matter regional volumes from the group whole brain analysis were also extracted. Demographic and extracted regional brain volumes were analyzed using SPSS (IBM-SPSS, Chicago, IL) and ANOVA. Post-hoc group comparisons were analyzed with Dunnett's T3, and ANOVA with covariates (ANCOVA) used Bonferroni correction for post hoc comparison, verified using boot-strap procedures. Regression analyses assessed behavior-brain volume relationships.
Results
Demographic and behavioral data (Table 1.) Eating disorder groups had similar age compared to controls, but recovered-anorexia were older than anorexia individuals.
Table 1.
Demographic variables
| Control Women (A) n = 24 | Anorexia Nervosa Women (B) n = 19 | Recovered-Anorexia Nervosa Women (C) n = 24 | Bulimia Nervosa Women (D) n = 20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | P-value | ||
| Age (years) | 27.4 | 6.3 | 23.1 | 5.8 | 30.3 | 8.1 | 25.2 | 5.3 | 4.7 | 0.004 | B<C** |
| BMI (kg/m2) | 21.6 | 1.3 | 16.0 | 1.1 | 20.8 | 2.4 | 22.6 | 5.7 | 17.4 | <0.001 | A, C, D>B*** |
| Education (years) | 16.6 | 2.1 | 14.5 | 2.4 | 16.9 | 2.7 | 16.0 | 3.0 | 3.4 | 0.020 | A, C>B* |
| Novelty Seeking | 17.9 | 5.2 | 13.7 | 6.7 | 18.1 | 6.1 | 22.1 | 6.7 | 6.0 | 0.001 | B<D** |
| Harm Avoidance | 9.6 | 4.0 | 23.7 | 5.4 | 15.5 | 6.5 | 23.0 | 5.8 | 32.6 | <0.001 | A<B, D***, A<C**, B>C***, C<D*** |
| Reward Dependence | 17.0 | 3.7 | 15.2 | 2.9 | 18.2 | 2.7 | 16.0 | 4.7 | 2.9 | 0.037 | C>B** |
| Depression | 1.1 | 0.9 | 24.4 | 10.6 | 4.5 | 4.2 | 24.5 | 11.3 | 57.5 | <0.001 | A<B, D***, A<C**, B, D>C*** |
| Drive for Thinness | 2.6 | 3.4 | 19.2 | 6.7 | 8.5 | 6.4 | 23.1 | 4.5 | 66.7 | <0.001 | A<B, D***, A<C**, B>C***, C<D*** |
| Bulimia | 0.8 | 1.2 | 3.7 | 4.0 | 2.3 | 2.5 | 22.7 | 5.3 | 182.2 | <0.001 | A<D***, A<B*, B<D***, C<D*** |
| Body Dissatisfaction | 4.4 | 4.3 | 24.4 | 9.3 | 10.5 | 8.1 | 30.7 | 8.0 | 56.6 | <0.001 | A<B, D***, A<C*, B>C***, C<D*** |
| Punishment Sensitivity | 4.4 | 2.8 | 7.2 | 3.8 | 5.8 | 3.3 | 8.4 | 3.6 | 5.6 | 0.002 | A<D** |
| Reward Sensitivity | 4.0 | 1.9 | 13.2 | 4.2 | 6.6 | 4.1 | 12.4 | 3.9 | 32.5 | <0.001 | A<B, D***, A<C*, B>C***, C<D*** |
| State Anxiety | 32.7 | 11.8 | 50.4 | 9.7 | 45.0 | 9.4 | 47.8 | 12.8 | 11.5 | <0.001 | A<B, C, D*** |
| Trait Anxiety | 33.9 | 11.4 | 51.7 | 9.7 | 43.6 | 6.9 | 56.0 | 10.9 | 21.5 | <0.001 | A<B, D***, A<C**, B>C*, C<D*** |
| Sucrose Sweetness | 8.33 | 0.16 | 8.88 | 0.19 | 8.17 | 0.16 | 8.70 | 0.17 | 3.6 | 0.017 | B>C* |
| Sucrose Pleasantness | 4.92 | 0.05 | 4.19 | 0.62 | 4.63 | 0.51 | 5.45 | 0.55 | 0.80 | 0.477 | |
| Medication Use | |||||||||||
| SSRI | 0 | 6 | 5 | 9 | |||||||
| Atypical Antipsychotic | 0 | 1 | 0 | 0 | |||||||
| SSRI + Atypical Antipsychotic | 0 | 2 | 0 | 4 | |||||||
| Comorbid Diagnoses | |||||||||||
| Major Depression | 0 | 2 | 0 | 4 | |||||||
| Anxiety Disorder | 0 | 4 | 0 | 6 | |||||||
| Major Depression + Anxiety Disorder | 0 | 6 | 0 | 6 | |||||||
Dunnett T3 post hoc test
p<0.05
p<0.01
p<0.001
Body Mass Index (weight in kg/height in m2) was lower in anorexia. Measures for eating pathology, mood and anxiety as well as reward sensitivity were typically increased in the eating disorder groups. Sucrose pleasantness was similar across groups but sweetness rating was greater in anorexia compared to recovered-anorexia individuals.
