Abstract
Here, we aimed to study serum heat shock protein (HSP) 70 levels in diabetic patients with and without albuminuria. We performed a 1:1 matched case control study on 40 diabetic patients with albuminuria as cases and 40 age, sex, body mass index matched diabetic patients without albuminuria (normoalbuminuria) as controls. Normoalbuminuria was defined as urinary albumin excretion rate <15 mg/12 h, and albuminuria was defined as urinary albumin excretion rate between 100–400 mg/12 h. Patients with albuminuria had a higher HSP70 than controls (0.83 ± 0.50 vs. 0.63 ± 0.06; p = 0.02), while they did not differ in any other studied variables. In ten of the studied pairs, the controls had higher HSP70 levels than cases (reverse relationship). Patients in the “direct relationship group” had higher HbA1c values than the patients in the “reverse relationship group” (8.9 ± 0.3 vs. 7.3 ± 0.6, p = 0.04). Cases in the reverse pairs had a lower low density lipoprotein cholesterol levels than their controls. The odds ratio of HSP70 in the prediction of albuminuria was (28.69 (3.2–250.1), p = 0.002). In conclusion, we have shown an increased HSP70 levels in diabetic patients with albuminuria.
Keywords: Heat shock protein 70, type 2 diabetes, Albuminuria
Introduction
Heat shock proteins (HSP) play important roles in the protection of cells during stressful conditions (Li and Srivastava 2004). Extensive literature relates abnormalities of HSP’s and type 2 diabetes (Nakhjavani et al. 2011; Nakhjavani et al. 2010; Kavanagh et al. 2011). Recent studies have shown the role of these molecules in the pathogenesis of diabetic nephropathy (Calabrese et al. 2007; Buraczynska et al. 2009). In an interesting study by Barutta and collaborators, it was shown that diabetes induces a localized increase in HSP’s in parts of the kidney and blocks HSP induction in other parts of the kidney (Barutta et al. 2008). Considering the divergence of intracellular and extracellular HSP70 family in type 2 diabetes (Rodrigues-Krause et al. 2012), we aimed to study serum HSP70 levels in diabetic patients with and without albuminuria.
Materials and methods
We performed a 1:1 matched case control study to discover the importance of HSP70 in the prediction of albuminuria in patients with type 2 diabetes. From the information of the diabetic patients attending Vali Asr hospital during January 2010 to August 2012, we obtained information of all diabetic patients with and without albuminuria. Diabetes was diagnosed according to the criteria of the American diabetes association (Diagnosis and classification of diabetes mellitus 2009). For each patient with documented albuminuria, we randomly selected one control matched by age, sex, and body mass index (BMI). Using this data base of 963 patients, 40 diabetic patients with albuminuria as cases and 40 age, sex, and BMI matched diabetic patients without albuminuria (normoalbuminuria) as controls were selected. Normoalbuminuria was defined as urinary albumin excretion rate <15 mg/12 h, and albuminuria was defined as urinary albumin excretion rate between 100–400 mg/12 h. Patients were instructed in the collection of timed 24-h urine for the measurement of urinary albumin excretion and were told to return on the morning after the end of the urine collection. Exclusion criteria were type 1 diabetes, acute or chronic renal failure, glomerulonephritis, congestive heart failure, acute infections, pregnancy, diabetic ketoacidosis, nonketonic hyperosmolar diabetes, thyroid disorders, and hospital admission in recent 3 months. None of the studied women were on hormone replacement therapy. None of the patients were on any antiinflammatory drugs, and none were using ethanol. There were no comorbid disease such as asthma, rheumatoid arthritis, and other autoimmune diseases present. Estimation of glomerular filtration rate was calculated using the chronic kidney disease epidemiology collaboration formula (Levey et al. 2009).
Demographic and anthropometric data including age, sex, duration of diabetes, height and waist circumference, weight in light clothing, and blood pressure in sitting position were recorded. Blood pressure was measured twice after 5 min average. The BMI (kilogram per square meter) was calculated according to the Quetelet formula. All participants gave written informed consent before participation. The research was carried out according to the principles of the Declaration of Helsinki; the local ethics review committee of Tehran University of Medical Science approved the study protocol.
Blood samples
Blood samples were collected after almost 12 h of fasting and serum creatinine, fasting blood sugar, total cholesterol, triglyceride, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, and HbA1c were measured. Glucose measurements (intra-assay coefficient of variants (CV) 2.1 %, inter-assay CV 2.6 %) were carried out using the glucose oxidase method. Creatinine was measured using calibrated Jaffe method (Parsazmoon, Karaj, Iran). Cholesterol, HDL, LDL, and triglyceride were determined using direct enzymatic methods (Parsazmun, Karaj, Iran). HbA1c was estimated by high-pressure liquid chromatography method. Serum creatinine was measured using direct colorimetric method. HSP70 was measured using a quantitative sandwich ELISA immunoassay (EKS-715, Stressgen). The intra- and inter-assay CV ranged between 4.5 and 7 %.