Total brain volumes were similar across groups (Table 2.).
Table 2.
Total brain and regional brain gray matter and white matter volumes
| Anatomical Region | Control Women (A) | Anorexia Nervosa Women (B) | Recovered-Anorexia Nervosa Women (C) | Bulimia Nervosa Women (D) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |||
| Whole Brain Volumes | ||||||||||||
| GM Volume (cm3) | 629.7 | 62.1 | 651.8 | 37.9 | 639.4 | 49.5 | 662.4 | 40.6 | 1.8 | 0.149 | ||
| WM Volume (cm3) | 494.0 | 44.3 | 503.8 | 41.0 | 493.0 | 56.3 | 503.3 | 68.2 | 0.3 | 0.856 | ||
| CSF Volume (cm3) | 226.8 | 30.6 | 246.3 | 33.1 | 226.5 | 26.6 | 233.2 | 28.0 | 2.0 | 0.118 | ||
| Total Intracranial Volume (cm3) | 1350.5 | 105.3 | 1402.0 | 71.6 | 1358.8 | 107.0 | 1398.9 | 117.8 | 1.5 | 0.232 | ||
| Regional GM Volumes | MNI Coordinates | |||||||||||
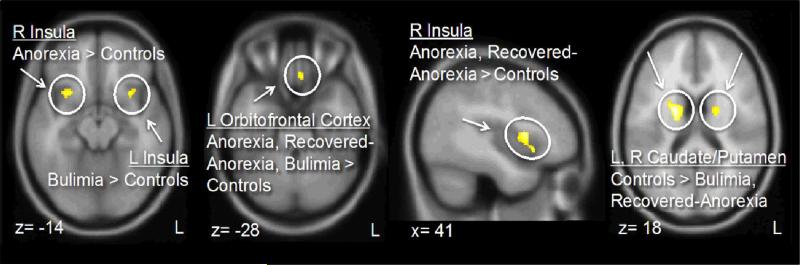
| L Orbitofrontal Cortex | x=-6, y=29, z=-26 | 0.479 | 0.071 | 0.553 | 0.091 | 0.538 | 0.066 | 0.585 | 0.072 | 7.9 | <0.001 | B>A*, D>A***, C>A* |
| R Anterior Ventral Insula | x=30, y=14, z=-12 | 0.704 | 0.079 | 0.806 | 0.084 | 0.754 | 0.084 | 0.777 | 0.104 | 5.3 | 0.002 | B>A*** |
| R Anterior, Middle Insula | x=42, y= 9, z= 4 | 0.597 | 0.058 | 0.679 | 0.066 | 0.645 | 0.063 | 0.630 | 0.069 | 6.1 | 0.001 | B>A***, C>A* |
| L Anterior Ventral Insula | x=-29, y=12, z=-17 | 0.703 | 0.059 | 0.753 | 0.077 | 0.729 | 0.066 | 0.769 | 0.070 | 4.0 | 0.011 | D>A** |
| R Dorsal Caudate | x=21, y=-3, z=19 | 0.173 | 0.019 | 0.170 | 0.017 | 0.153 | 0.018 | 0.147 | 0.021 | 10.2 | <0.001 | A>D***, A>C**, B>D**, B>C* |
| L Dorsal Caudate | x=-20, y=-3, z=18 | 0.308 | 0.027 | 0.295 | 0.032 | 0.281 | 0.034 | 0.270 | 0.036 | 5.7 | 0.001 | A>D**, A>C* |
| R Dorsal Putamen | x=20, y=0, z=12 | 0.173 | 0.013 | 0.168 | 0.016 | 0.157 | 0.017 | 0.151 | 0.015 | 9.2 | <0.001 | A>D***, A>C***, B>D** |
| R Dorsal Putamen | x=23, y=0, z=15 | 0.088 | 0.008 | 0.086 | 0.009 | 0.080 | 0.009 | 0.076 | 0.008 | 9.1 | <0.001 | A>D***, A>C**, B>D** |
| Regional WM Volumes | MNI Coordinates | |||||||||||
| R Medial Temporal Lobe | x=27, y=-19, z=-5 | 0.770 | 0.057 | 0.754 | 0.052 | 0.727 | 0.056 | 0.713 | 0.044 | 5.3 | 0.002 | A>D** |
| R Inferior Temporal Lobe | x=57, y=-20, z=-21 | 0.594 | 0.047 | 0.585 | 0.046 | 0.565 | 0.044 | 0.546 | 0.034 | 9.3 | <0.001 | A>B**, A>C***, D>B**, D>C** |
| R Inferior Parietal Lobe | x=55, y=-48, z=35 | 0.347 | 0.065 | 0.353 | 0.081 | 0.278 | 0.056 | 0.285 | 0.056 | 8.0 | <0.001 | A>D**, A>C**, B>D*, B>C** |
| Manually Traced Orbitofrontal Gyrus Rectus Volume, mm3 | 868 | 247 | 1128 | 438 | 1205 | 384 | 1282 | 449 | 5.1 | 0.003 | A<B*, C**, D*** | |
Dunnett T3 post hoc test
p<0.05
p<0.01
p<0.001.
Values for regional brain volumes are fractions of the respective brain tissue type per volume.
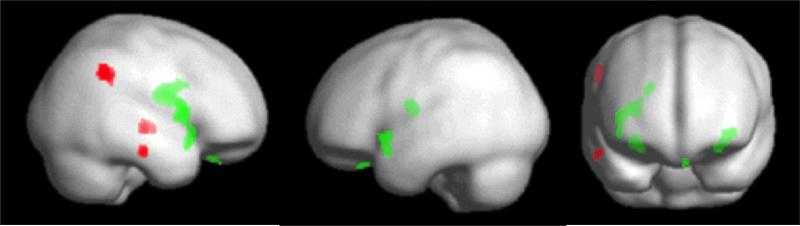
Gray matter results (Figures 1., 2., Table 2.) indicated increased orbitofrontal gyrus rectus volume in all eating disorder groups, reduced caudate and putamen volume in bulimia and recovered-anorexia, and increased insula volume in anorexia and recovered-anorexia (right) and bulimia (left) compared to controls.
Figure 1.
Areas of significant group difference; green indicates gray matter and red indicates white matter alterations.
Figure 2.
Areas of significant gray matter differences across groups.
Manually traced gyrus rectus volume (ANCOVA including total intracranial volume as covariate) confirmed increased volume in eating disorders compared to controls (Table 2.).
White matter results (Figure 1., Table 2.) showed reduced inferior temporal white matter in anorexia and recovered-anorexia compared to controls, inferior parietal volume reduced in bulimia and recovered-anorexia, and reduced medial temporal lobe white matter in bulimia compared to controls.