Statistical analysis
The statistical package SPSS 17 for windows (Chicago, IL, USA) was used for the primary analysis of the cases and controls. Kolmogorove–Smirnov test was employed to study the distribution of the variables. As HSP70 had a skew distribution, the logarithmic transformed values of HSP70 were used for further analysis in the conditional regression model. The data was individually matched. All the studied population was eligible for the analysis. Quantitative variables are presented as mean ± standard error of mean. Qualitative variables are presented as number and percent. Independent sample t test (for quantitative variables, normally distributed), Mann–Whitney U test (for quantitative variables, skewed distribution), and chi-square test (for qualitative variables) were employed to compare cases and controls. Conditional logistic regression analysis was employed to provide the value of HSP70 in the prediction of albuminuria.
Results
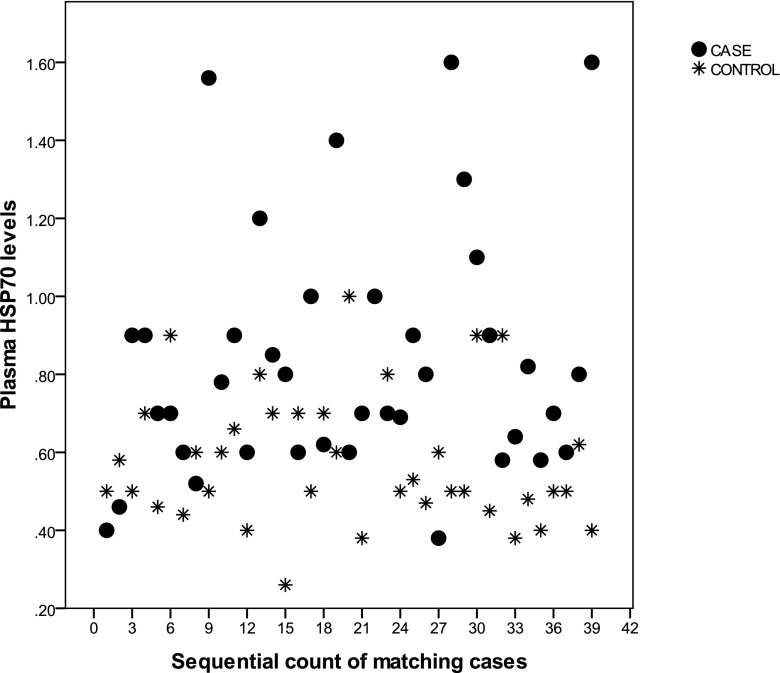
Patients with albuminuria had a higher HSP70 than controls (0.83 ± 0.50 vs. 0.63 ± 0.06; p = 0.02) (Fig. 1), while they did not differ in any other studied variables. In ten of the studied pairs, the controls had higher HSP70 levels than cases (reverse relationship). So the studied population was stratified according to direct relationship (pairs in which cases had higher HSP70 levels than controls) and reverse relationship. Patients in the “direct relationship group” had higher HbA1c values than the patients in the “reverse relationship group” (8.9 ± 0.3 vs. 7.3 ± 0.6, p = 0.04). There were no significant differences between the groups and other studied variables. Cases in the reverse pairs had a lower LDL levels than their controls (Table 1).
Fig. 1.
Presenting serum HSP70 levels in pairs of diabetic patients with albuminuria (cases) and without albuminuria (controls), 1:1 matched by age, sex, and BMI
Table 1.