Regression results
Age predicted negatively gray matter volumes in controls in the right insula (x=30, y=14, y=-12, r=-0.437, p<0.033) and left gyrus rectus (r=-0.411, p<0.048), in anorexia in left gyrus rectus (r=-0.830, p<0.001) and right putamen (r=-0.473, p<0.041), in recovered-anorexia in the left gyrus rectus (r=-0.453, p<0.026).
BMI predicted negatively in bulimia right caudate (r=-0.473, p<0.035) and in recovered-anorexia left insula gray matter (r=-0.510, p<0.011).
State (r=-0.441, p<0.031) and trait (r=-0.419, p<0.042) anxiety were negatively predicted in controls by left anterior ventral insula gray matter, but not in the eating disorders groups.
Sensitivity to Reward was in all eating disorder groups positively predicted by right putamen gray matter (MNI coordinates x=20, y=0, z=12, and x=23, y=0, z=15), AN: r=0.620, p<0.005, and r=0.554, p<0.014; BN: r=0.543, p<0.013, and r=0.443, p<0.050, AN-REC: r=0.420, p<0.041, and r=0.397, p<0.055.
Other variables including illness or recovery duration, or binge/purge episodes did not predict brain volume measures.
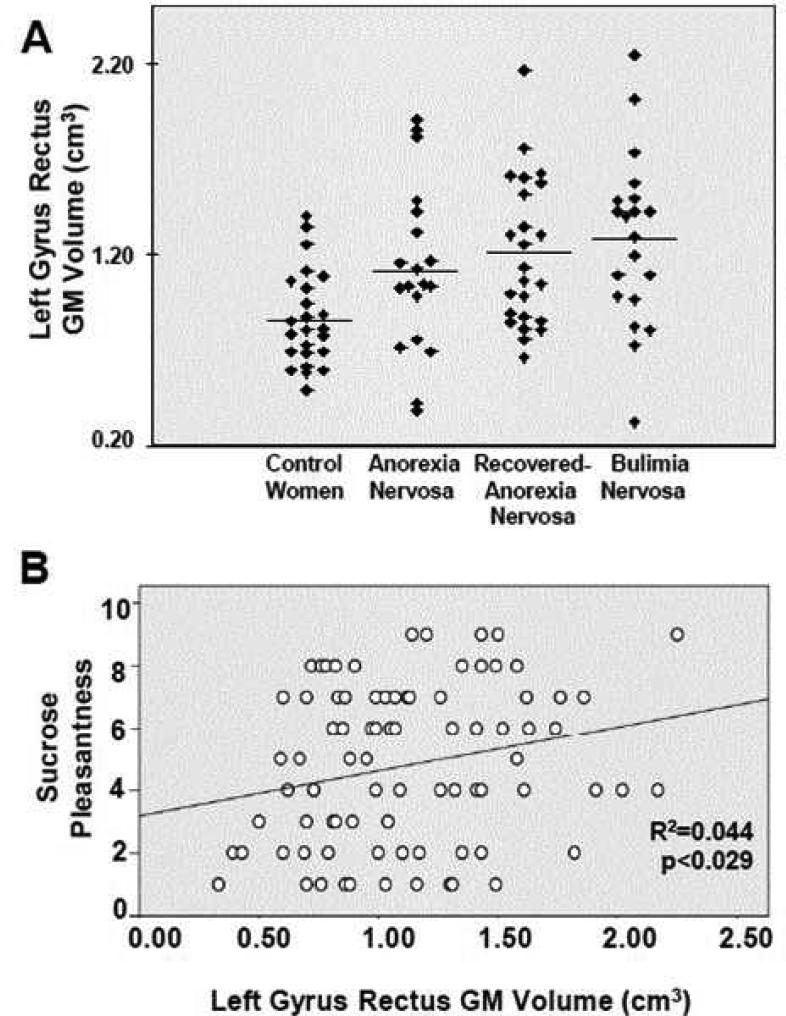
Gyrus rectus volume predicted significantly positively Sucrose Pleasantness in controls (r=0.419, F=4.970, p<0.03), in eating disorder groups combined (r=0.268, F=4.50, p<0.038), as well as all study subjects together (Figure 3.), but not in eating disorder groups separately.
Figure 3.
A. Manually drawn gyrus rectus gray matter volumes indicate larger volume in anorexia, recovered-anorexia and bulimia nervosa. B. Taste pleasantness correlated significantly with gyrus rectus volume across all groups.
There were no significant correlations between white matter volume and body mass index or behavior.
Discussion
The results of this large and well-controlled study implicate both overlapping and distinct brain morphology in two phases of anorexia as well as bulimia nervosa. Findings that show a link between brain structure and both sensitivity to reward and taste pleasantness support the notion that marked neurological underpinnings are associated with phenotypes exhibited across eating disorders. Specifically, VBM results indicate that anorexia and recovered-anorexia as well as bulimia nervosa are associated with increased left orbitofrontal gyrus rectus gray matter volume. Anorexia is associated with right, and bulimia with left increased anterior ventral insula gray matter volume, anorexia and recovered-anorexia have increased gray matter in the right anterior middle insula, and bulimia and recovered-anorexia but not anorexia are associated with decreased dorsal caudate and putamen gray matter volumes. Notably, in all three eating disorder groups putamen gray matter was positively related to sensitivity to reward. White matter was reduced in bulimia in the medial temporal, in anorexia and recovered-anorexia in the inferior temporal and in recovered-anorexia and bulimia in the parietal lobe.
The present study in adults is in line with more recent reports on normal total gray and white matter volumes (5, 6) and finds a distinct pattern of increased and decreased regional cortical and subcortical brain volumes in anorexia, recovered-anorexia and bulimia nervosa. However, we did not find alterations of the cingulate or temporal cortex as did previous studies (4).
Several factors distinguish our study from past investigations. First we used more accurate analysis software (20, 21), and to our knowledge no study in anorexia and only one study in bulimia nervosa (5) has used this method. Improved gray/white matter separation and reduced white matter volume underlying gray matter might have contributed to reduced gray matter results in the past, which may be supported by our finding of reduced white matter in anorexia, recovered-anorexia and bulimia nervosa. Second, we report only gray matter areas that survived stringent anatomical region based small volume correction as significant. Third, anorexia and bulimia nervosa individuals were in a strict inpatient or partial hospital program where they had normal food and fluids for 7-10 days before brain imaging and were prevented from binge or purge behavior. Fluid changes significantly affect gray matter changes (23), and our study protocol helps reduce acute effects of nutritional depletion. Fourth, in a highly conservative approach we used age, depression and anxiety, medication use and total intracranial volume as covariates, and to our knowledge this is the first eating disorder brain imaging study that includes all those covariates. Lastly, this is the largest study of restricting type anorexia and recovered-anorexia as well as bulimia nervosa on gray and white matter brain structure to date. In summary, our study procedures were very stringent and we think that they contributed to improved results.
All three eating disorder groups had increased left orbitofrontal cortex gyrus rectus volume, suggesting that this is potentially a trait marker for anorexia, and maybe also for bulimia nervosa, although this will need to be studied in bulimia after recovery as well. Those findings support previous research implicating the orbitofrontal cortex across eating disorder groups (14, 17). The gyrus rectus is the medial part of the orbitofrontal cortex (22). It is further defined by a caudal agranular and dysgranular layer (area 14) that transitions antero-superiorly into the granular layer (area 11) (24). The agranular and dysgranular layers have fiber connection to the hippocampus, amygdala, cingulate and insular cortex (25), areas important for taste as well as reward, motivation and emotion processing. In line with those functional aspects of the orbitofrontal cortex may be the finding of orbitofrontal cortex gyrus rectus volume predicting pleasantness of sucrose solution. Greater gyrus rectus volume predicted stronger pleasant experience, which is consistent with previous research (26). The orbitofrontal cortex is important in food intake control (26). It is possible that larger orbitofrontal gyrus rectus in eating disorders is associated with stronger sensory experience of food stimuli, which could be experienced as overwhelming - as supported by increased reward and punishment sensitivity (18) - which could trigger cognitively driven food avoidance. Importantly, the medial orbitofrontal cortex has been associated with food avoidance (13) and this region therefore may be a key structure in eating disorder pathology. Eating disorder phenotype differences with restriction in anorexia nervosa and episodic binge eating in bulimia on the contrary may be driven by insula and basal ganglia differences between the two disorders (18, 19).