Presenting the characteristics of patients with and without albuminuria
| Pairs with direct relationship | Pairs with reverse relationship | ||||
|---|---|---|---|---|---|
| With albuminuria (n = 30) | Without albuminuria (n = 30) | With albuminuria (n = 10) | Without albuminuria (n = 10) | ||
| Age (years) | 57 ± 2.1 | 58 ± 2.4 | 55 ± 3.6 | 58 ± 2.5 | |
| Female (n, %) | 14 | 14 | 5 | 5 | |
| Family history diabetes (n) | 14 | 20 | 8 | 7 | |
| Statins | 7 | 16 | 5 | 5 | |
| Drug diabetes | Glibenclamide | 5 | 2 | 3 | 1 |
| Glibenclamide + metformin | 1 | 6 | 3 | 1 | |
| Metformin + insulin | 15 | 11 | 2 | 6 | |
| Insulin | 1 | 6 | 2 | 1 | |
| Metformin | 8 | 5 | 0 | 1 | |
| Smoking (n) | 1 | 1 | 0 | 2 | |
| Diabetes duration (years) | 11 ± 1.6 | 9 ± 1.1 | 4 ± 2.0 | 9 ± 2.1 | |
| BMI (kg/m2) | 26 ± 0.7 | 26 ± 0.8 | 27 ± 1.6 | 26 ± 1.1 | |
| Waist circumference (cm) | 93 ± 1.8 | 92 ± 2.0 | 93 ± 2.8 | 92 ± 2.0 | |
| Systolic blood pressure (mmHg) | 123 ± 3.6 | 126 ± 4.5 | 122 ± 5.0 | 112 ± 5.6 | |
| Diastolic blood pressure (mmHg) | 72 ± 1.7 | 73 ± 2.3 | 70 ± 2.3 | 70 ± 2.6 | |
| HbA1c (%) | 9.3 ± 0.4 | 8.6 ± 0.4 | 6.5 ± 0.4 | 8.0 ± 0.7 | |
| Fasting blood sugar (mg/dl) | 217 ± 16.4 | 184 ± 12.6 | 133 ± 11.1 | 182 ± 39.2 | |
| HSP 70 (ng/ml) | 0.93 ± 0.05* | 0.51 ± 0.02 | 0.57 ± 0.03** | 0.96 ± 0.19 | |
| HDL (mg/dl) | 39 ± 1.1 | 41 ± 2.0 | 41 ± 2.6 | 42 ± 3.6 | |
| LDL (mg/dl) | 103 ± 5.8 | 103 ± 8.5 | 88 ± 9.1*** | 122 ± 9.3 | |
| Triglyceride (mg/dl) | 199 ± 25.9 | 169 ± 23.6 | 150 ± 18.1 | 139 ± 17.9 | |
| Cholesterol (mg/dl) | 191 ± 9.1 | 174 ± 9.7 | 168 ± 10.6 | 202 ± 14.7 | |
| eGFR (ml/min) | 65 ± 3.5 | 67 ± 5.0 | 70 ± 3.1 | 75 ± 10 | |
When comparing patients with and without albuminuria, pairs with direct relationship are the pairs which HSP70 is higher in cases, and pairs with reverse relationship are the pairs which HSP70 is higher in controls
*p < 0.001; **p < 0.05; ***p < 0.01
We then studied the value of HSP70 in the prediction of albuminuria using conditional logistic regression model. The HSP70 values were logarithmic transformed for this analysis. The Odds ratio of HSP70 in the prediction of albuminuria was (28.69 (3.2–250.1), p = 0.002).
Discussion
Our findings demonstrated that in a matched case–control design, patients with albuminuria have a higher serum HSP70 levels compared to those without albuminuria. Moreover, we showed that HSP70 is a significant and almost the only predictor of albuminuria in the conditional logistic regression model.
Why HSPs are increased in diabetic nephropathy. The primary explanation is their protective role against oxidative damage caused by diabetes or any other disease (Brezniceanu et al. 2010) (Kulkarni et al. 2012). Dai and collaborators have shown strong correlation between slit membrane density and HSP25 levels in the kidney of diabetic mice (Dai et al. 2006). Inconsistent with our findings, Calabrese and collaborators have shown an increased serum HSP70 levels in nonuremic type 2 diabetic patients with nephropathy. Serum HSP70 levels were positively correlated with markers of oxidative stress in line with an upregulation of these molecules in lymphocytes (Calabrese et al. 2007). In vitro studies on type 1 diabetes model have shown localized increase of HSPs in parts of the kidney. While diabetes induces HSP70 expression in the medulla, it does not modulate HSP expression in the glomeruli (Barutta et al. 2008). It is also shown that hyperthermia therapy reduces proteinuria in diabetic rat model of type 2 diabetes (Kokura et al. 2007).
On the other hand, this increase in HSP expression may also take part into the pathogenesis of diabetic nephropathy. Glomerulosclerosis and tubulointerstitial fibrosis are the main structural changes found in diabetic nephropathy. In situ hybridization of the renal tubular epithelium showed active expression of HSP47 mRNA (Abe et al. 2004). HSP47 is viewed as a collagen triggering HSP and could contribute to nephropathy. Abe and Li’s observation showed that HSP47 contribute to the fibrosis in experimental diabetic nephropathy (Liu et al. 2001; Abe et al. 2004). In other disease such as pulmonary fibrosis, there is an increased HSP47 induction, which is associated with collagen deposition (Iwashita et al. 2000). We have previously shown the duality of HSP70 behavior in low and high inflammatory states (Nakhjavani et al. 2012). These observations have questioned the classic role of these molecules as chaperons. Whether HSPs prevent or induce diabetic nephropathy in the presence of oxidative stress/chronic inflammation remain to be elucidated by future studies.