The cause for increased orbitofrontal gyrus rectus volume is unclear. One potential explanation is that the trajectory of orbitofrontal gray matter development in eating disorders may be delayed, reaching peak volume later than in controls and thus resulting in greater cortical thickness and volume (27). Another possibility could be effects of repeated food restriction in the eating disorder groups, but this will need to be tested further.
Anorexia and recovered-anorexia individuals showed increased volumes in the right anterior ventral/middle insula, which connects to ventral striatal and orbitofrontal reward pathways (26). Previous research implicated insula function in eating disorders (2) and altered insula structure could underlie altered function. The anterior ventral insula is connected to the amygdala (28), and has been associated with fear response (29). It also aids in connecting complex perceptual inputs to generate internal emotional states (30). Thus, altered insula volume could contribute to a dysfunction in the regulation of anxiety by the insula, contributing to high trait anxiety in anorexia nervosa. Functional imaging taste reward studies suggested excessive insula activation in anorexia (18) and increased size could mediate excessive, overwhelming taste stimulus transmission and subsequent input into reward-processing brain regions.
Right anterior insula has also been associated with self-recognition, the “abstract representation of oneself”(31) and interoceptive awareness (32). The fixed perception of being fat while severely underweight in anorexia nervosa (33) could thus be related to right sided increased, dysfunctional anterior ventral insula volume. Left anterior ventral insula activation is related to gastric distention (34) and self-reported fullness (35). Thus, altered anterior insula size could interfere with normal interoception in bulimia, which may contribute to a reduced ability to sense “fullness” or satiation and then trigger the urge to purge after excessive food intake and guilt experienced over eating.
Dorsal caudate and putamen volumes were reduced in recovered-anorexia and bulimia, but not anorexia. Nevertheless, in all three eating disorder groups right putamen gray matter volume correlated significantly positively with sensitivity to reward. The dorsal striatum has been widely associated with supporting rewarding behaviors based on previous experience (36) and reduced brain volume in that region might alter reward motivated behaviors. Dorsal striatum activation responds to reward and punishment (37) and contributes to reward based decision making (38). It is rich in dopamine D1 and D2 receptors, which code reward response, but those receptors have opposing effects (39). Thus, dopamine receptor expression may be affected by altered dorsal striatal volume and be related to altered sensitivity to reward in eating disorders.
Various right-sided white matter regions showed reduced volume in the eating disorder groups. The functionality of such alterations is unclear but the fact that the recovered-anorexia group showed reduced volumes in the right temporal and parietal lobe suggest either long lasting or premorbid volume reductions in anorexia. The right sided reduced inferior parietal lobe/temporo-parietal junction area in recovered-anorexia and bulimia has been associated with fiber-paths connecting with the insula, especially in women (40), further indicating an involvement of insula related brain circuitry in eating disorders.
Limitations
Although this is the largest structural imaging study contrasting anorexia, recovered-anorexia and bulimia nervosa to date, replication is needed. Some of the results are in contrast to previous studies that found reduced brain volumes, although in our study we found both increased and decreased gray, as well as decreased white matter volumes. The brain analysis method we used shows improved accuracy (20, 21) and we do not believe that there is a methodological systematic error. Especially this approach does not depend on standard template assumptions but normalizes the images to a from the specific study population created template, and therefore improving tissue segmentation accuracy. The manual tracing of gyrus rectus volume also supports the whole brain VBM results. Furthermore, the results in anorexia and normal weight recovered-anorexia point toward consistent brain alterations. The reason for increased gray and reduced white matter volume is unclear. However, a reason could be altered brain maturation in eating disorders. During development, gray matter in adolescence decreases (indicative of synaptic pruning), beginning in puberty in sensorimotor areas and then spreading during late adolescence into ‘higher-order’ cortical regions while white matter increases, indicative of thicker myelin sheathes, increased axonal diameter, and improved organization of white matter tracts improving signal transduction (27). A delay or incomplete maturation of brain structures in eating disorders could be responsible for the results in this study and would fit the developmental perspective. This poses an interesting future research direction and suggest the need for longitudinal imaging studies in these groups. However, while we made every effort to reduce effects of acute malnutrition, past or more recent effects of underfeeding may also have contributed to the differences across groups. Age, comorbidity and use of medication are a concern and potential confounds in brain imaging studies. We accounted for those factors by using them as covariates in the brain imaging analysis, and it is possible that inclusion of those covariates contributed to the fact that we did not find for instance alterations in cingulate or temporal cortex. Sucrose pleasantness was similar across groups, and sweetness perception was similar between the eating disorder groups and control women. However, the anorexia nervosa group rated sweetness higher compared to recovered-anorexia individuals. The meaning of difference between anorexia groups is uncertain and needs future exploration. Regression analysis between brain gray matter volumes and demographic and behavioral data suggested various significant relationships as discussed above. However, most of those results would not have survived multiple comparison correction and should be viewed as preliminary until further replication.
In summary, anorexia and recovered-anorexia nervosa as well as bulimia nervosa are associated with increased orbitofrontal gyrus rectus gray matter volume, which could be a trait related alteration. The strong correlations between gyrus rectus volume and taste pleasantness might suggest overstimulation in eating disorders to sensory input possibly contributing to food avoidance. Increased right anterior ventral insula volume in anorexia and recovered-anorexia versus increased left anterior ventral insula volume in bulimia nervosa distinguished the two disorders. Furthermore, the dorsal putamen appears to be important in modulating reward sensitivity in eating disorders. Future studies that integrate brain structure and function will be needed to disentangle how brain volume affects behavior.
Supplementary Material
Acknowledgements
A Davis Foundation Award of the Klarman Family Foundation Grants Program in Eating Disorders, NIMH grant K23 MH080135, and NIMH grant R01 MH096777 provided funding for all aspects of the study to Dr. Frank.
We are grateful to all the individuals who participated in this study. We further thank Dr. Joel Yager for his very thoughtful feedback and discussion.
Footnotes
Disclosures
Drs. Frank, Hagman and Mittal report no competing interests. Ms. Shott reports no competing interests.
References
- 1.American Psychiatric Association . Handbook of Psychiatric Measures. 4 ed. American Psychiatric Association; Washington DC: 2000. Diagnostic and Statistical Manual of Mental Disorders - Text Revision (DSM-IV-TR). [Google Scholar]
- 2.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 3.Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483(7387):92–5. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, Israel M, Berlim M, Giampietro V, Simmons A, Treasure J, Campbell I, Schmidt U. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. European eating disorders review : the journal of the Eating Disorders Association. 2012;20(2):94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 5.Schafer A, Vaitl D, Schienle A. Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage. 2010;50(2):639–43. doi: 10.1016/j.neuroimage.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 6.Brooks SJ, Barker GJ, O'Daly OG, Brammer M, Williams SC, Benedict C, Schioth HB, Treasure J, Campbell IC. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joos A, Kloppel S, Hartmann A, Glauche V, Tuscher O, Perlov E, Saum B, Freyer T, Zeeck A, Tebartz van Elst L. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182(2):146–51. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Suchan B, Busch M, Schulte D, Gronemeyer D, Herpertz S, Vocks S. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behavioural brain research. 2010;206(1):63–7. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Friederich HC, Walther S, Bendszus M, Biller A, Thomann P, Zeigermann S, Katus T, Brunner R, Zastrow A, Herzog W. Grey matter abnormalities within cortico limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59(2):1106–13. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Roberto CA, Mayer LE, Brickman AM, Barnes A, Muraskin J, Yeung LK, Steffener J, Sy M, Hirsch J, Stern Y, Walsh BT. Brain tissue volume changes following weight gain in adults with anorexia nervosa. The International journal of eating disorders. 2011;44(5):406–11. doi: 10.1002/eat.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner A, Greer P, Bailer U, Frank G, Henry S, Putnam K, Meltzer CC, Ziolko SK, Hoge J, McConaha C, Kaye WH. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biological Psychiatry. 2006;59(3):291–3. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Bosanac P, Kurlender S, Stojanovska L, Hallam K, Norman T, McGrath C, Burrows G, Wesnes K, Manktelow T, Olver J. Neuropsychological study of underweight and “weight-recovered” anorexia nervosa compared with bulimia nervosa and normal controls. The International journal of eating disorders. 2007;40(7):613–21. doi: 10.1002/eat.20412. [DOI] [PubMed] [Google Scholar]
- 13.Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(32):10799–808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SC, Campbell IC, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161(7):1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 15.Kringelbach ML, O'Doherty J, Rolls E, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 16.Gizewski ER, Rosenberger C, de Greiff A, Moll A, Senf W, Wanke I, Forsting M, Herpertz S. Influence of satiety and subjective valence rating on cerebral activation patterns in response to visual stimulation with high-calorie stimuli among restrictive anorectic and control women. Neuropsychobiology. 2010;62(3):182–92. doi: 10.1159/000319360. [DOI] [PubMed] [Google Scholar]
- 17.Stein D, Gross-Isseroff R, Besserglick R, Ziv A, Mayer G, Yaroslavsky A, Toledano A, Voet H, Weizman A, Hermesh H. Olfactory function and alternation learning in eating disorders. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22(9):615–24. doi: 10.1016/j.euroneuro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O'Reilly RC. Anorexia Nervosa and Obesity are Associated with Opposite Brain Reward Response. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank GK, Reynolds JR, Shott ME, O'Reilly RC. Altered temporal difference learning in bulimia nervosa. Biological Psychiatry. 2011;70(8):728–35. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One. 2012;7(9):e45081. doi: 10.1371/journal.pone.0045081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos A, Chaddad-Neto F, Joaquim AF, Campos-Filho JM, Mattos JP, Ribas GC, Oliveira E. The microsurgical anatomy of the gyrus rectus area and its neurosurgical implications. Arquivos de neuro-psiquiatria. 2009;67(1):90–5. doi: 10.1590/s0004-282x2009000100021. [DOI] [PubMed] [Google Scholar]
- 23.Blasel S, Pilatus U, Magerkurth J, von Stauffenberg M, Vronski D, Mueller M, Woeckel L, Hattingen E. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology. 2012 doi: 10.1007/s00234-011-1001-9. [DOI] [PubMed] [Google Scholar]
- 24.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature neuroscience. 2012;15(1):13–9. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. The Journal of comparative neurology. 1992;323(3):341–58. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 26.Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta physiologica Hungarica. 2008;95(2):131–64. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- 27.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. The Journal of comparative neurology. 2005;490(2):101–18. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan MA, LeDoux JE. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiology of learning and memory. 1999;72(3):244–51. doi: 10.1006/nlme.1999.3907. [DOI] [PubMed] [Google Scholar]
- 30.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54(5):515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 31.Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Bredart S. Here I am: the cortical correlates of visual self-recognition. Brain research. 2007;1143:169–82. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 33.Konstantakopoulos G, Varsou E, Dikeos D, Ioannidi N, Gonidakis F, Papadimitriou G, Oulis P. Delusionality of body image beliefs in eating disorders. Psychiatry research. 2012 doi: 10.1016/j.psychres.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 35.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Korner J, Bauman A, Fowler JS, Thanos PK, Volkow ND. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–31. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 37.Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(31):8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(20):7349–56. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucyi A, Moayedi M, Weissman-Fogel I, Hodaie M, Davis KD. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS One. 2012;7(4):e35589. doi: 10.1371/journal.pone.0035589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paxinos G, Mai JK. The human nervous system. 2nd ed. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- 42.Cacioppo JT, Berntson GG. Social neuroscience : key readings. Psychology Press; New York: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.