The principal limitation of the current study is the lack of follow up, which preclude the determination of the direction of causality; however, we took advantage of a relatively large sample size and close similarity between groups in most of the potentially confounding variables. These patients were overweight, with central obesity. The mean waist circumference was about 93 cm. The mean BMI in was 27, which is not in the obesity range. As previously reported, the cut off values for obesity and waist circumference differ in Iranian population compared to other societies (Delavari et al. 2009). This could be the reason for a surprisingly low BMI and high waist circumference values in the current study. In conclusion, we have shown an increased HSP70 levels in diabetic patients with albuminuria.
Contributor Information
Afsaneh Morteza, Phone: +98-21-8841791, FAX: +98-21-64432466, Email: aafsaneh03@gmail.com, Email: afsaneh.morteza@gmail.com.
Manouchehr Nakhjavani, Email: nakhjavanim@tums.ac.ir.
Mehrdada Larry, Email: mehrdad.larry@gmail.com.
Arash Aghajani Nargesi, Email: arash.aan@gmail.com.
Alireza Esteghamati, Email: esteghamati@tums.ac.ir.
References
- Abe K, Li K, Sacks SH, Sheerin NS. The membrane attack complex, C5b-9, up regulates collagen gene expression in renal tubular epithelial cells. Clin Exp Immunol. 2004;136(1):60–66. doi: 10.1111/j.1365-2249.2004.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Pinach S, Giunti S, Vittone F, Forbes JM, Chiarle R, Arnstein M, Perin PC, Camussi G, Cooper ME, Gruden G. Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295(6):F1817–1824. doi: 10.1152/ajprenal.90234.2008. [DOI] [PubMed] [Google Scholar]
- Brezniceanu ML, Lau CJ, Godin N, Chenier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21(6):943–954. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraczynska M, Swatowski A, Buraczynska K, Dragan M, Ksiazek A. Heat shock protein gene polymorphisms and the risk of nephropathy in patients with Type 2 diabetes. Clin Sci (Lond) 2009;116(1):81–86. doi: 10.1042/CS20070411. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Di Mauro M, Stella AM, Castellino P. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12(4):299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Natarajan R, Nast CC, LaPage J, Chuang P, Sim J, Tong L, Chamberlin M, Wang S, Adler SG. Glucose and diabetes: effects on podocyte and glomerular p38MAPK, heat shock protein 25, and actin cytoskeleton. Kidney Int. 2006;69(5):806–814. doi: 10.1038/sj.ki.5000033. [DOI] [PubMed] [Google Scholar]
- Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. (2009) First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care 32(6):1092–7. doi:10.2337/dc08-1800 [DOI] [PMC free article] [PubMed]
- Diagnosis and classification of diabetes mellitus Diabetes Care. 2009;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita T, Kadota J, Naito S, Kaida H, Ishimatsu Y, Miyazaki M, Ozono Y, Kohno S. Involvement of collagen-binding heat shock protein 47 and procollagen type I synthesis in idiopathic pulmonary fibrosis: contribution of type II pneumocytes to fibrosis. Hum Pathol. 2000;31(12):1498–1505. doi: 10.1053/hupa.2000.20378. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300(5):E894–901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia. 2007;23(3):259–265. doi: 10.1080/02656730601176824. [DOI] [PubMed] [Google Scholar]
- Kulkarni OP, Ryu M, Kantner C, Sardy M, Naylor D, Lambert D, Brown R, Anders HJ. Recombinant chaperoning ten suppresses cutaneous lupus and lupus nephritis in MRL-(Fas)lpr mice. Nephrol Dial Transplant. 2012;27(4):1358–1367. doi: 10.1093/ndt/gfr544. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Srivastava P (2004) Heat-shock proteins. Curr Protoc Immunol Appendix 1:Appendix 1T. doi:10.1002/0471142735.ima01ts58 [DOI] [PubMed]
- Liu D, Razzaque MS, Cheng M, Taguchi T. The renal expression of heat shock protein 47 and collagens in acute and chronic experimental diabetes in rats. Histochem J. 2001;33(11–12):621–628. doi: 10.1023/A:1016398200087. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Asgarani F, Khalilzadeh O, Ghazizadeh Z, Bathaie SZ, Esteghamati A. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498(1):107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15(6):959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Meysamie A, Esteghamati A, Khalilzadeh O, Esfahanian F, Khajeali L, Feiz F. Serum heat shock protein 70 and oxidized LDL in patients with type 2 diabetes: does sex matter? Cell Stress Chaperones. 2011;16(2):195–201. doi: 10.1007/s12192-010-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]